On the Microstructure Development under Cyclic Temperature Conditions during WAAM of Microalloyed Steels
Abstract
1. Introduction
2. Experimental Procedure
2.1. Investigated Materials
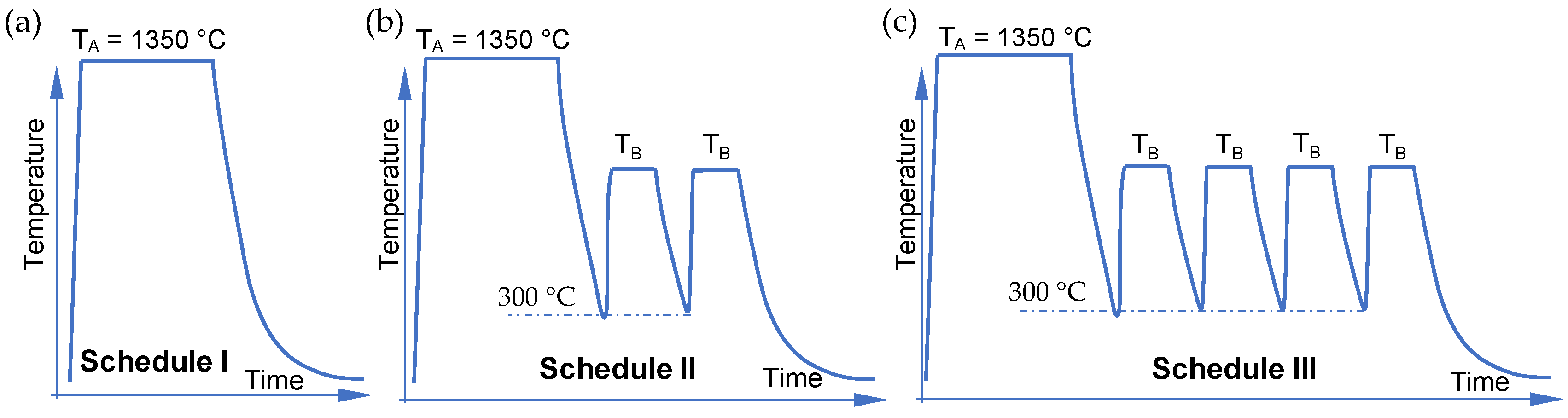
2.2. Dilatometry and Heat Treatment
2.3. Microstructure Investigations
2.4. Hardness Test
3. Results and Discussions
3.1. Microstructure Charactersitics
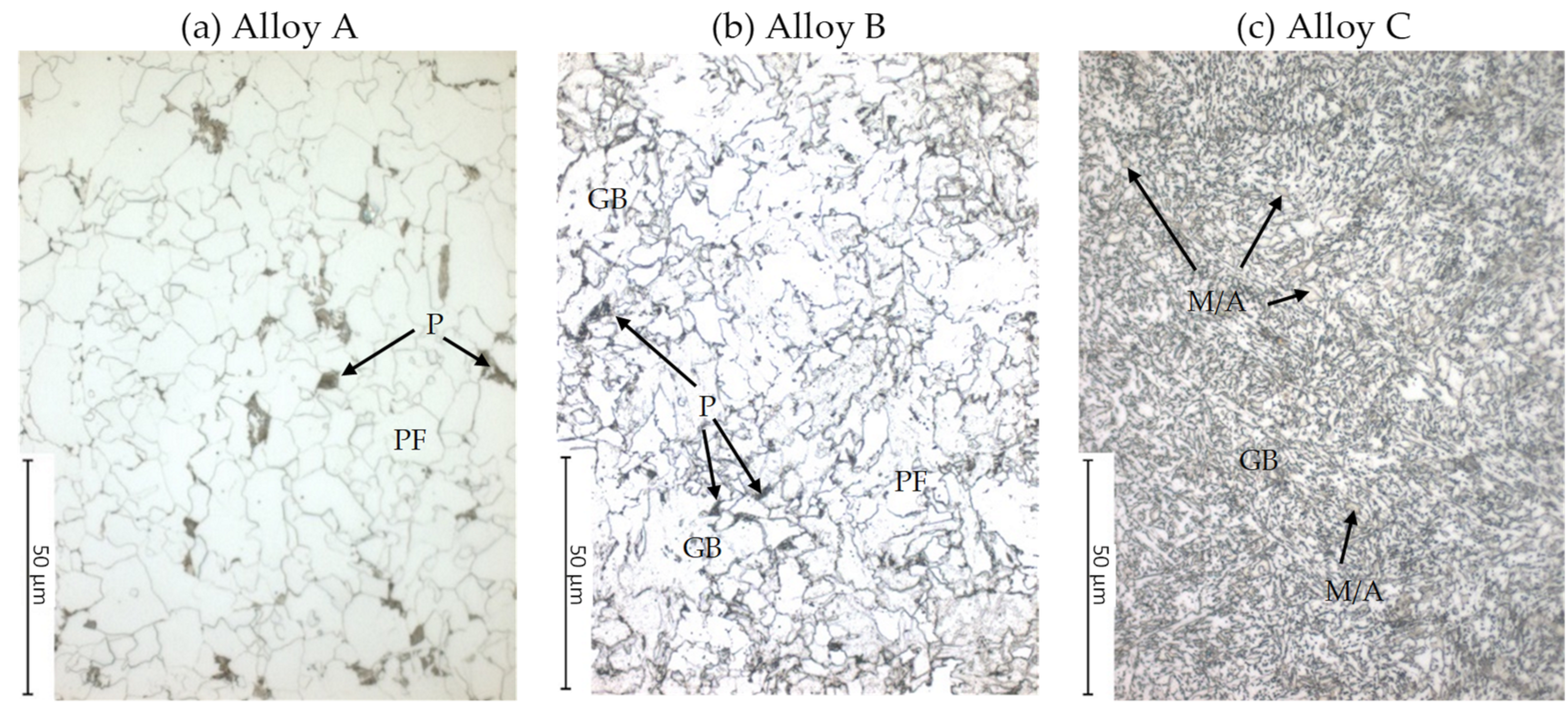
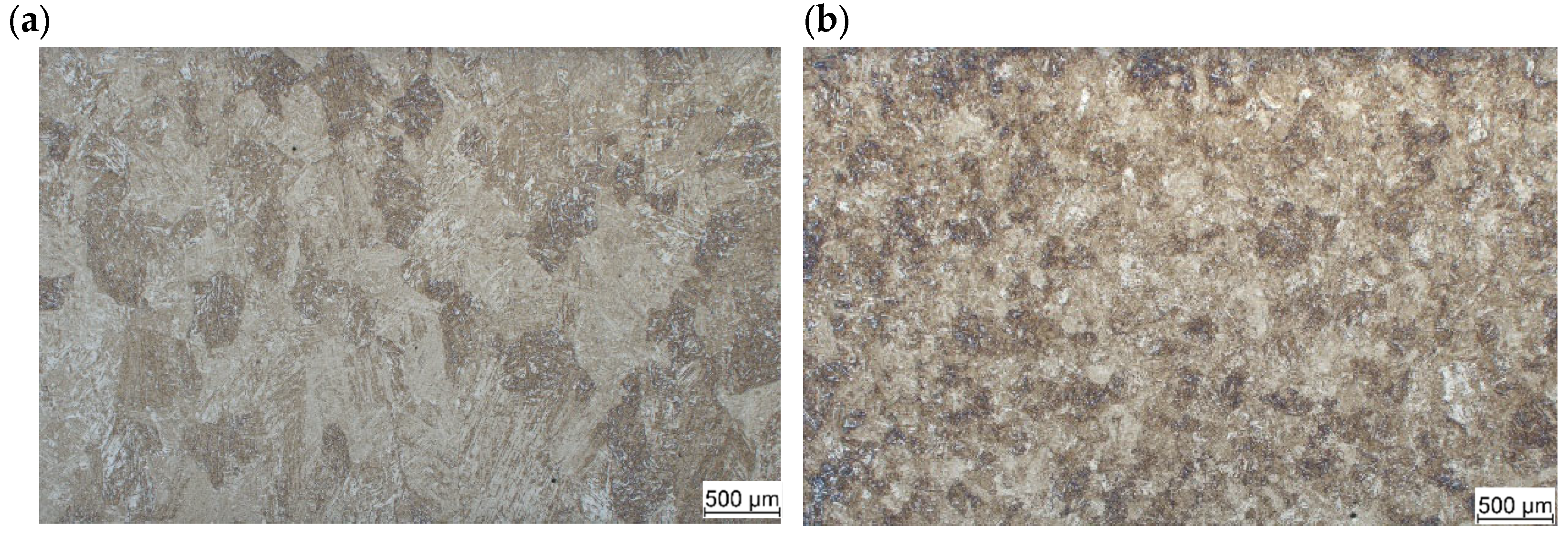
3.1.1. As Received Microstructure
3.1.2. Microstructure Development during Reheating and Austenitization
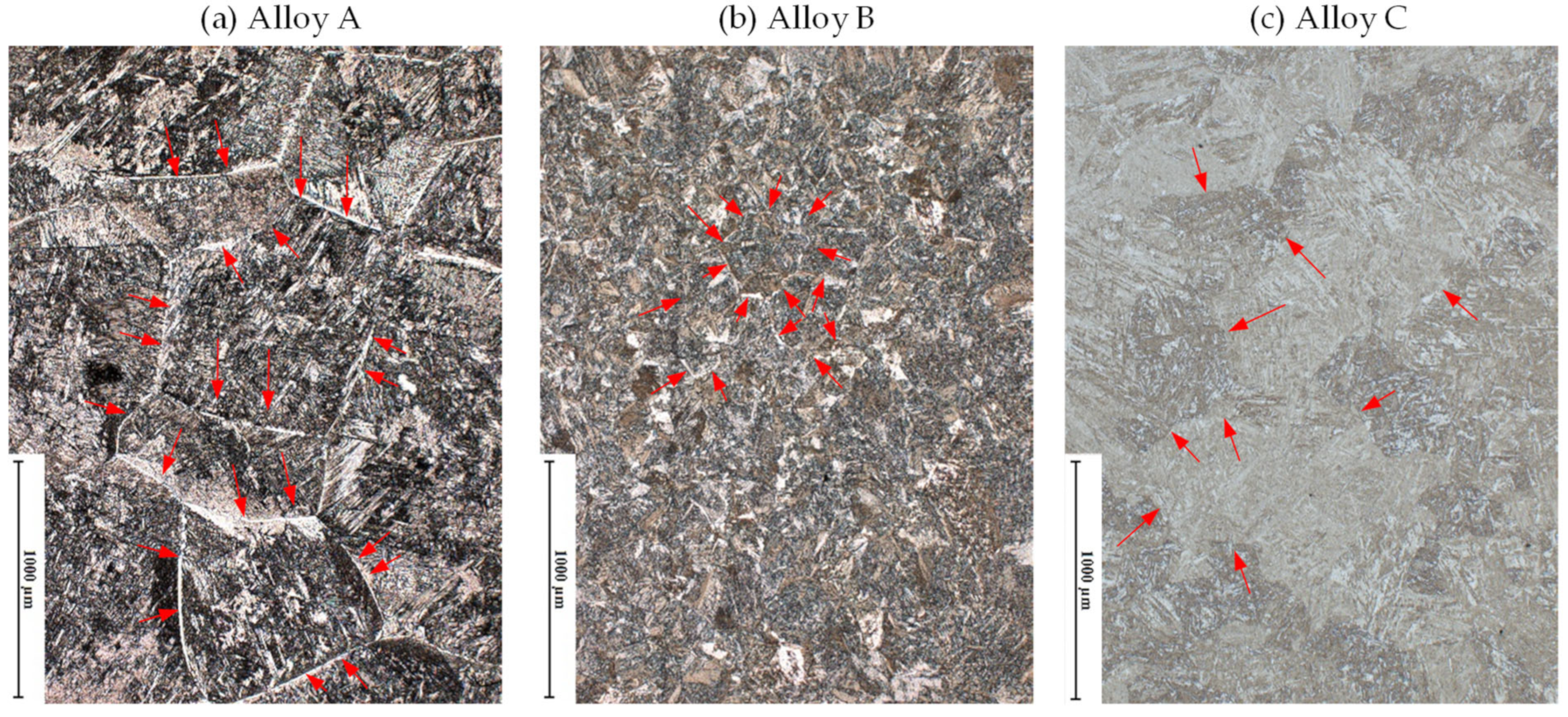
3.1.3. Microstructure Development during Applying Schedule I
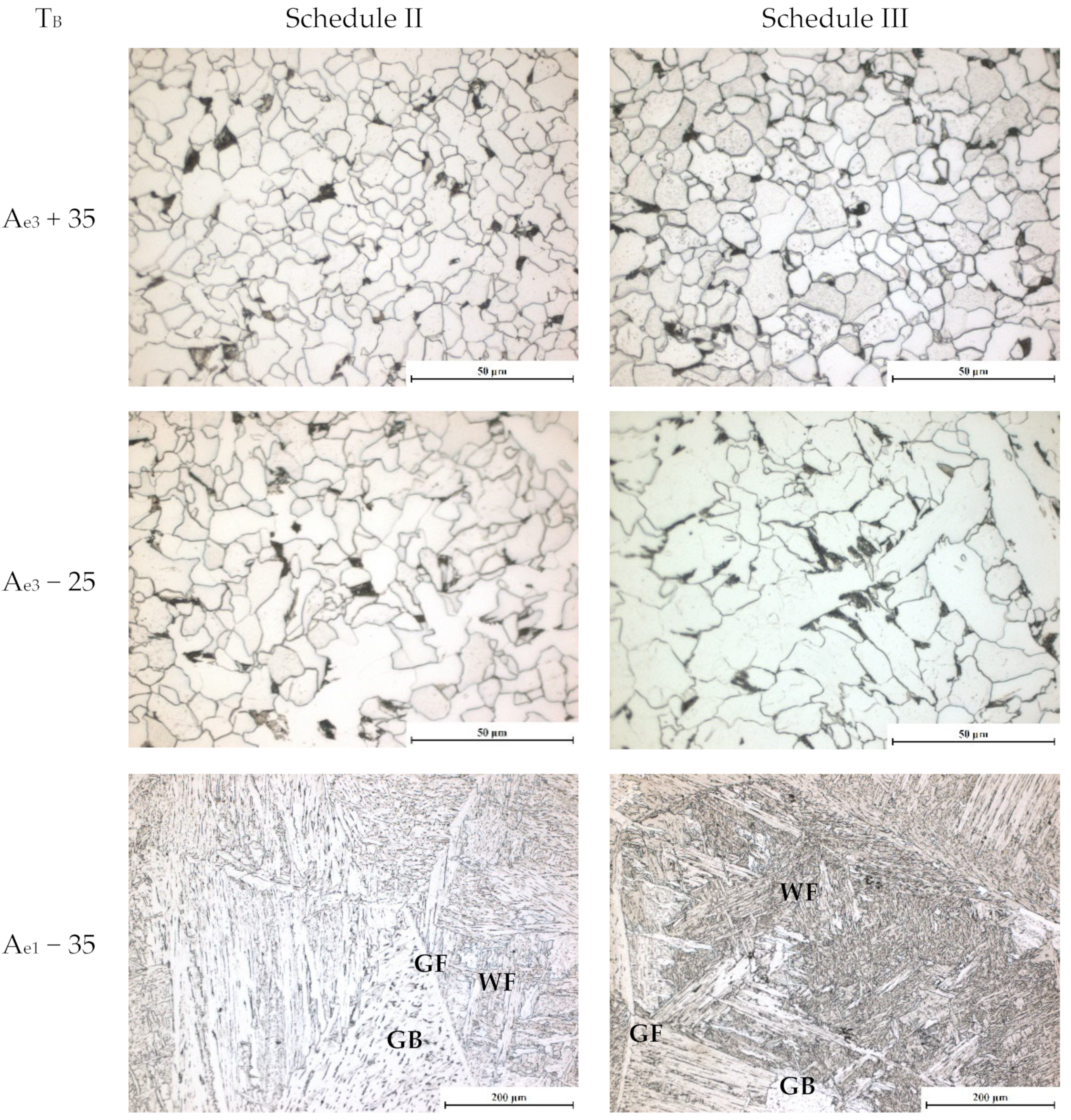
3.1.4. Microstructure Development during Applying Schedule II
3.1.5. Microstructure Developed after Applying Schedule III
3.2. Hardness Characteristics
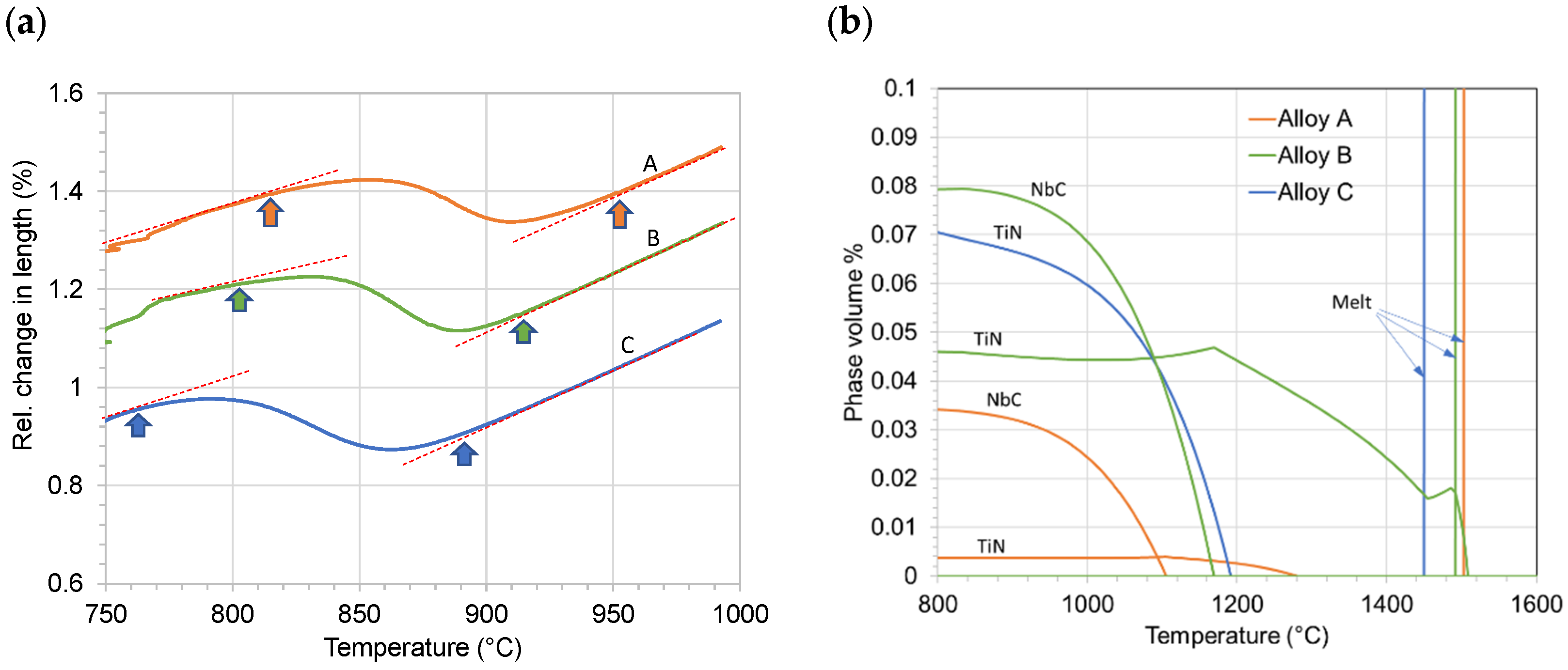
3.3. Transformation Temperatures during the Thermal Cycles
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treutler, K.; Wesling, V. The Current State of Research of Wire Arc Additive Manufacturing (WAAM): A Review. Appl. Sci. 2021, 11, 8619. [Google Scholar] [CrossRef]
- Mbodj, N.G.; Abuabiah, M.; Plapper, P.; El Kandaoui, M.; Yaacoubi, S. Bead Geometry Prediction in Laser-Wire Additive Manufacturing Process Using Machine Learning: Case of Study. Appl. Sci. 2021, 11, 11949. [Google Scholar] [CrossRef]
- Richter, A.; Scheck, M.; Gehling, T.; Bohn, C.; Wesling, V.; Rembe, C. Erfassung geometrischer Daten des Schmelzbades zur Regelung eines WAAM-Prozesses. tm Tech. Mess. 2021, 88, s95–s100. [Google Scholar] [CrossRef]
- Richter, A.; Gehling, T.; Treutler, K.; Wesling, V.; Rembe, C. Real-time measurement of temperature and volume of the weld pool in wire-arc additive manufacturing. Meas. Sens. 2021, 17, 100060. [Google Scholar] [CrossRef]
