The Growth of Intermetallic Compounds and Its Effect on Bonding Properties of Cu/Al Clad Plates by CFR
Abstract
1. Introduction
2. Experimental Materials and Methods
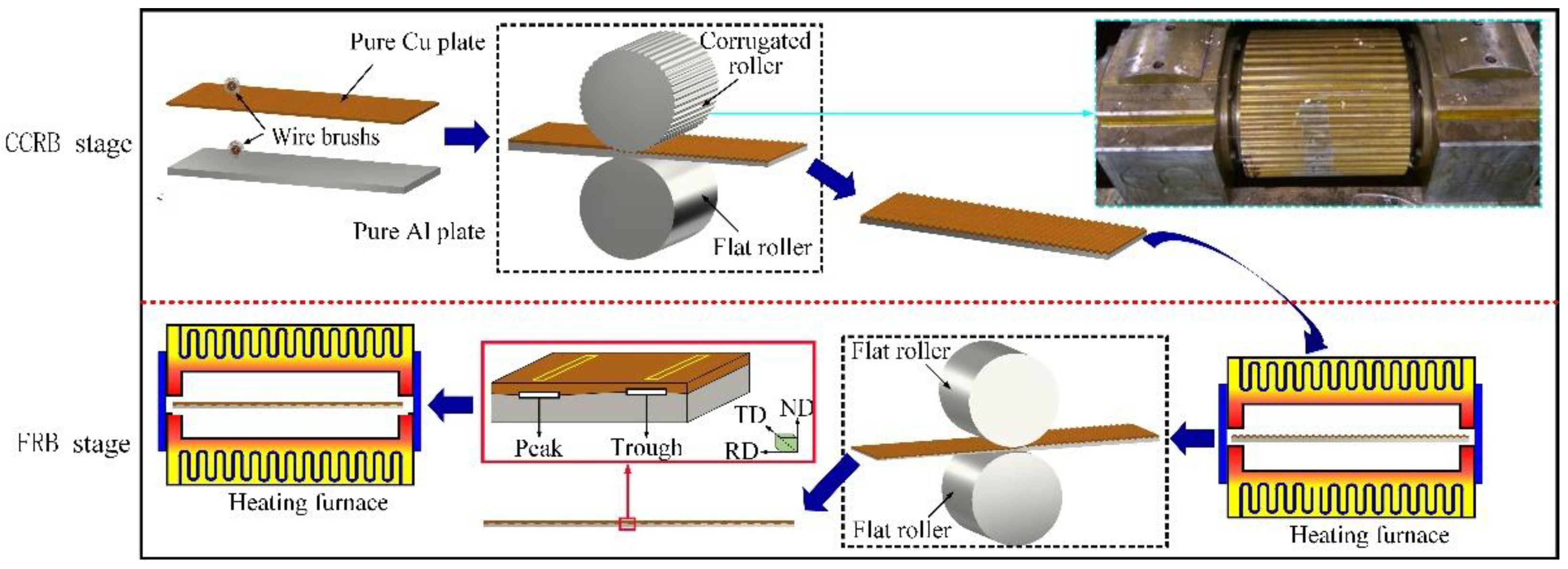
2.1. Materials Preparation
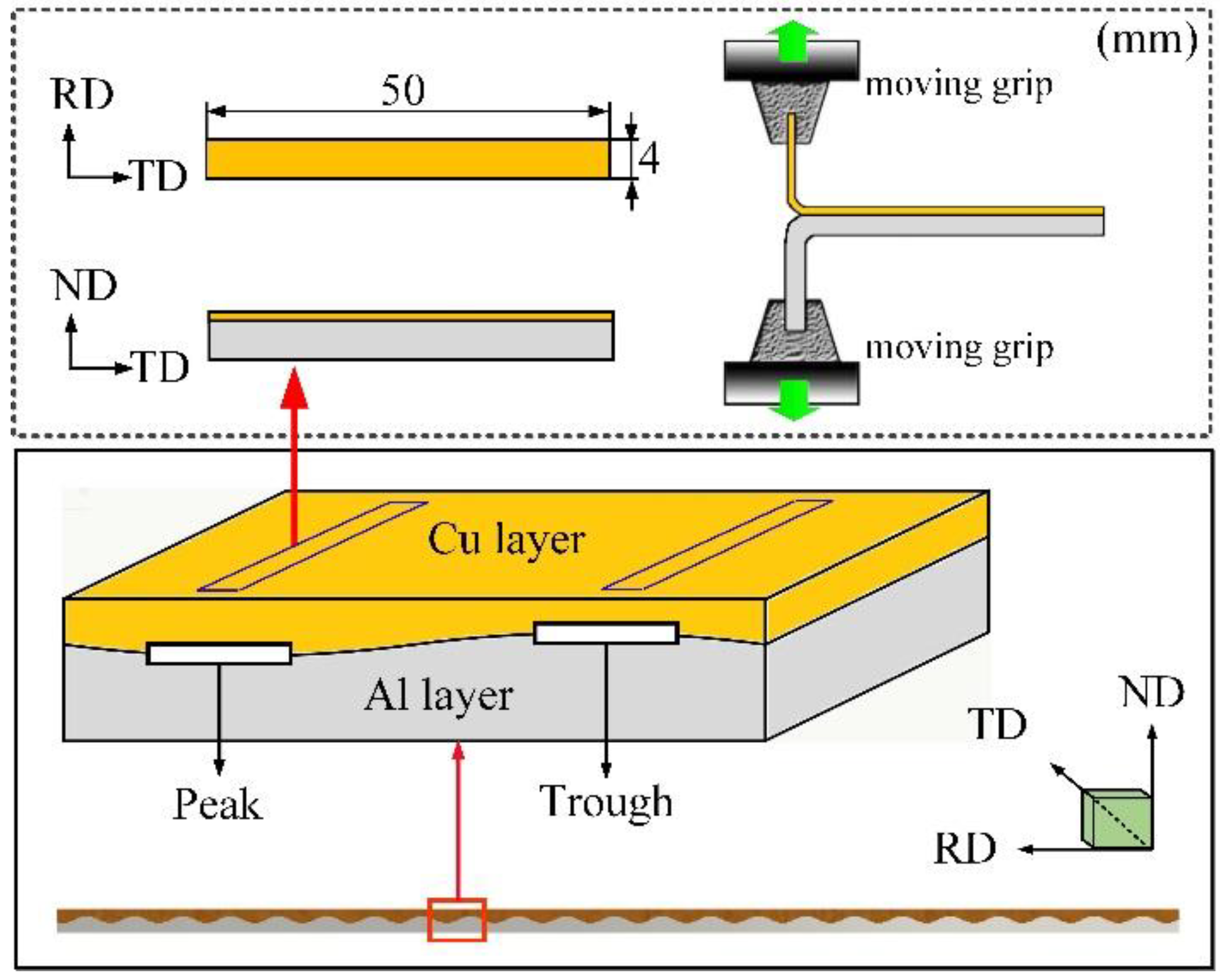
2.2. Mechanical Properties
2.3. Microstructural Characterization
3. Results and Discussion
3.1. Interface Microstructure of Cu/Al Clad Plate after CCRB and FRB
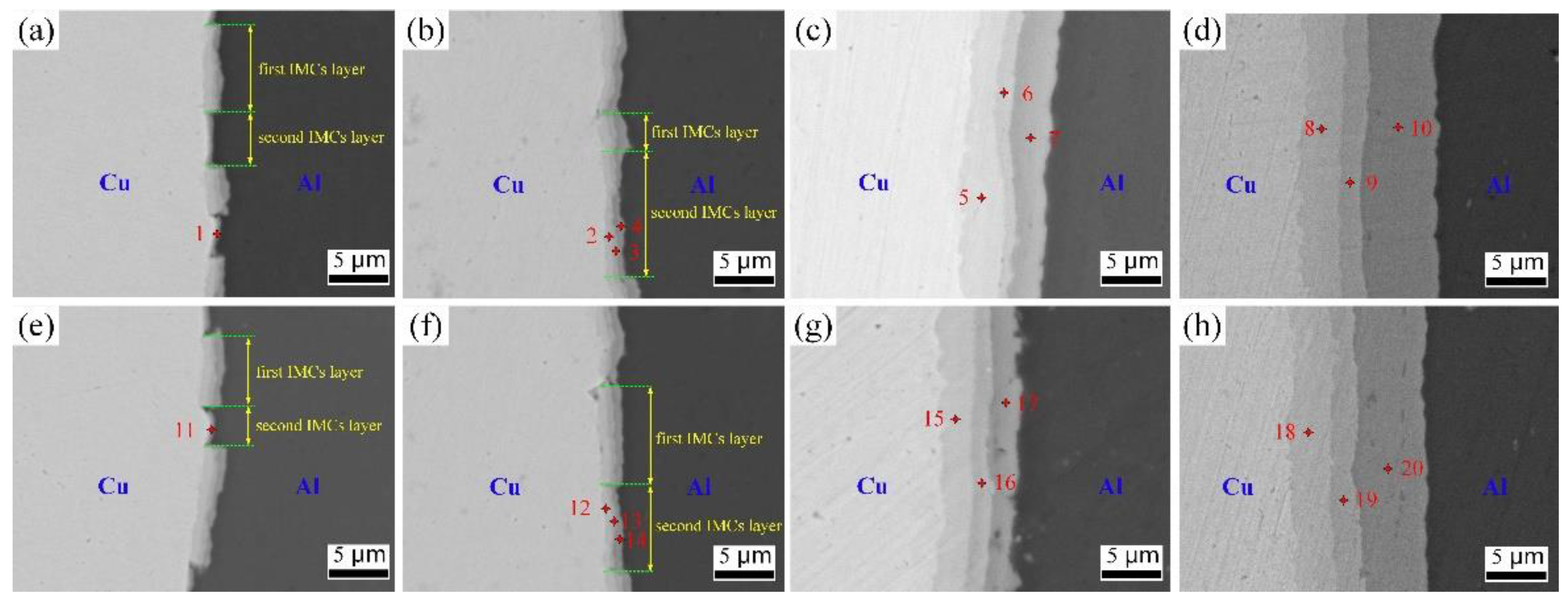
3.2. Interface Structure Evolution after Annealing
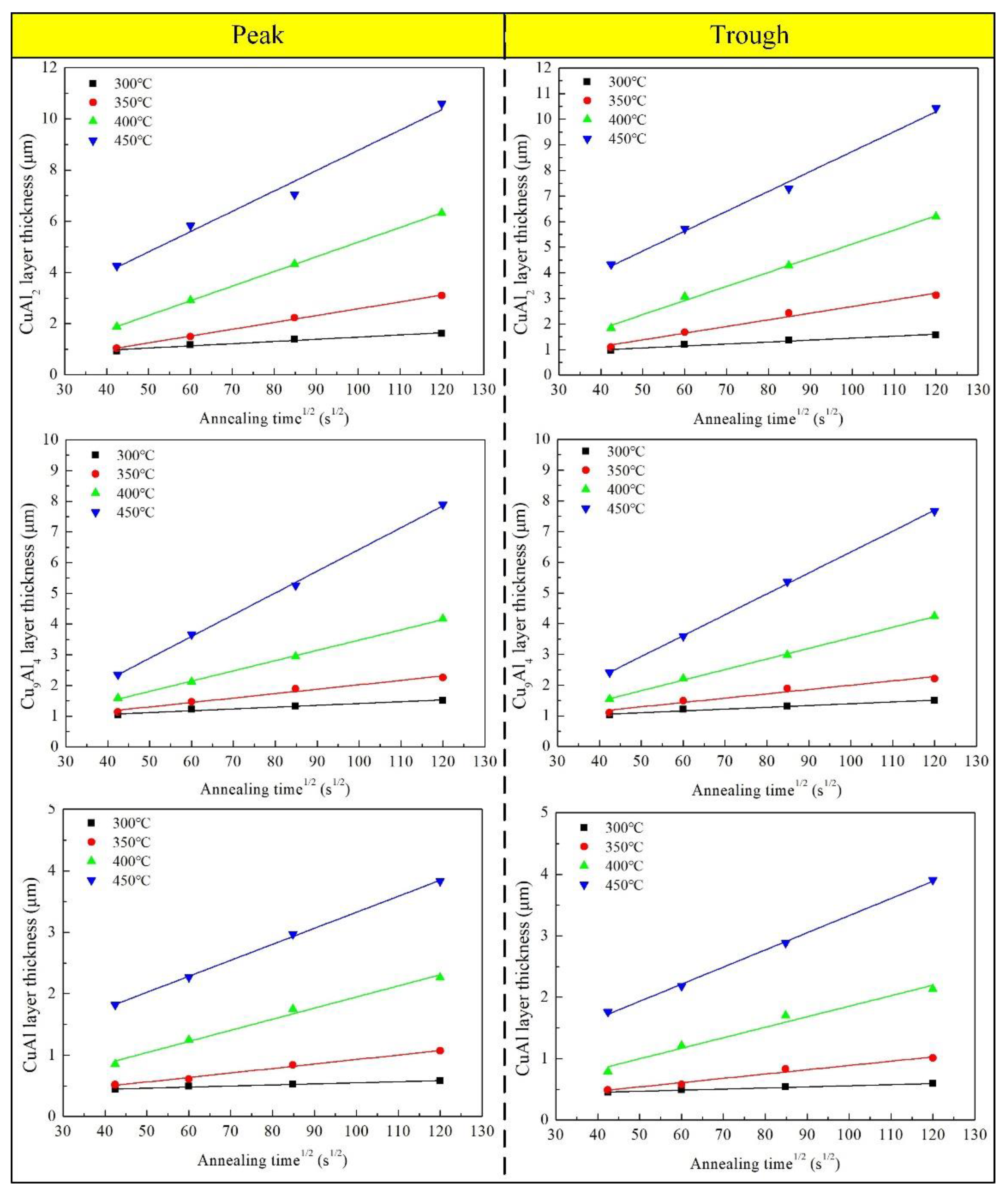
3.3. Growth of Interface IMCs Layer during Annealing
3.4. Effect of Annealing Time on Interface Structure and Bonding Properties
3.4.1. Interface Microstructure Evolution
3.4.2. Bonding Performance
3.4.3. Microstructure of Peel Surface
4. Conclusions
- (1)
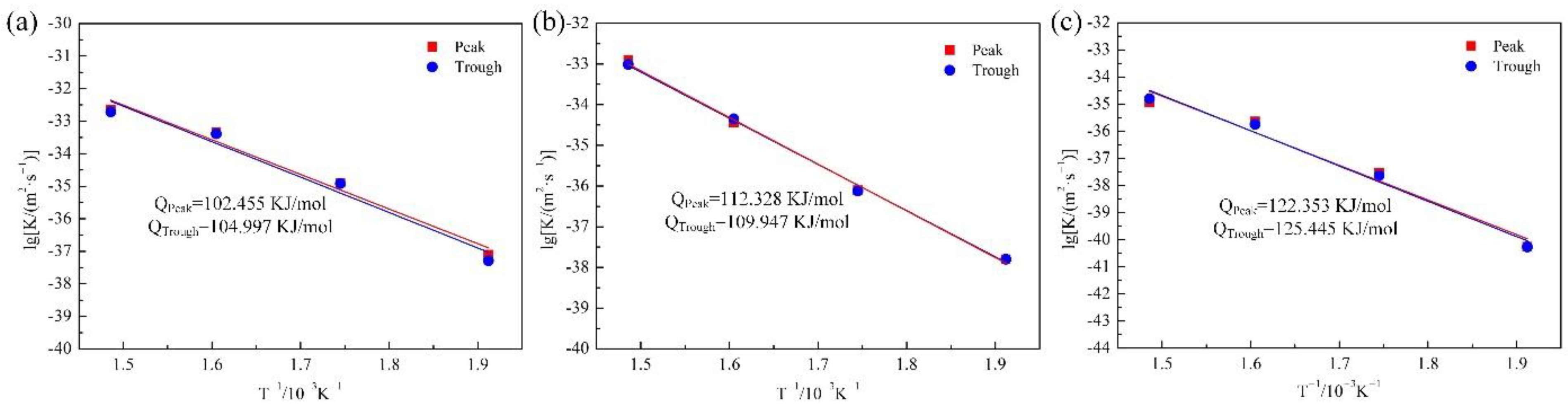
- The growth activation energies calculated for the Cu9Al4, CuAl2, and CuAl phases were 112.328 KJ/mol, 102.455 KJ/mol, and 122.353 KJ/mol and 109.947 KJ/mol, 104.997 KJ/mol, and 125.445 KJ/mol at the peak and trough, respectively. Among them, the growth activation energy at the peak position was slightly higher than that at the trough position. The activation energy sequence of the three IMCs was CuAl > Cu9Al4 > CuAl2.
- (2)
- The formation of the first IMCs layer was significantly restrained at the early stages of annealing by a greater atomic concentration gradient in the new bonding interface area, which caused a slight rise in the IMCs layer’s growth activation energy.
- (3)
