Synthesis and Catalytic Studies of Nanoalloy Particles Based on Bismuth, Silver, and Rhenium
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
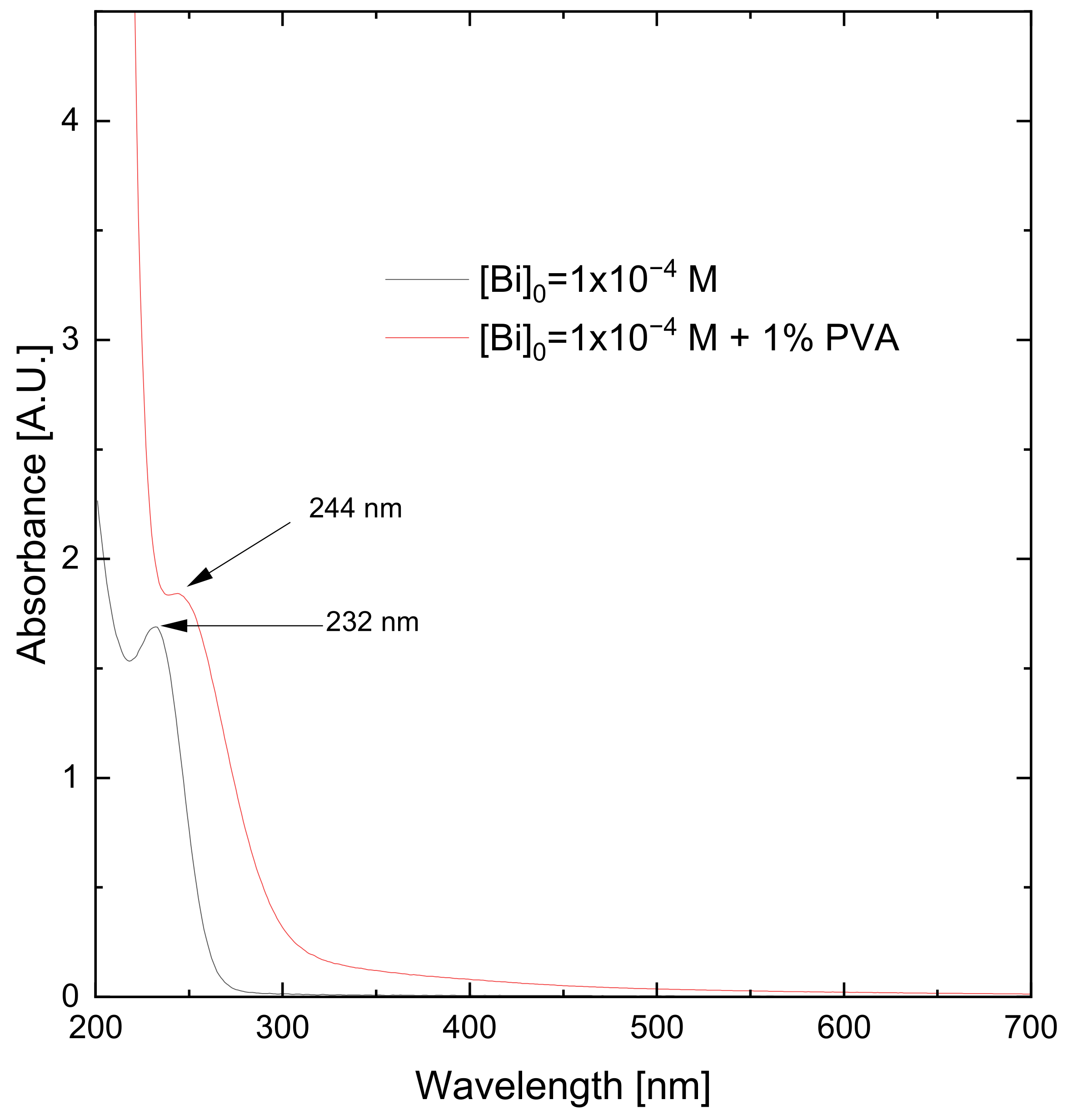
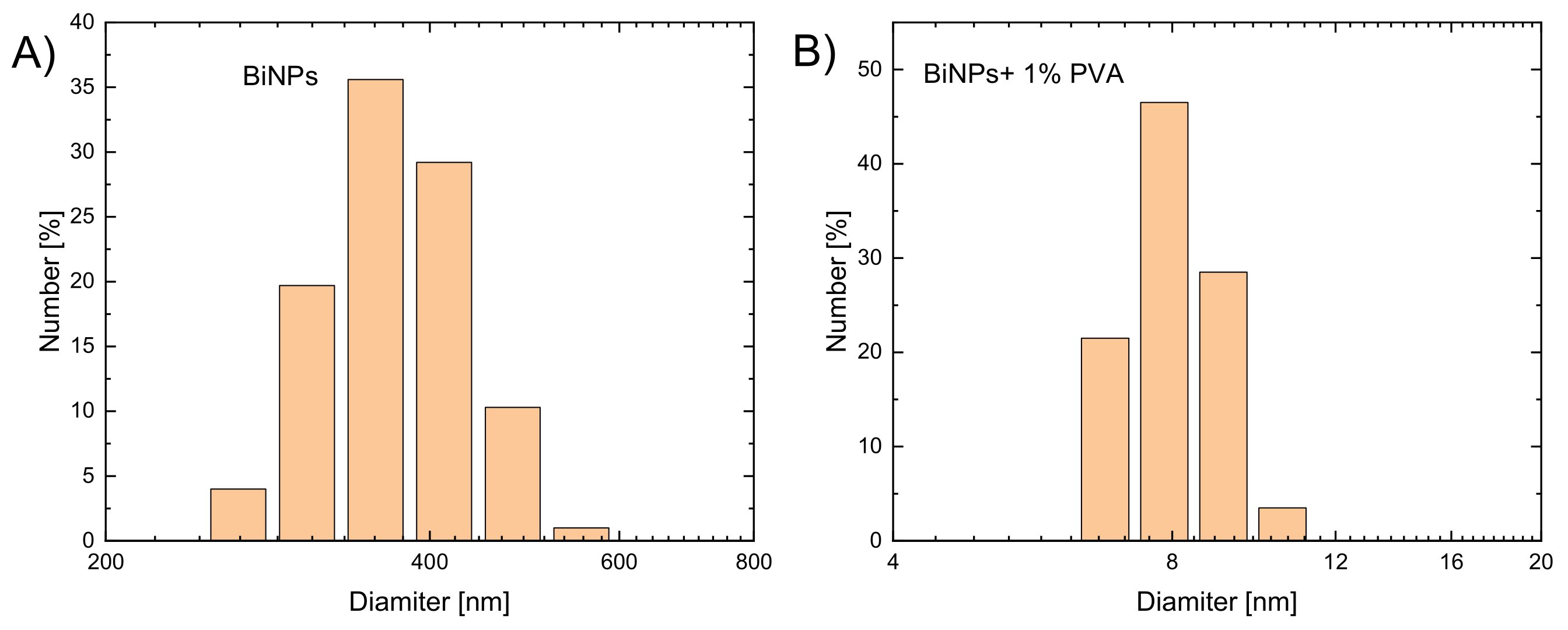
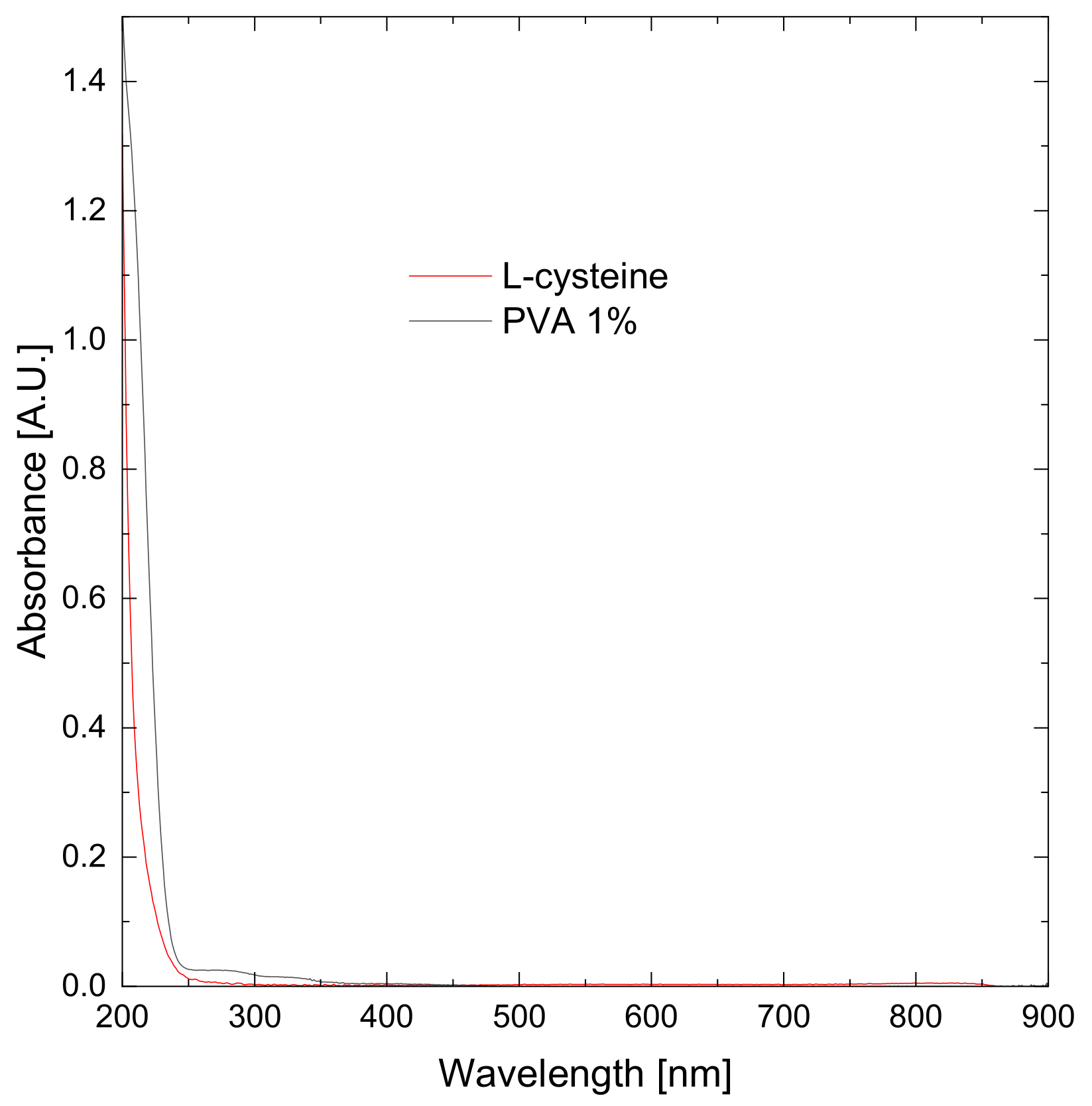
3.1. Synthesis of Bismuth Nanoparticles
- where:ks–Rayleigh scattering coefficientm–number of scattering particles,d–particle diameter (nm),n–refractive index,λ–wavelength (nm)
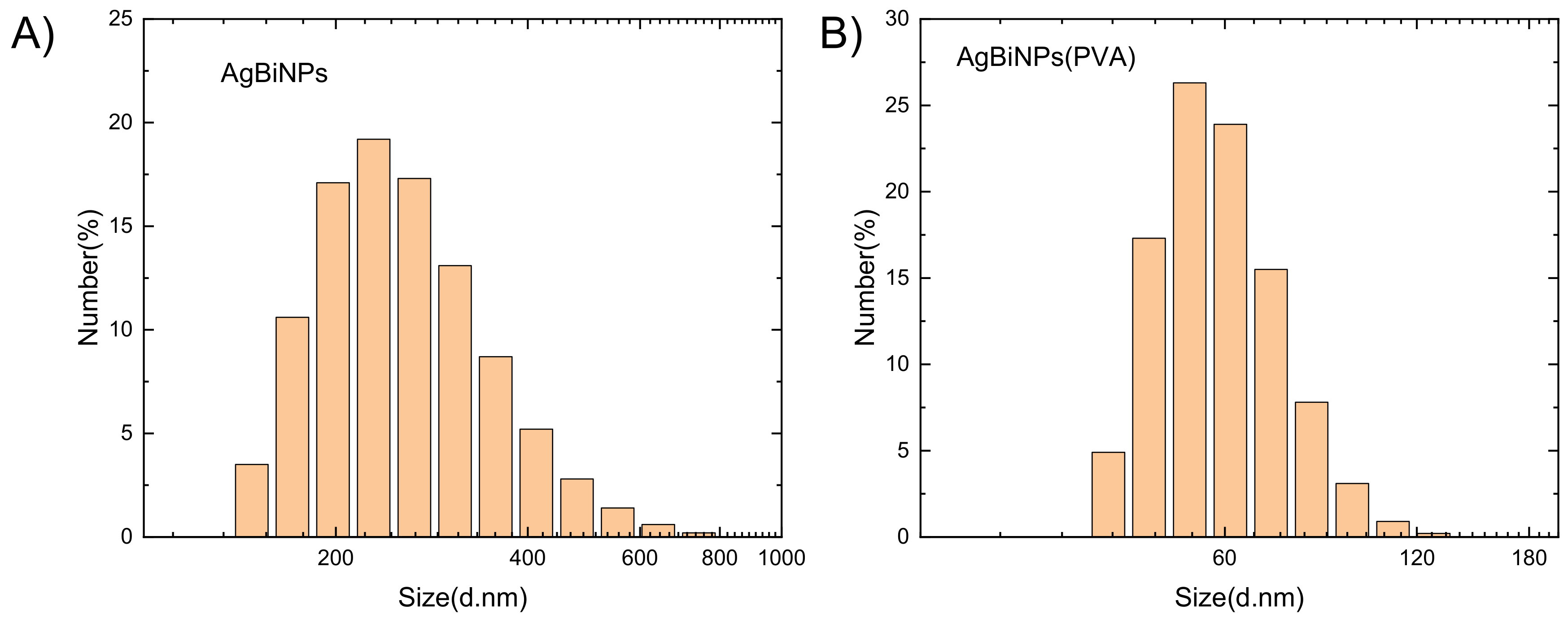
3.2. Synthesis of Bismuth–Silver Nanoparticles
3.3. Synthesis of Bismuth–Rhenium Nanoparticles
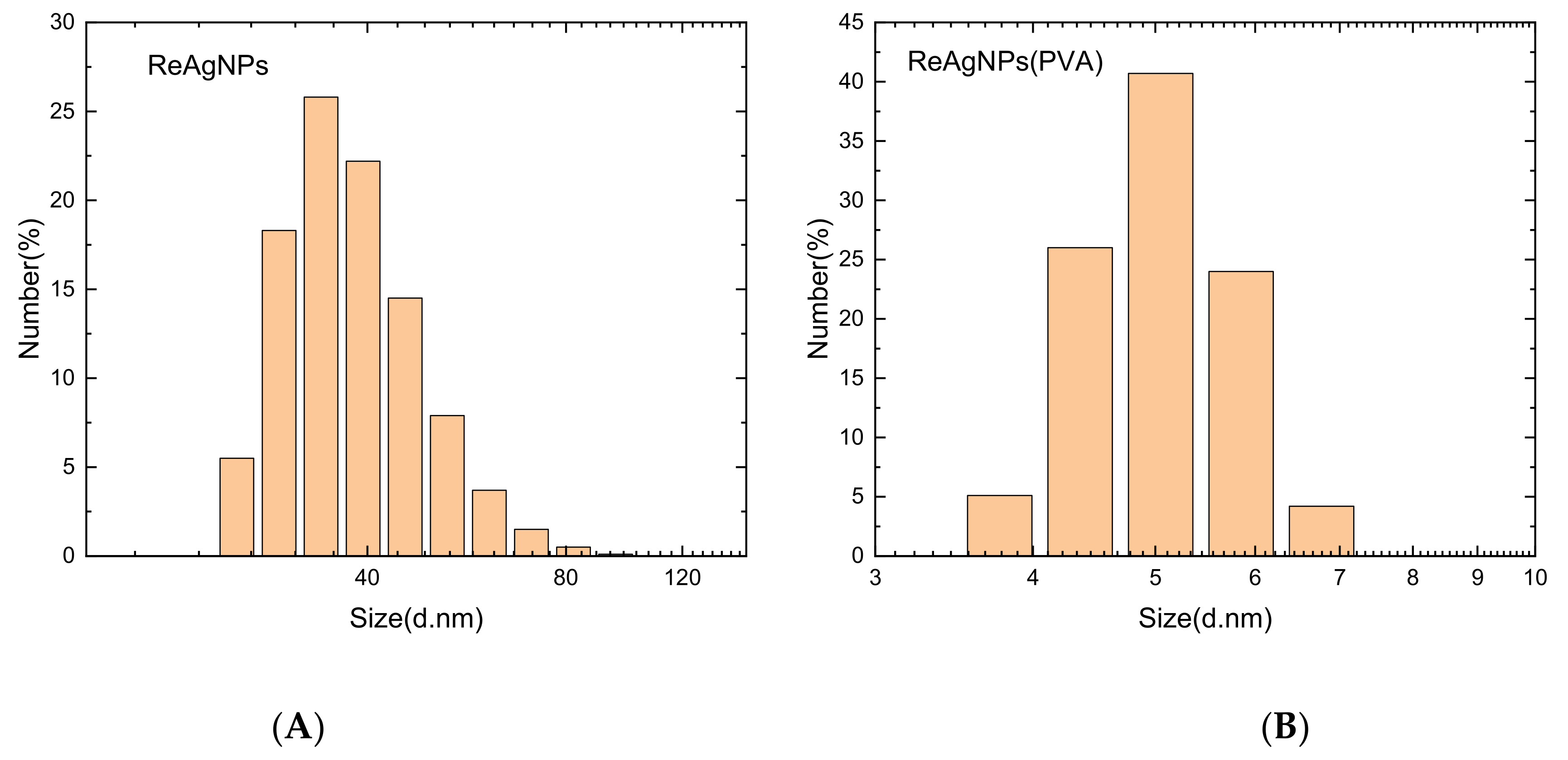
3.4. Synthesis of Silver–Rhenium Nanoparticles
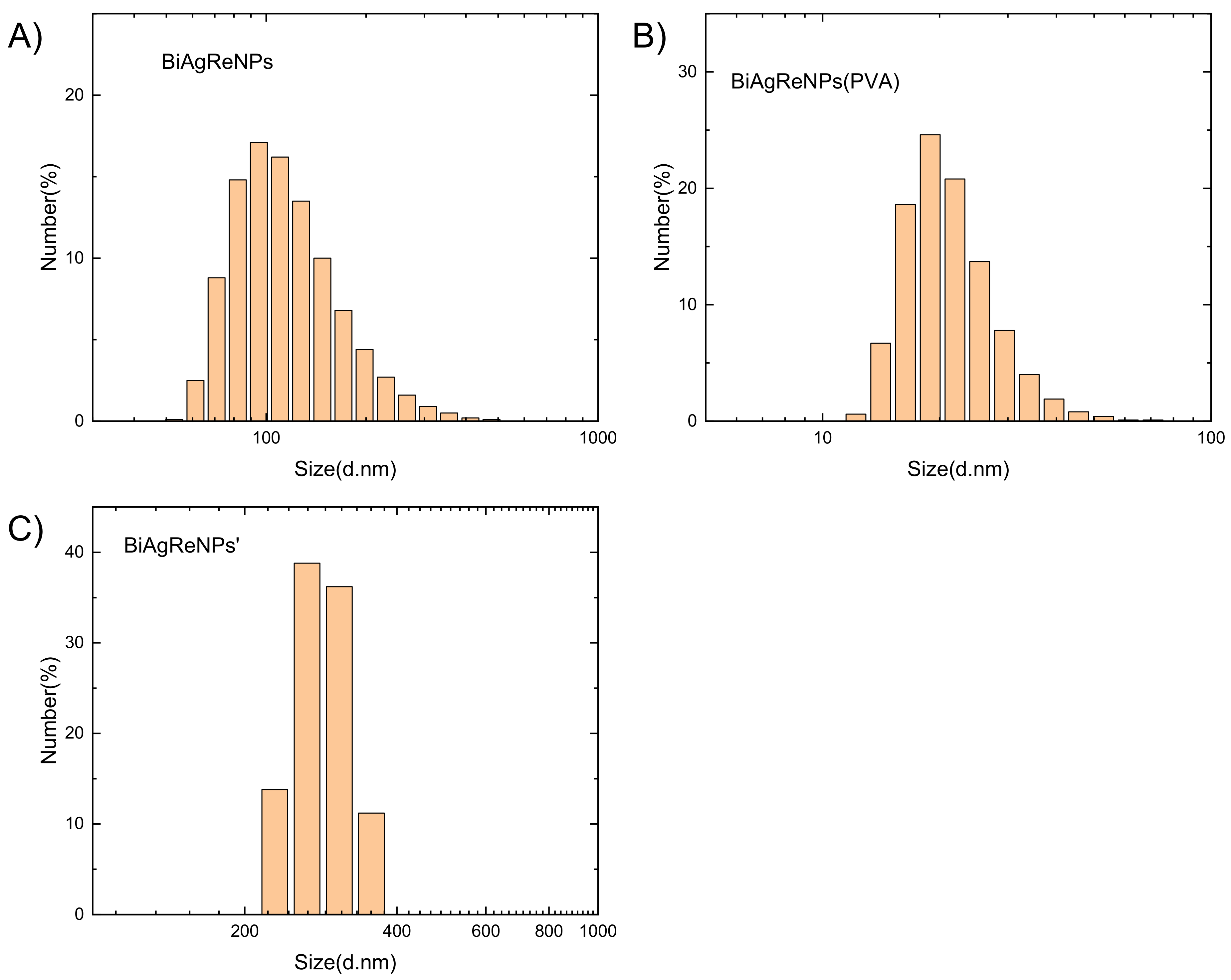
3.5. Synthesis of Bismuth–Silver-Rhenium Nanoparticles
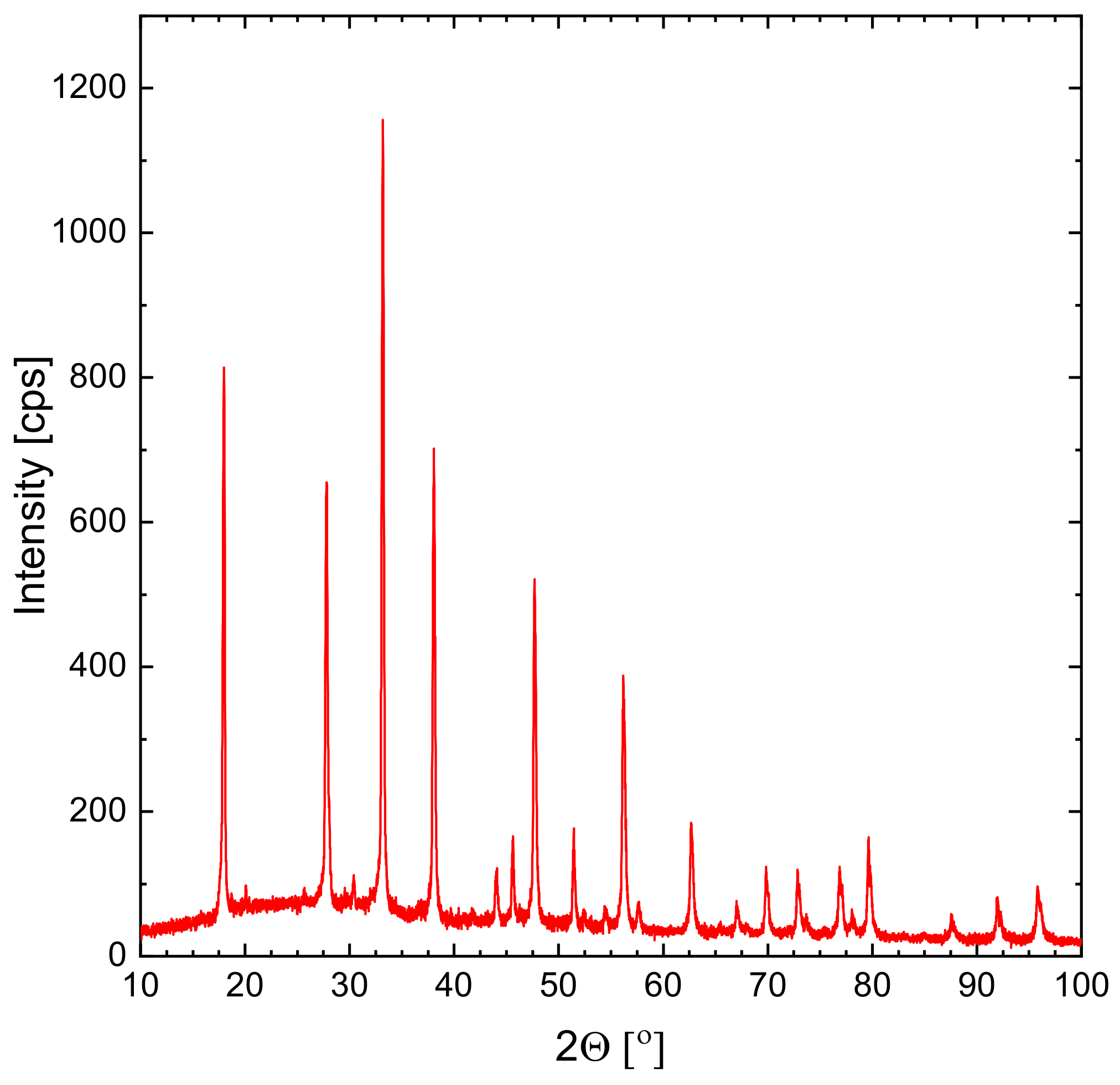
3.6. XRD Analysis of Ag–Bi–Re Nanoparticles
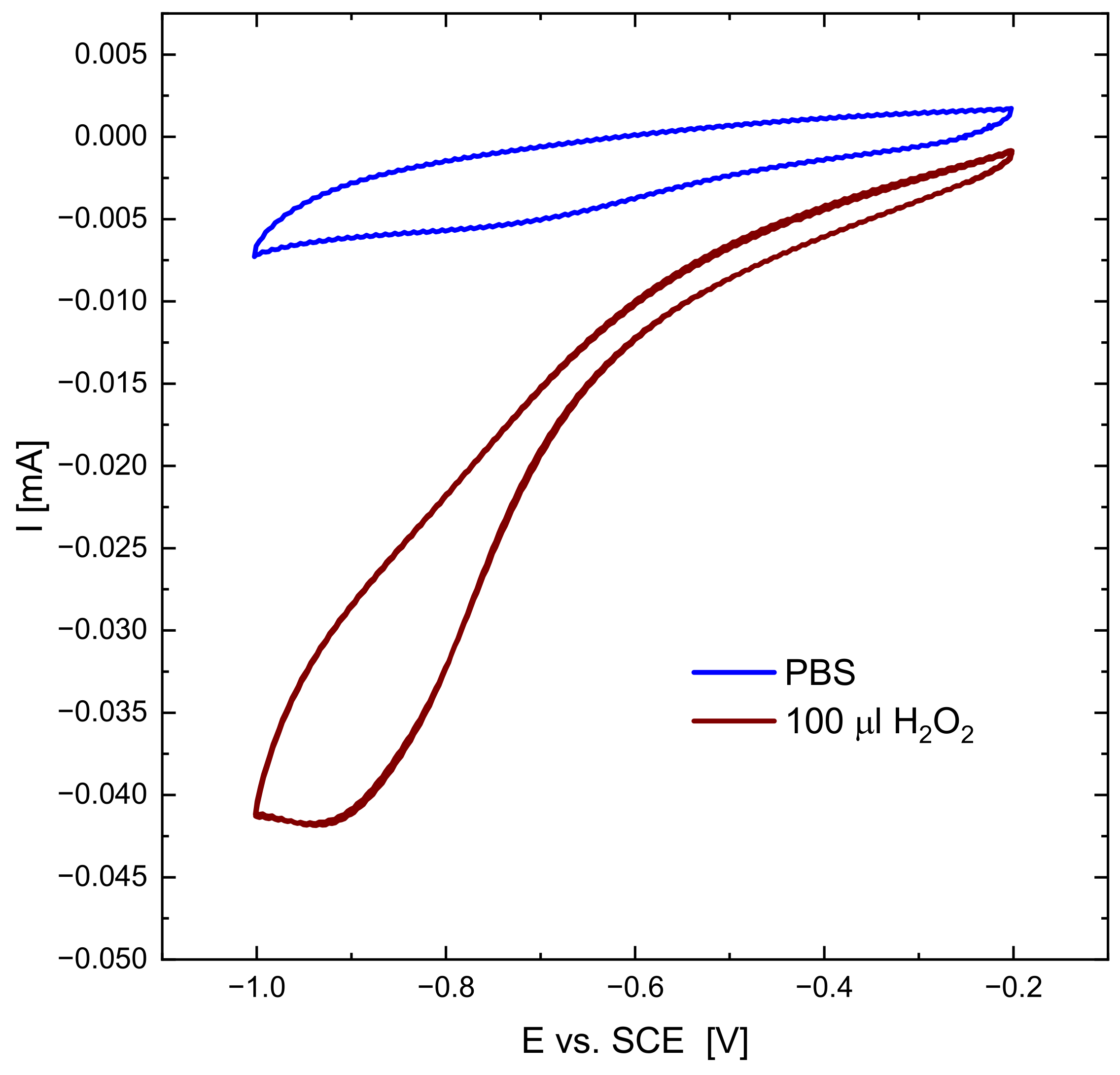
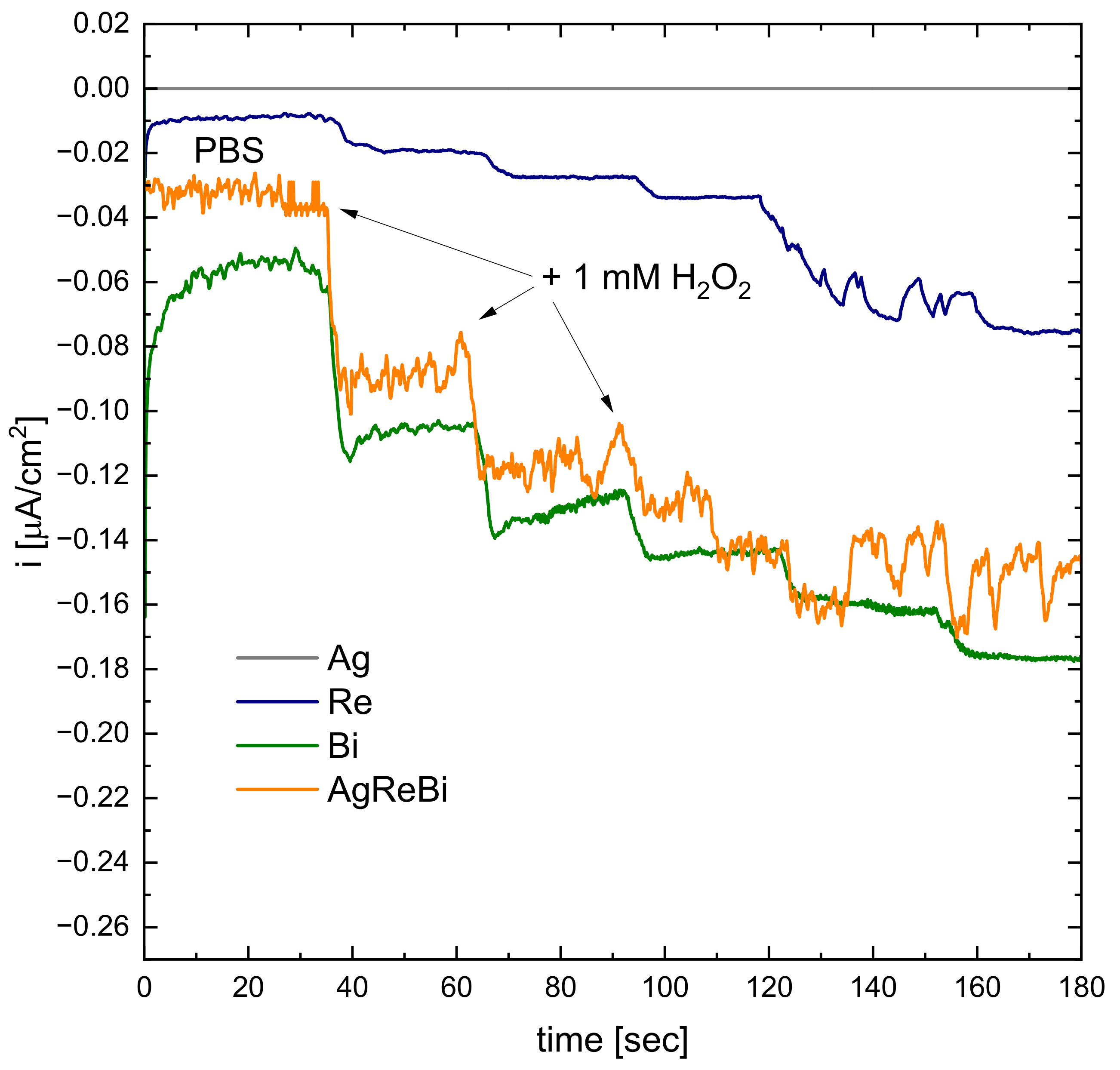
3.7. Catalytic Properties Studies
- where:Ni–number of particles in Ri radiusMm,Bi/Ag/Re−molar mass of metal precursorC0,Bi(III)/Ag(I)/Re(VII)–initial concentration of precursor saltV–the value of suspension used in the synthesis of the electrode (in this case 30 µL)Bi/Ag/Re–density of the metal precursorRi–radius of “i” fraction of nanoparticlesFri–a fraction of nanoparticles in %
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, C.; Pal, T. Retracted Article: Recent advances of metal–metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater. Chem. A 2017, 5, 9465–9487. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Rajendran, S.; Show, P.L.; Manavalan, K.; Naushad, M. Metal/metal oxide nanocomposites for bactericidal effect: A review. Chemosphere 2021, 272, 128607. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Rani, N.; Saini, K. A review on transition metal oxides based nanocomposites, their synthesis techniques, different morphologies and potential applications. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1225, 012004. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.Z.; Biradar, A.V.; Peng, D.L.; Zboril, R.; Varma, R.S. Core–shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef] [PubMed]