- Richter, A.; Rembe, C.; Gehling, T.; Treutler, K.; Wesling, V. Echtzeittemperaturmessung bei additivem Lichtbogenschweißen/Real-time temperature measurement at wire arc additive welding. tm Tech. Mess. 2019, 86, 112–116. [Google Scholar] [CrossRef]
- Richter, A.; Scheck, M.; Bohn, C.; Rembe, C. Erfassung der Schmelzbadfläche mit Korrektur der Perspektive zur Prozessregelung eines Wire and Arc Additive Manufacturing. tm Tech. Mess. 2022, 89, 525–533. [Google Scholar] [CrossRef]
- Scheck, M.; Franz, J.; Richter, A.; Gehling, T.; Treutler, K.; Beitler, S.; Wesling, V.; Rembe, C.; Bohn, C. Identification and Modeling of Wire Arc Additive Manufacturing under consideration of Interpass Temperature. In Proceedings of the 2022 UKACC 13th International Conference on Control (CONTROL), Plymouth, UK, 20–22 April 2022; IEEE: Toulouse, France, 2022; pp. 219–225. [Google Scholar] [CrossRef]
- Ehlers, R.; Treutler, K.; Wesling, V. SAT Solving with Fragmented Hamiltonian Path Constraints for Wire Arc Additive Manufacturing. In Theory and Applications of Satisfiability Testing—SAT 2020; Pulina, L., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 492–500. [Google Scholar]
- Wächter, M.; Leicher, M.; Hupka, M.; Leistner, C.; Masendorf, L.; Treutler, K.; Kamper, S.; Esderts, A.; Wesling, V.; Hartmann, S. Monotonic and Fatigue Properties of Steel Material Manufactured by Wire Arc Additive Manufacturing. Appl. Sci. 2020, 10, 5238. [Google Scholar] [CrossRef]
- Kühne, R.; Feldmann, M.; Citarelli, S.; Reisgen, U.; Sharma, R.; Oster, L. 3D printing in steel construction with the automated Wire Arc Additive Manufacturing. Ce/Papers 2019, 3, 577–583. [Google Scholar] [CrossRef]
