Corrosion Behavior of Al10Cr30Fe25Mn30Ti5 High-Entropy Alloy: Microstructural, Electrochemical, and Surface Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Microstructure Characterization
2.2. Electrochemical Techniques
2.3. Surface Characterization Techniques
3. Results
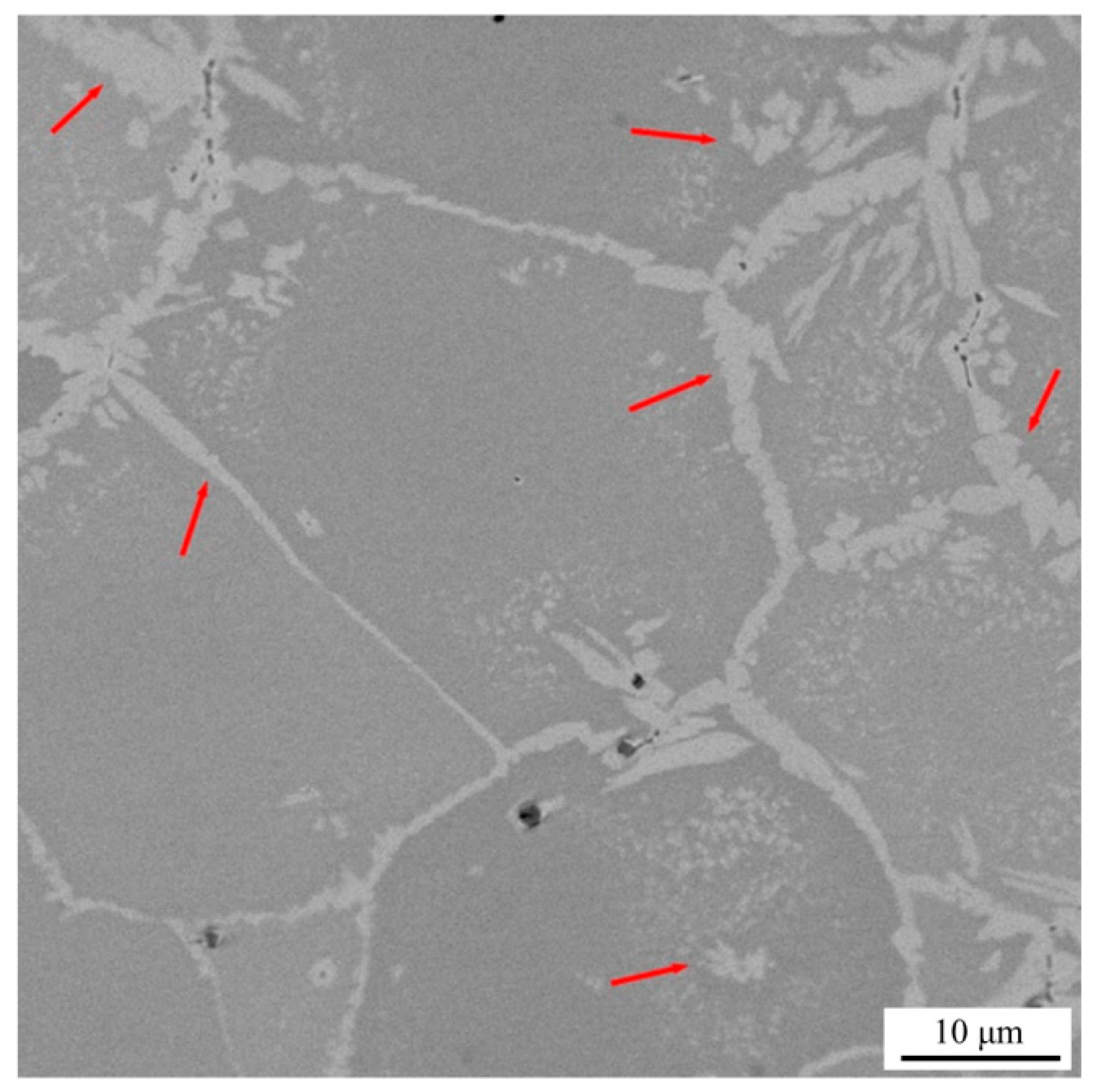
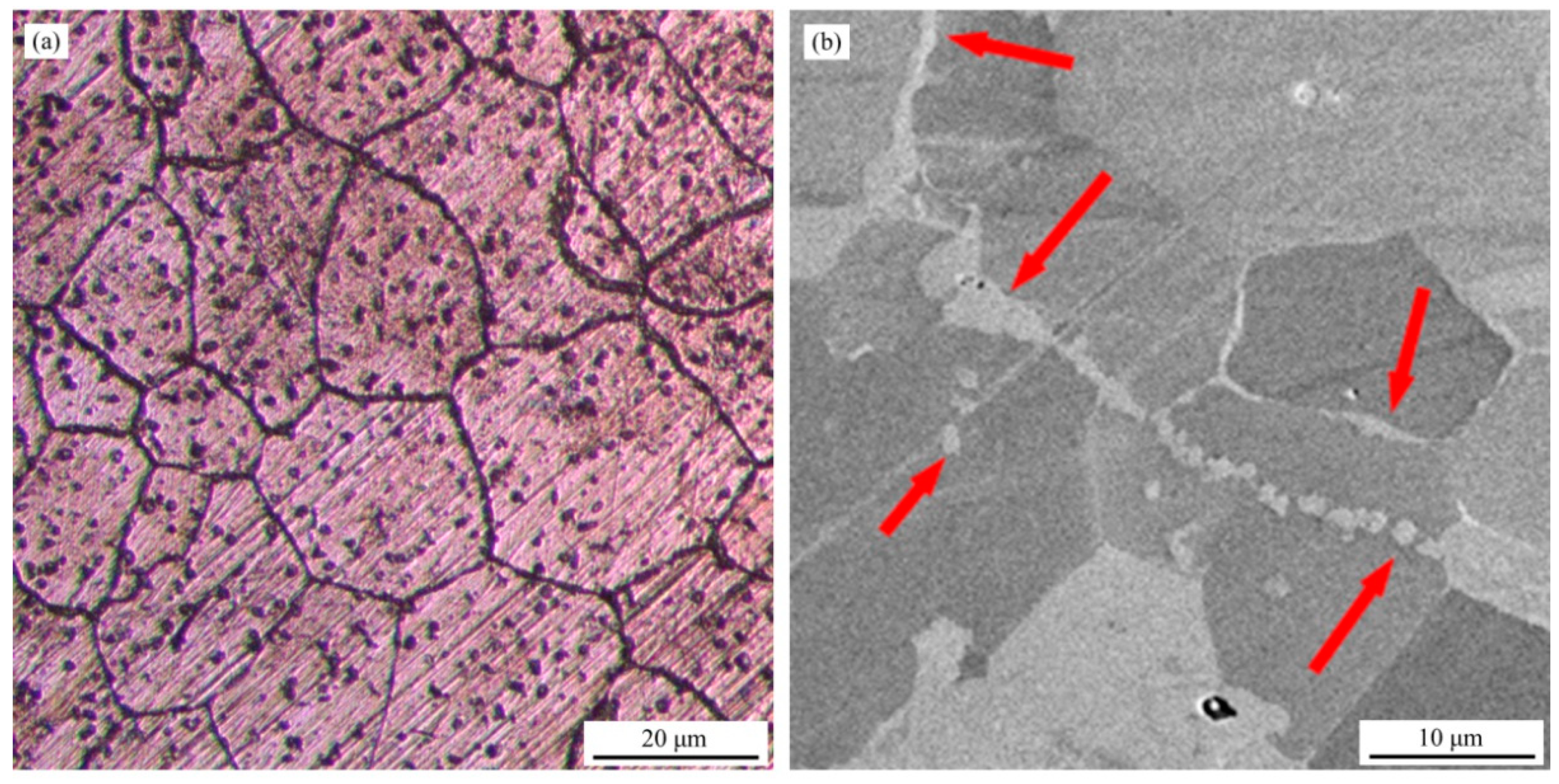
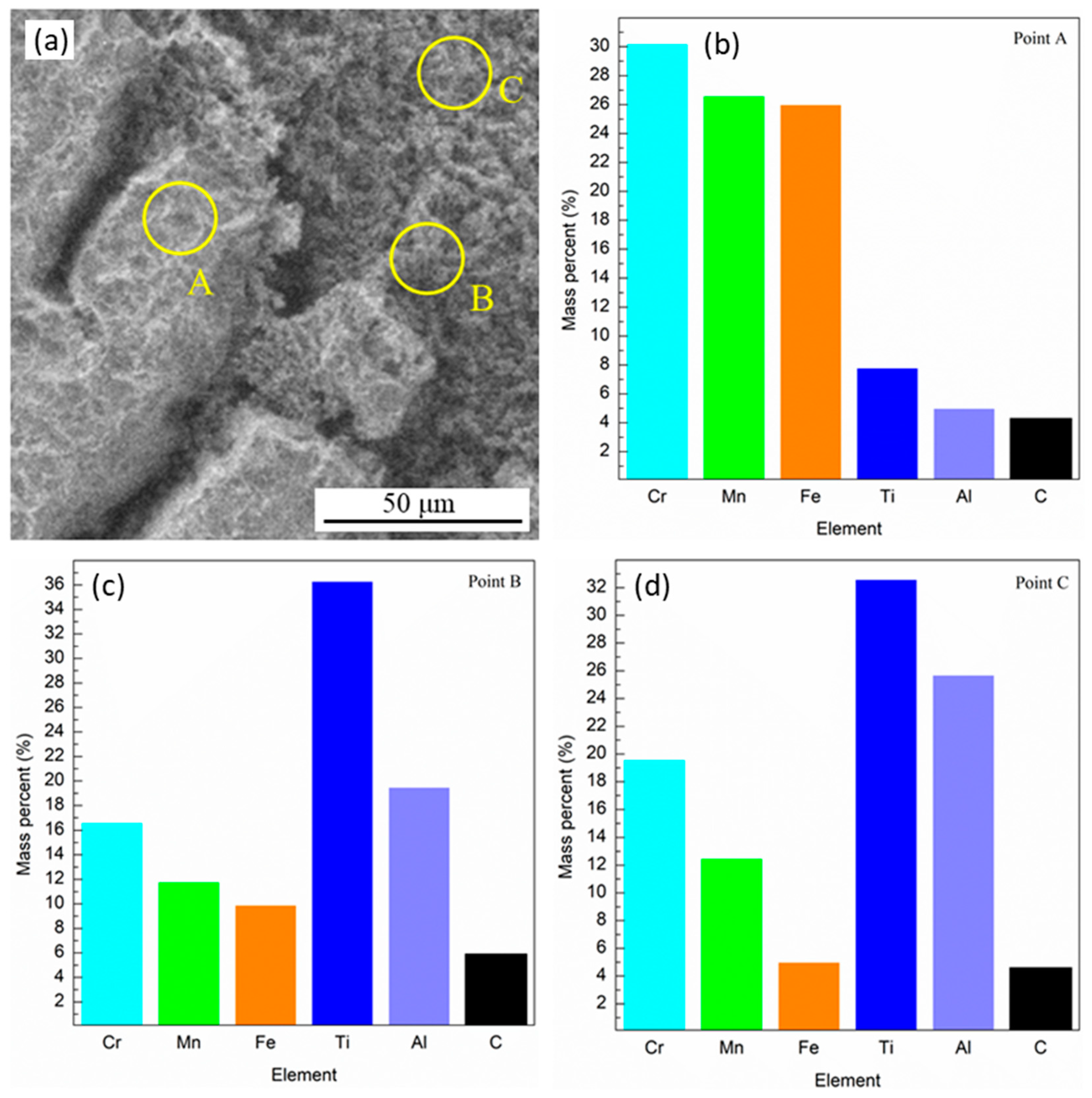
3.1. Microstructure and Hardness