- The maximum average peel strength at the peak and trough interfaces annealing at 350 °C for 10 min reached 53.07 N/mm and 41.23 N/mm, respectively. Furthermore, the average peel strength of the interface gradually decreased as the annealing period was extended, and numerous brittle IMCs layers formed at the interface.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mroz, S.; Wierzba, A.; Stefanik, A.; Szota, P. Effect of asymmetric accumulative roll-bonding process on the microstructure and strength evolution of the AA1050/AZ31/AA1050 multilayered composite materials. Materials 2020, 13, 5401. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Hong, S.I. Effect of heat treatment on the bending behavior of tri-layered Cu/Al/Cu composite plates. Mater. Des. 2013, 47, 590–598. [Google Scholar] [CrossRef]
- Mo, T.; Chen, Z.; Zhou, Z.; Liu, J.; He, W.; Liu, Q. Enhancing of mechanical properties of rolled 1100/7075 Al alloys laminated metal composite by thermomechanical treatments. Mater. Sci. Eng. A 2021, 800, 140313. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, D.; Cao, X.; Yang, W.; Wang, W. Influence of multi-pass rolling and subsequent annealing on the interface microstructure and mechanical properties of the explosive welding Mg/Al composite plates. Mater. Sci. Eng. A 2018, 723, 97–108. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, B.; Xin, Y.; Hong, R.; Yu, H.; Liu, Q. Microstructure and mechanical behavior of a Mg AZ31/Al 7050 laminate composite fabricated by extrusion. Mater. Sci. Eng. A 2015, 640, 454–459. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Pramana, S.S.; White, T.J.; Chen, Z.; Acoff, V.L. Behavior of aluminum oxide, intermetallics and voids in Cu–Al wire bonds. Acta Mater. 2011, 59, 5661–5673. [Google Scholar] [CrossRef]
- Ghalandari, L.; Mahdavian, M.M.; Reihanian, M. Microstructure evolution and mechanical properties of Cu/Zn multilayer processed by accumulative roll bonding (ARB). Mater. Sci. Eng. A 2014, 593, 145–152. [Google Scholar] [CrossRef]
- Abedi, R.; Akbarzadeh, A. Bond strength and mechanical properties of three-layered St/AZ31/St composite fabricated by roll bonding. Mater. Des. 2015, 88, 880–888. [Google Scholar] [CrossRef]
- Gao, X.; Bian, L.; Zhao, J.; Han, J.; Wang, T.; Huang, Q. Interface-position-dependent structure evolution and mechanical behavior of corrugated cold rolled Cu/Al clad plate: Experiment and simulation. J. Mater. Res. Technol. 2021, 13, 216–227. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Bian, L.; Huang, Q. Microstructural evolution and mechanical behavior of Mg/Al laminated composite sheet by novel corrugated rolling and flat rolling. Mater. Sci. Eng. A 2019, 765, 138318. [Google Scholar] [CrossRef]
- Sheng, L.Y.; Yang, F.; Xi, T.F.; Lai, C.; Ye, H.Q. Influence of heat treatment on interface of Cu/Al bimetal composite fabricated by cold rolling. Compos. Part B-Eng. 2011, 42, 1468–1473. [Google Scholar] [CrossRef]
- Wang, T.; Li, S.; Ren, Z.; Han, J.; Huang, Q. A novel approach for preparing Cu/Al laminated composite based on corrugated roll. Mater. Lett. 2019, 234, 79–82. [Google Scholar] [CrossRef]