- Plangger, J.; Schabhüttl, P.; Vuherer, T.; Enzinger, N. CMT Additive Manufacturing of a High Strength Steel Alloy for Application in Crane Construction. Metals 2019, 9, 650. [Google Scholar] [CrossRef]
- Schroepfer, D.; Treutler, K.; Boerner, A.; Gustus, R.; Kannengiesser, T.; Wesling, V.; Maus-Friedrichs, W. Surface finishing of hard-to-machine cladding alloys for highly stressed components. Int. J. Adv. Manuf. Technol. 2021, 114, 1427–1442. [Google Scholar] [CrossRef]
- Treutler, K.; Lorenz, S.; Hamje, J.; Wesling, V. Wire and Arc Additive Manufacturing of a CoCrFeMoNiV Complex Concentrated Alloy Using Metal-Cored Wire—Process, Properties, and Wear Resistance. Appl. Sci. 2022, 12, 6308. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, D.; Wu, B.; Cuiuri, D.; Li, H.; Norrish, J. Arc Welding Processes for Additive Manufacturing: A Review. In Transactions on Intelligent Welding Manufacturing; Springer: Singapore, 2018; pp. 3–24. [Google Scholar]
- Wu, B.; Pan, Z.; Ding, D.; Cuiuri, D.; Li, H.; Xu, J.; Norrish, J. A review of the wire arc additive manufacturing of metals: Properties, defects and quality improvement. J. Manuf. Process. 2018, 35, 127–139. [Google Scholar] [CrossRef]
- Eissel, A.; Engelking, L.; Treutler, K.; Wesling, V.; Schröpfer, D.; Kannengießer, T. Modification of Co–Cr alloys to optimize additively welded microstructures and subsequent surface finishing. Weld. World 2022, 66, 2245–2257. [Google Scholar] [CrossRef]
- Leicher, M.; Treutler, K.; Wesling, V. Development of an Alternative Alloying Concept for Additive Manufacturing Using PVD Coating. Appl. Sci. 2022, 12, 6619. [Google Scholar] [CrossRef]