3.2. High-Energy X-ray Diffraction (HEXRD)
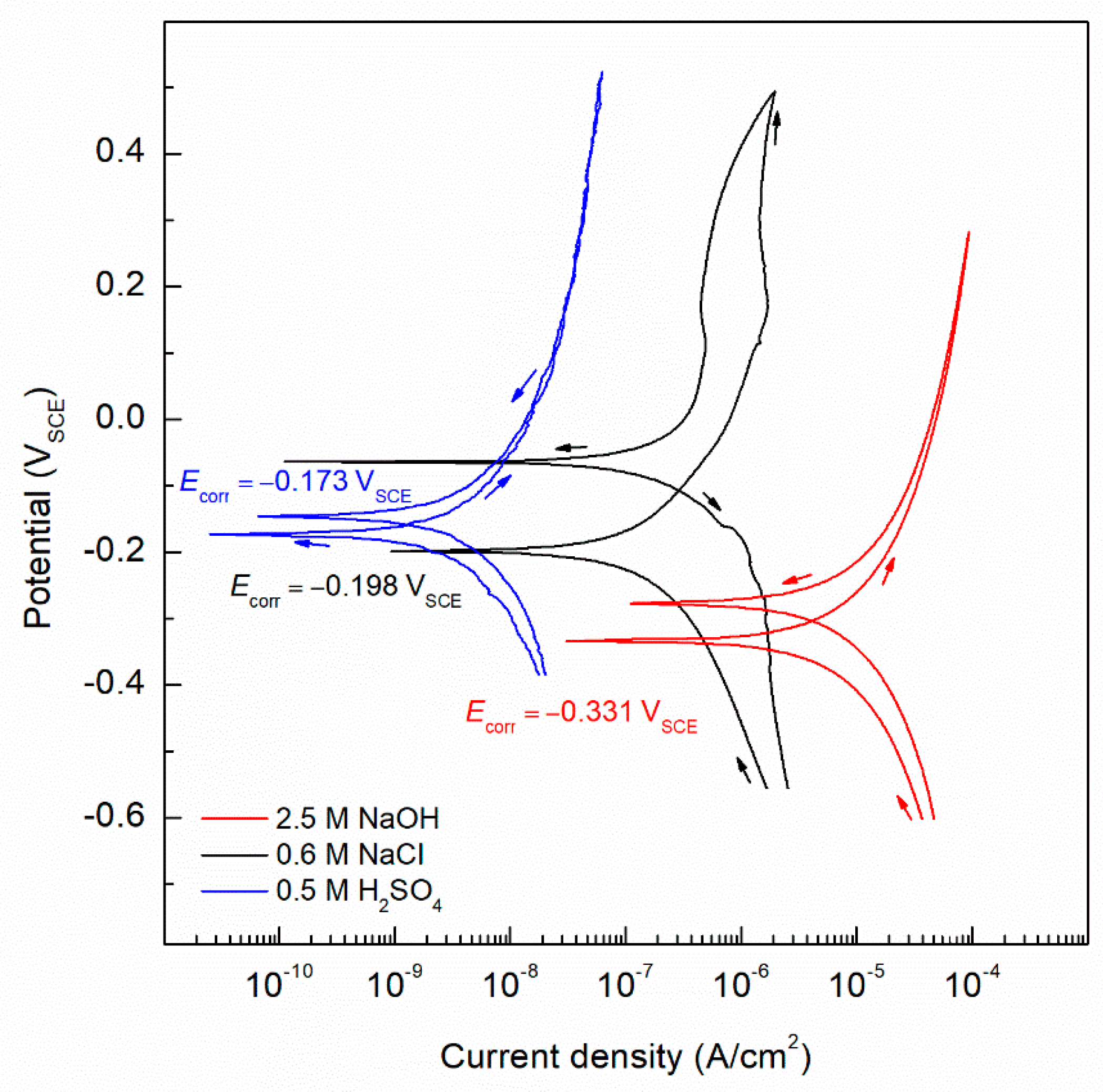
3.3. Cyclic Potentiodynamic Polarization (CPP)
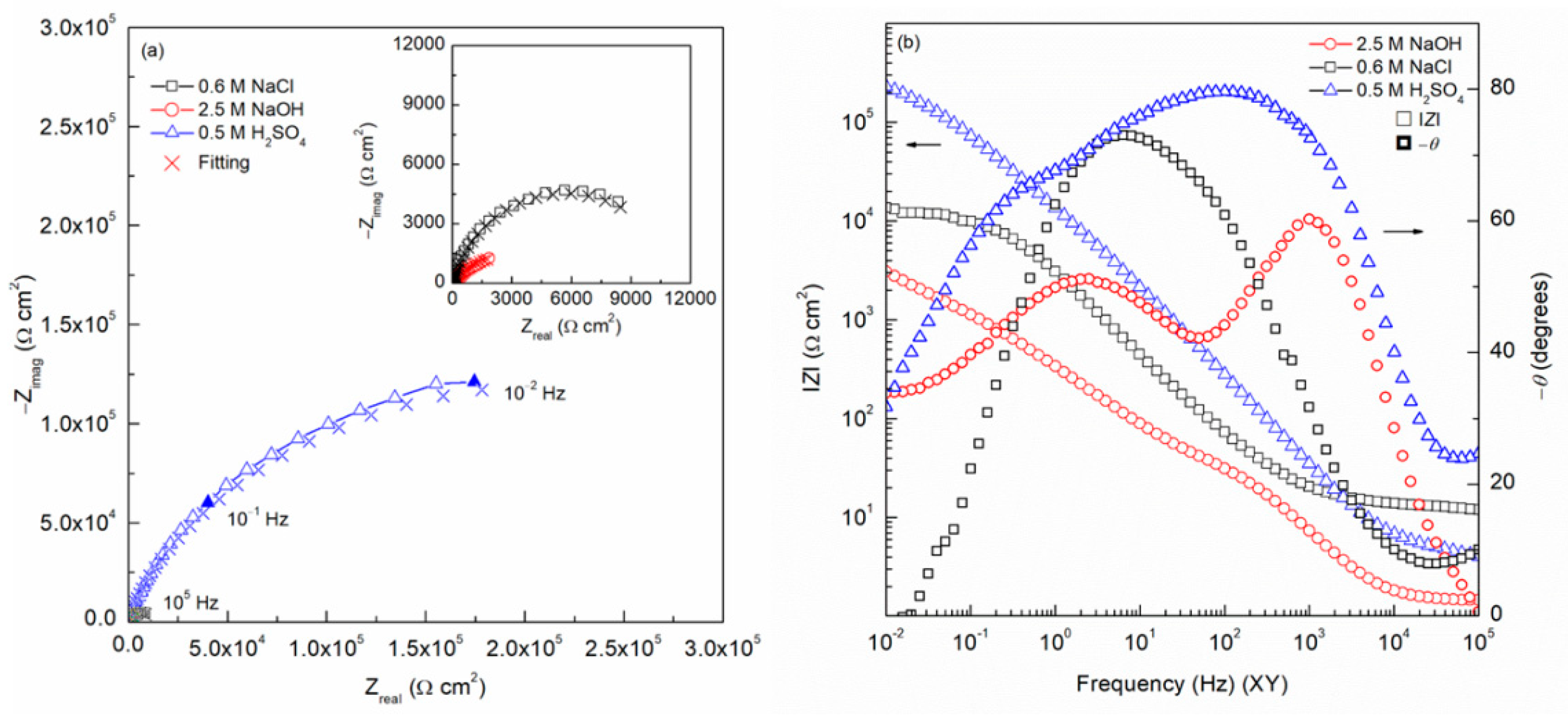