- Chang, D.; Wang, P.; Zhao, Y. Effects of asymmetry and annealing on interfacial microstructure and mechanical properties of Cu/Al laminated composite fabricated by asymmetrical roll bonding. J. Alloys Compd. 2020, 815, 152453. [Google Scholar] [CrossRef]
- Luo, C.; Liang, W.; Chen, Z.; Zhang, J.; Chi, C.; Yang, F. Effect of high temperature annealing and subsequent hot rolling on microstructural evolution at the bond-interface of Al/Mg/Al alloy laminated composites. Mater. Charact. 2013, 84, 34–40. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, H.L.; Hwang, W.S. Influence of interfacial structure development on the fracture mechanism and bond strength of aluminum/copper bimetal plate. Mater. Trans. 2006, 47, 1232–1239. [Google Scholar] [CrossRef]
- Heness, G.; Wuhrer, R.; Yeung, W.Y. Interfacial strength development of roll-bonded aluminium/copper metal laminates. Mater. Sci. Eng. A 2008, 483–484, 740–742. [Google Scholar] [CrossRef]
- Wang, T.; Gao, X.Y.; Zhang, Z.X.; Ren, Z.K.; Qi, Y.Y.; Zhao, J.W. Interfacial bonding mechanism of Cu/Al composite plate produced by corrugated cold roll bonding. Rare Metals 2021, 40, 1284–1293. [Google Scholar] [CrossRef]
- Topolski, K.; Szulc, Z.; Garbacz, H. Microstructure and properties of the Ti6Al4V/Inconel 625 bimetal obtained by explosive joining. J. Mater. Eng. Perform. 2016, 25, 3231–3237. [Google Scholar] [CrossRef]
- Srinivasan, R.; Karthik Raja, G. Experimental study on bending behaviour of aluminium-copper clad sheets in V-bending process. Mech. Ind. 2019, 20, 618. [Google Scholar] [CrossRef]