- Eissel, A.; Engelking, L.; Treutler, K.; Schroepfer, D.; Wesling, V.; Kannengiesser, T. Nickel-Iron-Alloy Modification to Enhance Additively Welded Microstructure for Subsequent Milling. In Proceedings of the 2nd International Conference on Advanced Joining Processes (AJP 2021), Sintra, Portugal, 21–22 October 2021, 1st ed.; Da Silva, L.F.M., Martins, P.A.F., Reisgen, U., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 85–99. [Google Scholar]
- Reisgen, U.; Sharma, R.; Mann, S.; Oster, L. Increasing the manufacturing efficiency of WAAM by advanced cooling strategies. Weld. World 2020, 64, 1409–1416. [Google Scholar] [CrossRef]
- Luo, J.; You, G.; Lu, D.; Zeng, S.; Peng, L.; Liu, Q. Effect of Modified Water-Bath Method on Microstructure and Mechanical Properties of Wire Arc Additive Manufactured Low-Carbon Low-Alloy Steel. Steel Res. Int. 2021, 92, 523. [Google Scholar] [CrossRef]
- Panchenko, O.; Kladov, I.; Kurushkin, D.; Zhabrev, L.; Ryl’Kov, E.; Zamozdra, M. Effect of thermal history on microstructure evolution and mechanical properties in wire arc additive manufacturing of HSLA steel functionally graded components. Mater. Sci. Eng. A 2022, 851, 143569. [Google Scholar] [CrossRef]
- Treutler, K.; Kamper, S.; Leicher, M.; Bick, T.; Wesling, V. Multi-Material Design in Welding Arc Additive Manufacturing. Metals 2019, 9, 809. [Google Scholar] [CrossRef]
- Pütz, R.D.; Pratesa, Y.; Oster, L.; Sharma, R.; Reisgen, U.; Zander, D. Microstructure and Corrosion Behavior of Functionally Graded Wire Arc Additive Manufactured Steel Combinations. Steel Res. Int. 2021, 92, 2100387. [Google Scholar] [CrossRef]
- Leicher, M.; Kamper, S.; Treutler, K.; Wesling, V. Multi-material design in additive manufacturing—Feasibility validation. Weld. World 2020, 64, 1341–1347. [Google Scholar] [CrossRef]