- Csapó, E.; Oszkó, A.; Varga, E.; Juhász, Á.; Buzás, N.; Kőrösi, L.; Majzik, A.; Dékány, I. Synthesis and characterization of Ag/Au alloy and core(Ag)–shell(Au) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 281–287. [Google Scholar] [CrossRef]
- Safaie, N.; Ferrier, R.C., Jr. Janus nanoparticle synthesis: Overview, recent developments, and applications. J. Appl. Phys. 2020, 127, 170902. [Google Scholar] [CrossRef]
- Song, Y.; Chen, S. Janus nanoparticles: Preparation, characterization, and applications. Chem. Asian J. 2014, 9, 418–430. [Google Scholar] [CrossRef]
- Ismail, A.M.; Csapó, E.; Janáky, C. Correlation between the work function of Au–Ag nanoalloys and their electrocatalytic activity in carbon dioxide reduction. Electrochim. Acta 2019, 313, 171–178. [Google Scholar] [CrossRef]
- Ermolov, L.V.; Slinkin, A.A. Strong metal-carrier interaction and its role in catalysis. Russ. Chem. Rev. 1991, 60, 331–344. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, H.; Park, J.Y. Charge Transport in Metal–Oxide Interfaces: Genesis and Detection of Hot Electron Flow and Its Role in Heterogeneous Catalysis. Catal. Lett. 2015, 145, 299–308. [Google Scholar] [CrossRef]
- Bonura, G.; Arena, F.; Mezzatesta, G.; Cannilla, C.; Spadaro, L.; Frusteri, F. Role of the ceria promoter and carrier on the functionality of Cu-based catalysts in the CO2-to-methanol hydrogenation reaction. Catal. Today 2011, 171, 251–256. [Google Scholar] [CrossRef]
- Guttikonda, M.; Dey, A.; Pandey, K.; Maity, S. Fabrication of metal matrix composites by powder metallurgy: A review. AIP Conf. Proc. 2018, 1952, 020041. [Google Scholar]