- Chen, C.; Ni, P.Y.; Jonsson, L.T.I.; Tilliander, A.; Cheng, G.G.; Jönsson, P.G. A Model Study of Inclusions Deposition, Macroscopic Transport, and Dynamic Removal at Steel–Slag Interface for Different Tundish Designs. Metall. Mater. Trans. B 2016, 47, 1916–1932. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, G.G. Delta-ferrite distribution in a continuous casting slab of Fe-Cr-Mn austenitic stainless steel. Metall. Mater. Trans. B 2017, 48, 2324–2333. [Google Scholar] [CrossRef]
- Han, Y.Q.; Ben, L.H.; Yao, J.J.; Wu, C.J. Microstructural characterization of Cu/Al composites and effect of cooling rate at the Cu/Al interfacial region. Int. J. Miner. Metall. Mater. 2015, 22, 94–101. [Google Scholar] [CrossRef]
- Yuan, X.G.; Lv, L.; Huang, H.J.; Zuo, X.J.; Liu, H. Effect of diffusion heat treatment on thickness of cold-rolled Cu/Al composite laminate interface. Appl. Mech. Mater. 2013, 310, 112–116. [Google Scholar] [CrossRef]
- Mao, Z.; Xie, J.; Wang, A.; Wang, W.; Li, Y.; Ma, D. Interfacial microstructure and bonding strength of copper/aluminum clad sheets produced by horizontal twin-roll casting and annealing. Mater. Res. Express. 2018, 6, 016505. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.H.; Chen, G.H.; Wang, R.M.; Miao, C.H.; Zheng, Z.X.; Tang, W.M. Formation and growth of Cu–Al IMCs and their effect on electrical property of electroplated Cu/Al laminar composites. Trans. Nonferr. Metal. Soc. 2016, 26, 3283–3291. [Google Scholar] [CrossRef]