- Samardžić, I.; Stoić, A.; Kozak, D.; Kladaric, I.; Dunđer, M. Application of Weld Thermal Cycle Simulator in Manufacturing Engineering. J. Manuf. Ind. Eng. 2013, 12, 2–177. [Google Scholar] [CrossRef]
- Celin, R.; Burja, J.; Kosec, G. A comparison of as-welded and simulated heat affected zone (HAZ) microstructures. Mater. Tehnol. 2016, 50, 455–460. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, S.; Huang, A.; Shi, Y. Microstructure and mechanical properties of high-strength low alloy steel by wire and arc additive manufacturing. Int. J. Miner. Metall. Mater. 2020, 27, 933–942. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Duarte, V.; Avila, A.J.; Telmo, G.S.; Miranda, R.M.; Oliveira, J.P. Wire and arc additive manu-facturing of HSLA steel: Effect of thermal cycles on microstructure and mechanical properties. Addit. Manuf. 2019, 27, 440–450. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, F.; Huang, R.; Yuan, D.; Guo, C.; Wang, J. Microstructure and mechanical properties of low-carbon high-strength steel fabricated by wire and arc additive manufacturing. Metals 2020, 10, 216. [Google Scholar] [CrossRef]
- Müller, J.; Hensel, J.; Dilger, K. Mechanical properties of wire and arc additively manufactured high-strength steel structures. Weld. World 2021, 66, 395–407. [Google Scholar] [CrossRef]
- Wesling, V.; Leicher, M.; Gräbner, M.; Treutler, K.; Lorenz, S.; Esderts, A.; Hupka, M.; Wächter, M.; Spitzer, K.H. Werkstoffkennwerte additiv gefertigter Strukturen. In Tagungsband 4. Niedersächsisches Symposium Materialtechnik: 25. Bis 26. Februar 2021; TU Clausthal: Clausthal-Zellerfeld, Germany, 2021. [Google Scholar]
- Trzaska, J.; Dobrzański, L.A. Modelling of CCT diagrams for engineering and constructional steels. J. Mater. Process. Technol. 2007, 192, 504–510. [Google Scholar] [CrossRef]