- Meiyanathan, M.; Ravichandran, M. Synthesis of Metal Matrix Composites via Powder Metallurgy Route: A Review. Mech. Mech. Eng. 2018, 22, 65–76. [Google Scholar]
- Idris, J.; Kabir, M.A. Powder Metallurgy Development for The Production of Metal Matrix Composite. In Proceedings of the Processing and Fabrication of Advanced Materials VIII, Singapore, 8–10 September 1999; pp. 921–931. [Google Scholar]
- Somani, N.; Sharma, N.; Sharma, A.; Gautam, Y.K.; Khatri, P.; Solomon, J. Allanson Agnal, Fabrication of Cu-SiC Composites using Powder Metallurgy Technique. Mater. Today Proc. 2018, 5 Pt 2, 28136–28141. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef]
- Abbas, M.; Hachemaoui, A.; Yahiaoui, A.; Mourad, A.-H.I.; Belfedal, A.; Cherupurakal, N. Chemical synthesis of nanocomposites via in-situ polymerization of aniline and iodoaniline using exchanged montmorillonite. Polym. Polym. Compos. 2020, 29, 982–991. [Google Scholar] [CrossRef]
- Pozdnyakov, A.A.-O.; Emel’yanov, A.I.; Korzhova, S.A.; Kuznetsova, N.P.; Bolgova, Y.I.; Trofimova, O.M.; Semenova, T.A.; Prozorova, G.-O. Green Synthesis of Stable Nanocomposites Containing Copper Nanoparticles Incorporated in Poly-N-vinylimidazole. Polymers 2021, 13, 3212. [Google Scholar] [CrossRef]
- Khanna, P.K.; Singh, N.; Charan, S.; Subbarao, V.V.V.S.; Gokhale, R.; Mulik, U.P. Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method. Mater. Chem. Phys. 2005, 93, 117–121. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Woehl, T.J.; Evans, J.E.; Arslan, I.; Ristenpart, W.D.; Browning, N.D. Direct in Situ Determination of the Mechanisms Controlling Nanoparticle Nucleation and Growth. ACS Nano 2012, 6, 8599–8610. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Mech, K.; Wojnicki, M.; Mania, I.; Gajewska, M.; Marzec, M. Influence of Experimental Conditions on Deposition of Silver Nanoparticles onto Surface of Graphene Oxide/Wpływ Warunków Eksperymentalnych Na Proces Osadzania Nanocząstek Srebra Na Powierzchni Tlenku Grafenu. Arch. Metall. Mater. 2015, 60, 2631–2635. [Google Scholar]