- Lee, W.B.; Bang, K.S.; Jung, S.B. Effects of intermetallic compound on the electrical and mechanical properties of friction welded Cu/Al bimetallic joints during annealing. J. Alloys Compd. 2005, 390, 212–219. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hwang, W.S. Effect of annealing on the interfacial structure of aluminum-copper joints. Mater. Trans. 2007, 48, 1938–1947. [Google Scholar] [CrossRef]
- Braunovic, M.; Alexandrov, N. Intermetallic compounds at aluminum-to-copper electrical interfaces: Effect of temperature and electric current. IEEE. Trans. Compon. Packag. Manuf. Technol. Part A 1994, 17, 78–85. [Google Scholar] [CrossRef]
- Hug, E.; Bellido, N. Brittleness study of intermetallic (Cu, Al) layers in copper-clad aluminium thin wires. Mater. Sci. Eng. A 2011, 528, 7103–7106. [Google Scholar] [CrossRef]



| Component Plate | Cu | Al | Ti | Bi | Sb | As | Fe | Sn | S | Si |
|---|---|---|---|---|---|---|---|---|---|---|
| T2Cu | >99 | - | - | 0.001 | 0.002 | 0.002 | 0.008 | 0.008 | 0.005 | - |
| 1060Al | - | >99.61 | 0.013 | - | - | - | 0.26 | - | - | 0.08 |
| Component Plate | Hardness (VHN) | Yield Strength (MPa) | Elongation (%) |
|---|---|---|---|
| T2Cu | 86 | 90 | 30.93% |
| 1060Al | 41 | 79 | 25.29% |
| Element | Point 1 | Point 2 | Point 3 | Point 4 | Point 5 | Point 6 | Point 7 | Point 8 | Point 9 | Point 10 |
| Cu | 39.45 | 62.56 | 48.59 | 34.84 | 67.37 | 51.94 | 37.82 | 68.35 | 47.92 | 38.67 |
| Al | 60.55 | 37.44 | 51.41 | 65.16 | 32.63 | 48.06 | 62.18 | 31.65 | 52.08 | 61.33 |