- Dobrzanski, L.A.; Trzaska, J. Application of neural networks for the prediction of continuous cooling transformation diagrams. Comput. Mater. Sci. 2004, 30, 251–259. [Google Scholar] [CrossRef]
- Wang, J.; van der Wolk, P.J.; van der Zwaag, S. Effects of carbon concentration and cooling rate on continuous cooling transformations predicted by artificial neural network. ISIJ Int. 1999, 39, 38–46. [Google Scholar] [CrossRef][Green Version]
- Kouraytem, N.; Li, X.; Tan, W.; Kappes, B.; Spear, A.D. Modeling process–structure–property relationships in metal additive manufacturing: A review on physics-driven versus data-driven approaches. J. Phys. Mater. 2021, 4, 032002. [Google Scholar] [CrossRef]
- Soliman, M.; Weidenfeller, B.; Palkowski, H. Metallurgical Phenomena during Processing of TRIP Steel. Steel Res. Int. 2009, 80, 57–65. [Google Scholar]
- Soliman, M. Microstructural Control and Properties Optimization of Microalloyed Pipeline Steel. Metals 2020, 10, 1499. [Google Scholar] [CrossRef]
- Bramfitt, B.L.; Benscoter, A.O. Metallographer’s Guide: Practices and Procedures for Irons and Steels; ASM International: Materials Park, OH, USA, 2002. [Google Scholar]
- Gomez, M.; Valles, P.; Medina, S.F. Evolution of microstructure and precipitation state during thermomechanical processing of a X80 microalloyed steel. Mater. Sci. Eng. A 2011, 528, 4761–4773. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Andrews, K.W. Empirical formulae for the calculation of some transformation temperatures. J. Iron Steel Inst. 1965, 203, 721. [Google Scholar]
- Zhou, T.; Yu, H.; Wang, S. Effect of microstructural types on toughness and microstructural optimization of ultra-heavy steel plate: EBSD analysis and microscopic fracture mechanism. Mater. Sci. Eng. A 2016, 658, 150–158. [Google Scholar] [CrossRef]