- Wojnicki, M.; Tokarski, T.; Hessel, V.; Fitzner, K.; Luty-Błocho, M. Continuous, monodisperse silver nanoparticles synthesis using microdroplets as a reactor. J. Flow Chem. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Gomez, C.; Hallot, G.; Pastor, A.; Laurent, S.; Brun, E.; Sicard-Roselli, C.; Port, M. Metallic bismuth nanoparticles: Towards a robust, productive and ultrasound assisted synthesis from batch to flow-continuous chemistry. Ultrason. Sonochem. 2019, 56, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Wojnicki, M.; Tokarski, T.; Hessel, V.; Fitzner, K.; Luty-Błocho, M. 2H and 4H silver colloidal suspension synthesis, as a new potential drug carrier. Chem. Eng. J. 2020, 382, 122922. [Google Scholar] [CrossRef]
- Chakraborty, I.; Shirodkar, S.N.; Gohil, S.; Waghmare, U.V.; Ayyub, P. The nature of the structural phase transition from the hexagonal (4H) phase to the cubic (3C) phase of silver. J. Phys. Condens. Matter 2014, 26, 115405. [Google Scholar] [CrossRef]
- Brzózka, A.; Szeliga, D.; Kurowska-Tabor, E.; Sulka, G.D. Synthesis of copper nanocone array electrodes and its electrocatalytic properties toward hydrogen peroxide reduction. Mater. Lett. 2016, 174, 66–70. [Google Scholar] [CrossRef]
- Skibińska, K.; Kutyła, D.; Kula, A.; Gajewska, M.; Marzec, M.M.; Żabiński, P. Hydrogen evolution reaction (HER) activity of conical Co–Fe alloy structures and their application as a sensitive and rapid sensor for H2O2 detection. Arch. Civ. Mech. Eng. 2022, 22, 76. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Li, J.G.; Chen, X.L.; Cheng, D.; Zhang, J.Y.; Yang, H.X. Highly Sensitive Electrochemical Detection of Hydrogen Peroxide Based on Polyethyleneimine-Au Nanoparticles-Zinc Protoporphyrin. J. Electrochem. Soc. 2019, 166, B631. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhang, X.M.; Chai, X.Y.; Wang, T.Q.; Cao, T.L.; Li, Y.N.; Zhang, L.Y.; Fan, F.Q.; Fu, Y.; Qi, W. An Electrochemical Sensor for H2O2 Based on Au Nanoparticles Embedded in UiO-66 Metal–Organic Framework Films. ACS Appl. Nano Mater. 2021, 4, 6103–6110. [Google Scholar] [CrossRef]
- Chen, S.H.; Yuan, R.; Chai, Y.Q.; Hu, F.X. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Xu, L.; Lian, M.L.; Chen, X.; Lu, Y.L.; Yang, W.S. Amperometric sensing of hydrogen peroxide via an ITO electrode modified with gold nanoparticles electrodeposited on a CoMn-layered double hydroxide. Microchim. Acta 2017, 184, 3989–3996. [Google Scholar] [CrossRef]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Köller, M.; Epple, M. The dissolution and biological effects of silver nanoparticles in biological media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef]
- Liu, H.; Weng, L.; Yang, C. A review on nanomaterial-based electrochemical sensors for H2O2, H2S and NO inside cells or released by cells. Microchim. Acta 2017, 184, 1267–1283. [Google Scholar] [CrossRef]
- Xi, J.B.; Zhang, Y.; Wang, N.; Wang, L.; Zhang, Z.Y.; Xiao, F.; Wang, S. Ultrafine Pd Nanoparticles Encapsulated in Microporous Co3O4 Hollow Nanospheres for In Situ Molecular Detection of Living Cells. ACS Appl. Mater. Interfaces 2015, 7, 5583–5590. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Migliorini, F.L.; Christinelli, W.A.; Correa, D.S. Detection of hydrogen peroxide (H2O2) using a colorimetric sensor based on cellulose nanowhiskers and silver nanoparticles. Carbohydr. Polym. 2019, 212, 235–241. [Google Scholar] [CrossRef]
- Verma, N.; Singh, M. A disposable microbial based biosensor for quality control in milk. Biosens. Bioelectron. 2003, 18, 1219–1224. [Google Scholar] [CrossRef]