| Element | Point 11 | Point 12 | Point 13 | Point 14 | Point 15 | Point 16 | Point 17 | Point 18 | Point 19 | Point 20 |
| Cu | 42.84 | 64.34 | 50.16 | 32.57 | 64.91 | 53.58 | 31.49 | 66.82 | 51.95 | 34.55 |
| Al | 57.16 | 35.66 | 49.84 | 67.43 | 35.09 | 46.42 | 68.51 | 33.18 | 48.05 | 65.45 |
| Position | Temperature (°C) | IMC | K (m2/s) | Position | Temperature (°C) | IMC | K (m2/s) |
|---|---|---|---|---|---|---|---|
| Peak | 300 | CuAl2 | 7.68 × 10−17 | Trough | 300 | CuAl2 | 6.39 × 10−17 |
| Cu9Al4 | 3.83 × 10−17 | Cu9Al4 | 3.83 × 10−17 | ||||
| CuAl | 3.26 × 10−18 | CuAl | 3.26 × 10−18 | ||||
| 350 | CuAl2 | 6.98 × 10−16 | 350 | CuAl2 | 6.85 × 10−16 | ||
| Cu9Al4 | 2.08 × 10−16 | Cu9Al4 | 2.05 × 10−16 | ||||
| CuAl | 5.03 × 10−17 | CuAl | 4.48 × 10−17 | ||||
| 400 | CuAl2 | 3.29 × 10−15 | 400 | CuAl2 | 3.16 × 10−15 | ||
| Cu9Al4 | 1.11 × 10−15 | Cu9Al4 | 1.21 × 10−15 | ||||
| CuAl | 3.30 × 10−16 | CuAl | 2.98 × 10−16 | ||||
| 450 | CuAl2 | 6.66 × 10−15 | 450 | CuAl2 | 6.18 × 10−15 | ||
| Cu9Al4 | 5.09 × 10−15 | Cu9Al4 | 4.59 × 10−15 | ||||
| CuAl | 6.71 × 10−16 | CuAl | 7.68 × 10−16 |
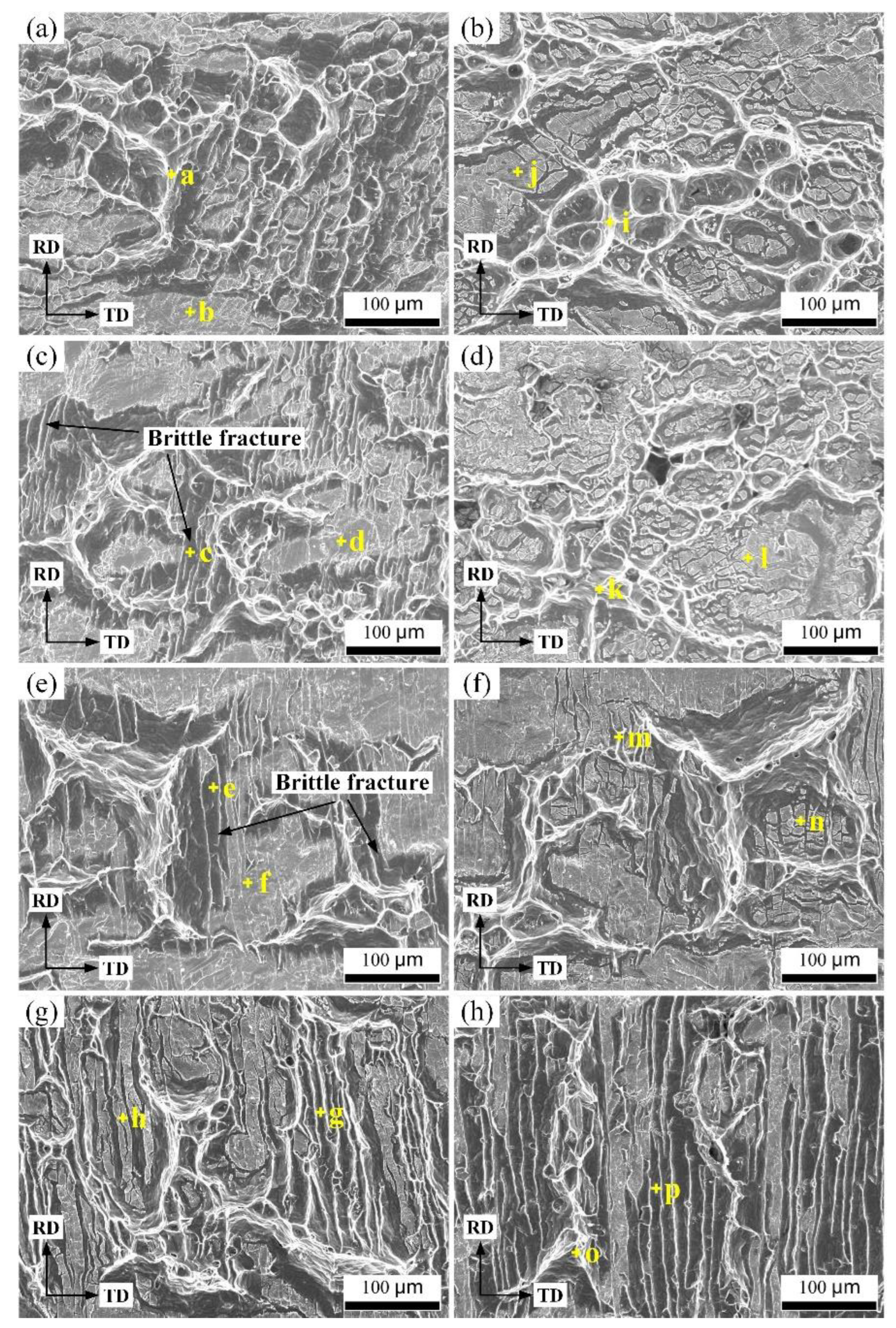
| Element | Point a | Point b | Point c | Point d | Point e | Point f | Point g | Point h | |
| Cu side | Cu | 3.48 | 66.36 | 25.33 | 65.31 | 17.67 | 64.58 | 78.36 | 59.32 |
| Al | 96.52 | 33.64 | 74.67 | 34.69 | 82.33 | 35.42 | 21.64 | 40.68 | |
| Element | Point i | Point j | Point k | Point l | Point m | Point n | Point o | Point p | |
| Al side | Cu | 2.64 | 43.61 | 7.64 | 48.41 | 23.45 | 48.67 | 8.47 | 35.78 |