- Tian, D.; Karjalainen, L.; Qian, B.; Chen, X. Cleavage Fracture Model for Granular Bainite in Simulated Coarse-Grained Heat-Affected Zones of High-Strength Low-Alloyed Steels. JSME Int. J. Ser. A Mech. Mater. Eng. 1997, 40, 179–188. [Google Scholar] [CrossRef][Green Version]
- Lescano, D.E.; Silvetti, S.P. Study of Microstructure and Tempered Martensite Embrittlement in AISI 15b41 Steel. Procedia Mater. Sci. 2012, 1, 134–140. [Google Scholar] [CrossRef][Green Version]
- Wu, Z.; Liu, Z.; Li, X. Microstructural effects on the fracture toughness of advanced high strength steels. Mater. Lett. 2020, 271, 127761. [Google Scholar] [CrossRef]
- Srinivas, M.; Malakondaiah, B.; RamaRao, P. Influence of polycrystal grain size on fracture toughness of and fatigue threshold in Armco iron Author. Eng. Fract. Mech. 1987, 28, 561–576. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Wu, H.; Lang, D.; Xiao, D.; Wang, Z.; Su, B.; Meng, D. The influence of impurities on the ductility and toughness of a low-temperature-aged U-Nb alloy. Mater. Sci. Eng. A 2018, 739, 1–16. [Google Scholar] [CrossRef]
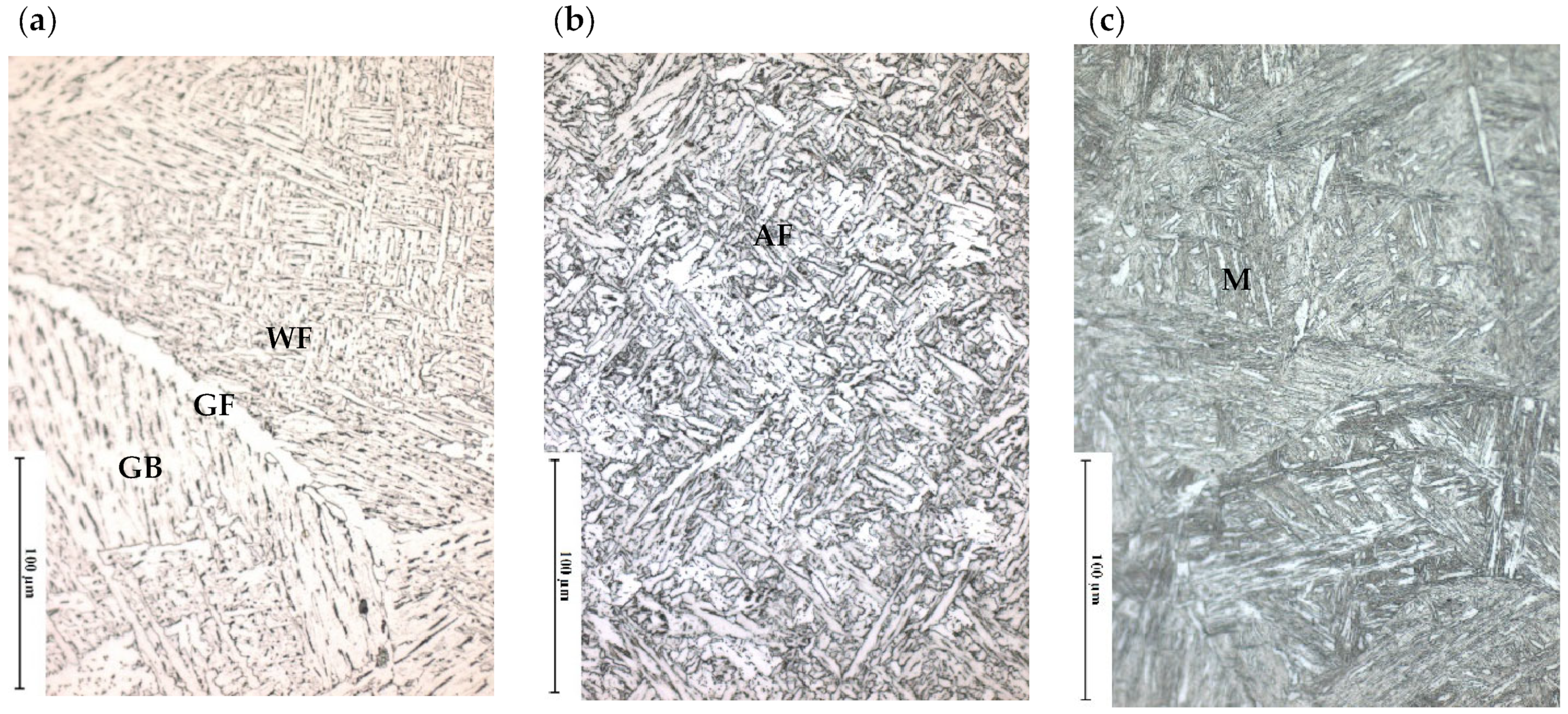

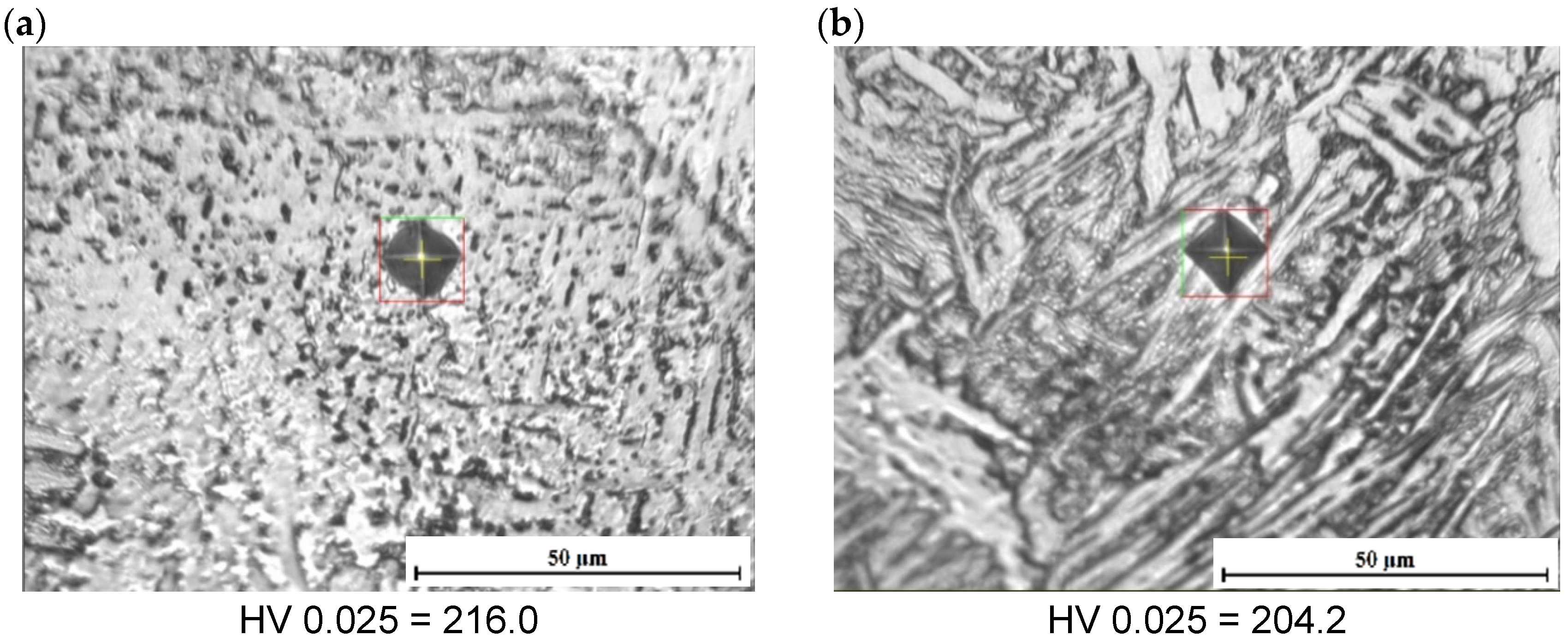
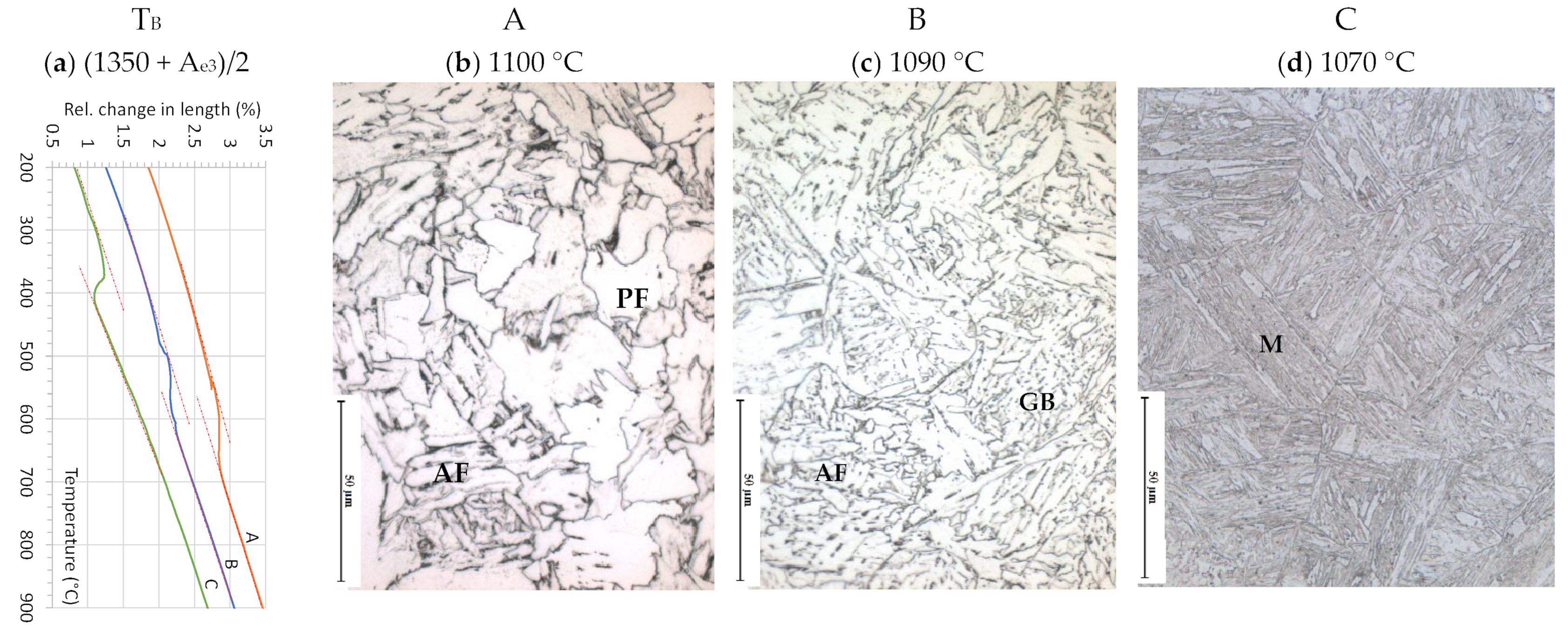
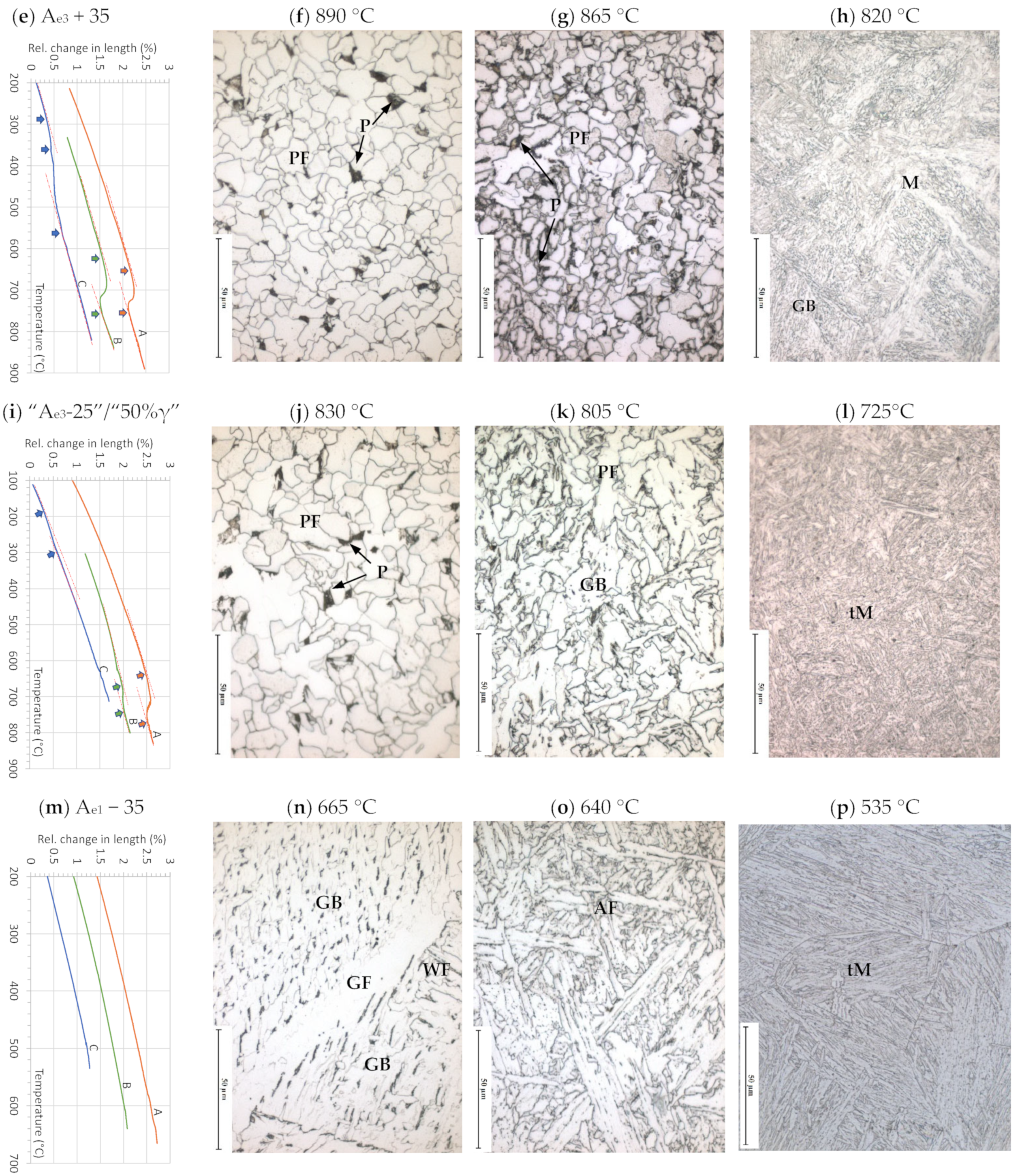


| Alloy | C | Si | Mn | Al | Cr | Ni | Mo | Ti | Nb | N |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 0.059 | 0.023 | 0.766 | 0.039 | 0.039 | 0.033 | 0.003 | 0.002 | 0.031 | 0.003 |
| B | 0.064 | 0.028 | 1.494 | 0.049 | 0.041 | 0.032 | 0.008 | 0.030 | 0.059 | 0.005 |
| C | 0.133 | 0.714 | 1.36 | 0.0056 | 0.340 | 2.24 | 0.563 | 0.033 | - | 0.005 |
| Temp. | A | B | C | |
|---|---|---|---|---|
| Ae | Ae1 | 700 | 675 | 570 |
| Ae3 | 855 | 830 | 785 | |