- Han, C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016, 40, 272–279. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.N.; Yang, B.C.; Liu, Z.X.; Liu, Q.Y.; Zhang, X. Colorimetric and ultrasensitive detection of H2O2 based on Au/Co3O4-CeOx nanocomposites with enhanced peroxidase-like performance. Sens. Actuators B Chem. 2018, 271, 336–345. [Google Scholar] [CrossRef]
- Burford, N.; Eelman, M.D.; Mahony, D.E.; Morash, M. Definitive identification of cysteine and glutathione complexes of bismuth by mass spectrometry: Assessing the biochemical fate of bismuth pharmaceutical agents. Chem. Commun. 2003, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Cheek, G.T.; Peña, D. Electrochemical Investigations of L-Cysteine Interactions with Bismuth Ions. J. Electrochem. Soc. 2020, 167, 155522. [Google Scholar] [CrossRef]
- Alekseev, V.; Semenov, A.; Pakhomov, P. Complexation of Ag+ Ions with L-Cysteine. Russ. J. Inorg. Chem. 2012, 57, 1041–1044. [Google Scholar] [CrossRef]
- Leung, B.O.; Jalilehvand, F.; Mah, V.; Masood, P.; Wu, Q. Silver(I) complex formation with cysteine, penicillamine, and glutathione. Inorg. Chem. 2013, 52, 4593–4602. [Google Scholar] [CrossRef]
- Kirsch, S.; Jankowsky, R.; Fau-Leibnitz, P.; Leibnitz, P.; Fau-Spies, H.; Spies, H.; Fau-Johannsen, B.; Johannsen, B. Crystal and solution structure of oxo rhenium(V) complexes with cysteine and cysteine methyl ester. J. Biol. Inorg. Chem. 1999, 4, 48–55. [Google Scholar] [CrossRef]
- Young, A.T. Rayleigh scattering. Appl. Opt. 1981, 20, 533–535. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Ochiai, B. Green synthesis of crystalline bismuth nanoparticles using lemon juice. RSC Adv. 2021, 11, 26683–26686. [Google Scholar] [CrossRef]
- Wojnicki, M.; Luty-Błocho, M.; Kotańska, M.; Wytrwal, M.; Tokarski, T.; Krupa, A.; Kołaczkowski, M.; Bucki, A.; Kobielusz, M. Novel and effective synthesis protocol of AgNPs functionalized using L-cysteine as a potential drug carrier. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 123–130. [Google Scholar] [CrossRef]