| Al | 97.36 | 56.39 | 92.36 | 51.59 | 76.55 | 41.33 | 91.53 | 64.22 |
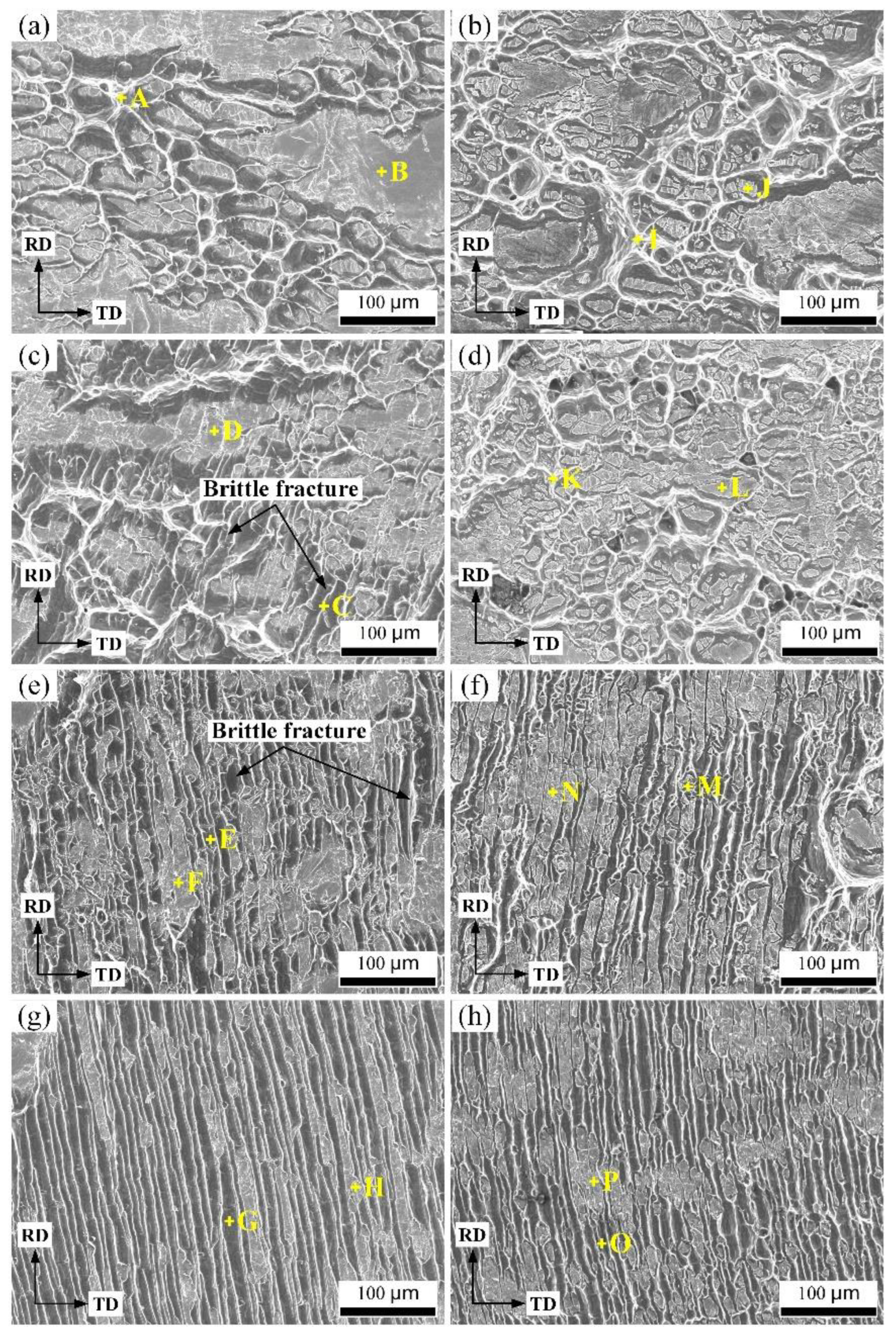
| Element | Point A | Point B | Point C | Point D | Point E | Point F | Point G | Point H | |
| Cu side | Cu | 5.62 | 61.88 | 25.41 | 41.86 | 30.29 | 52.61 | 22.37 | 48.62 |
| Al | 94.38 | 38.12 | 74.59 | 58.14 | 69.71 | 47.39 | 77.63 | 51.38 | |
| Element | Point I | Point J | Point K | Point L | Point M | Point N | Point O | Point P | |
| Al side | Cu | 5.37 | 36.76 | 16.34 | 42.18 | 22.31 | 42.85 | 20.38 | 54.86 |
| Al | 94.63 | 63.24 | 83.66 | 57.82 | 77.69 | 57.15 | 79.62 | 45.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Deng, G.; Zhai, W.; Li, S.; Gao, X.; Wang, T. The Growth of Intermetallic Compounds and Its Effect on Bonding Properties of Cu/Al Clad Plates by CFR. Metals 2022, 12, 1995. https://doi.org/10.3390/met12111995
Li L, Deng G, Zhai W, Li S, Gao X, Wang T. The Growth of Intermetallic Compounds and Its Effect on Bonding Properties of Cu/Al Clad Plates by CFR. Metals. 2022; 12(11):1995. https://doi.org/10.3390/met12111995
Chicago/Turabian StyleLi, Long, Guangping Deng, Weiguo Zhai, Sha Li, Xiangyu Gao, and Tao Wang. 2022. "The Growth of Intermetallic Compounds and Its Effect on Bonding Properties of Cu/Al Clad Plates by CFR" Metals 12, no. 11: 1995. https://doi.org/10.3390/met12111995
APA StyleLi, L., Deng, G., Zhai, W., Li, S., Gao, X., & Wang, T. (2022). The Growth of Intermetallic Compounds and Its Effect on Bonding Properties of Cu/Al Clad Plates by CFR. Metals, 12(11), 1995. https://doi.org/10.3390/met12111995