| TB | (1350 + Ae3)/2 | 1100 | 1090 | 1070 |
| Ae3 + 35 | 890 | 865 | 820 | |
| Ae3 − 25 | 830 | 805 | - | |
| 50% austenite | 780 | - | 725 | |
| Ae1 − 35 | 665 | 640 | 535 |
| Temp. | A | B | C | ||||
|---|---|---|---|---|---|---|---|
| Ae | Ae1 | 700 | 675 | 570 | |||
| Ae3 | 855 | 830 | 785 | ||||
| Ac | Ac1 | 816 | 804 | 763 | |||
| Ac3 | 954 | 924 | 890 | ||||
| Ar1/Ar3 MS/Mf | TA or TB | St | Fi | St | Fi | St | Fi |
| schedule I:1350 °C | 630 | 528 | 565 | 425 | 396 | 298 | |
| schedule II: (1350 + Ae3)/2 | 680 | 553 | 625 | 496 | 427 | 308 | |
| schedule II: Ae3+ 35 | 757 | 651 | 750 | 619 | 560 | 281 | |
| schedule II: Ae3 − 25/50%γ | 774 | 658 | 733 | 657 | 331 | 180 | |
| schedule II: Ae1 − 35 | - | - | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Soliman, M.; Treutler, K.; Wesling, V.; Spitzer, K.-H. On the Microstructure Development under Cyclic Temperature Conditions during WAAM of Microalloyed Steels. Metals 2022, 12, 1913. https://doi.org/10.3390/met12111913
Huang C, Soliman M, Treutler K, Wesling V, Spitzer K-H. On the Microstructure Development under Cyclic Temperature Conditions during WAAM of Microalloyed Steels. Metals. 2022; 12(11):1913. https://doi.org/10.3390/met12111913
Chicago/Turabian StyleHuang, Chang, Mohamed Soliman, Kai Treutler, Volker Wesling, and Karl-Heinz Spitzer. 2022. "On the Microstructure Development under Cyclic Temperature Conditions during WAAM of Microalloyed Steels" Metals 12, no. 11: 1913. https://doi.org/10.3390/met12111913
APA StyleHuang, C., Soliman, M., Treutler, K., Wesling, V., & Spitzer, K.-H. (2022). On the Microstructure Development under Cyclic Temperature Conditions during WAAM of Microalloyed Steels. Metals, 12(11), 1913. https://doi.org/10.3390/met12111913