- Van der Horst, C.; Silwana, B.; Iwuoha, E.; Somerset, V. Synthesis and Characterization of Bismuth-Silver Nanoparticles for Electrochemical Sensor Applications. Anal. Lett. 2015, 48, 1311–1332. [Google Scholar] [CrossRef]
- Wojnicki, M.; Luty-Błocho, M.; Dobosz, I.; Grzonka, J.; Pacławski, K.; Kurzydłowski, K.J.; Fitzner, K. Electro-Oxidation of Glucose in Alkaline Media on Graphene Sheets Decorated with Gold Nanoparticles. Mater. Sci. Appl. 2013, 2013, 162–169. [Google Scholar] [CrossRef]
- Maji, S.K.; Dutta, A.K.; Srivastava, D.N.; Paul, P.; Mondal, A.; Adhikary, B.; Adhikary, U. Electrocatalytic activity of silver nanoparticles modified glassy carbon electrode as amperometric sensor for hydrogen peroxide. J. Nanosci. Nanotechnol. 2013, 13, 4969–4974. [Google Scholar] [CrossRef]
- Bui, M.-P.; Pham, X.-H.; Han, K.; Li, C.; Kim, Y.S.; Seong, G. Electrocatalytic reduction of hydrogen peroxide by silver particles patterned on single-walled carbon nanotubes. Sens. Actuators B Chem. 2010, 150, 436–441. [Google Scholar] [CrossRef]
- Campbell, F.W.; Belding, S.R.; Baron, R.; Xiao, L.; Compton, R.G. Hydrogen Peroxide Electroreduction at a Silver-Nanoparticle Array: Investigating Nanoparticle Size and Coverage Effects. J. Phys. Chem. C 2009, 113, 9053–9062. [Google Scholar] [CrossRef]
- Brzózka, A.; Brudzisz, A.; Jeleń, A.; Kozak, M.; Wesół, J.; Iwaniec, M.; Sulka, G.D. A comparative study of electrocatalytic reduction of hydrogen peroxide at carbon rod electrodes decorated with silver particles. Mater. Sci. Eng. B 2021, 263, 114801. [Google Scholar] [CrossRef]
- Lin, D.-H.; Jiang, Y.-X.; Wang, Y.; Sun, S.-G. Silver Nanoparticles Confined in SBA-15 Mesoporous Silica and the Application as a Sensor for Detecting Hydrogen Peroxide. J. Nanomater. 2008, 2008, 473791. [Google Scholar] [CrossRef]
- Csapó, E.; Patakfalvi, R.; Hornok, V.; Tóth, L.T.; Sipos, Á.; Szalai, A.; Csete, M.; Dékány, I. Effect of pH on stability and plasmonic properties of cysteine-functionalized silver nanoparticle dispersion. Colloids Surf. B Biointerfaces 2012, 98, 43–49. [Google Scholar] [CrossRef]
- Khalkho, B.R.; Kurrey, R.; Deb, M.K.; Shrivas, K.; Thakur, S.S.; Pervez, S.; Jain, V.K. L-cysteine modified silver nanoparticles for selective and sensitive colorimetric detection of vitamin B1 in food and water samples. Heliyon 2020, 6, e03423. [Google Scholar] [CrossRef]









| Colloid | Ag | Re | Bi | AgReBi |
|---|---|---|---|---|
| Specific surface area (cm2/μL) | 412 | 119 | 28 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtaszek, K.; Skibińska, K.; Cebula, F.; Tokarski, T.; Escribà-Gelonch, M.; Hessel, V.; Wojnicki, M. Synthesis and Catalytic Studies of Nanoalloy Particles Based on Bismuth, Silver, and Rhenium. Metals 2022, 12, 1819. https://doi.org/10.3390/met12111819
Wojtaszek K, Skibińska K, Cebula F, Tokarski T, Escribà-Gelonch M, Hessel V, Wojnicki M. Synthesis and Catalytic Studies of Nanoalloy Particles Based on Bismuth, Silver, and Rhenium. Metals. 2022; 12(11):1819. https://doi.org/10.3390/met12111819
Chicago/Turabian StyleWojtaszek, Konrad, Katarzyna Skibińska, Filip Cebula, Tomasz Tokarski, Marc Escribà-Gelonch, Volker Hessel, and Marek Wojnicki. 2022. "Synthesis and Catalytic Studies of Nanoalloy Particles Based on Bismuth, Silver, and Rhenium" Metals 12, no. 11: 1819. https://doi.org/10.3390/met12111819
APA StyleWojtaszek, K., Skibińska, K., Cebula, F., Tokarski, T., Escribà-Gelonch, M., Hessel, V., & Wojnicki, M. (2022). Synthesis and Catalytic Studies of Nanoalloy Particles Based on Bismuth, Silver, and Rhenium. Metals, 12(11), 1819. https://doi.org/10.3390/met12111819