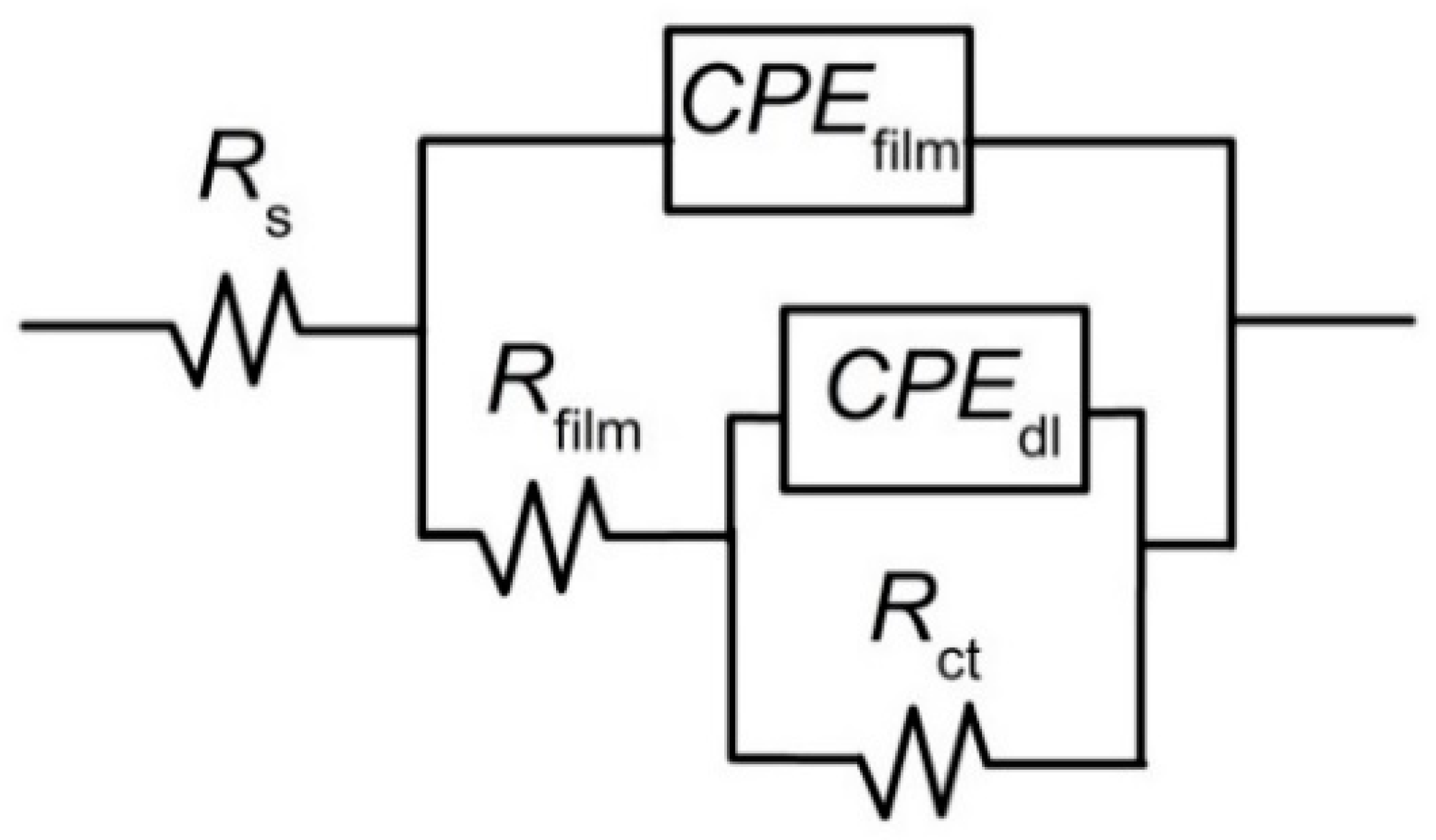
3.4. Electrochemical Impedance Spectroscopy (EIS)
4. Discussion
4.1. Microstructure and Hardness Analysis
4.2. Crystallographic HEXRD Analysis
4.3. Cyclic Potentiodynamic Polarization (CPP)
4.4. Electrochemical Impedance Spectroscopy (EIS)
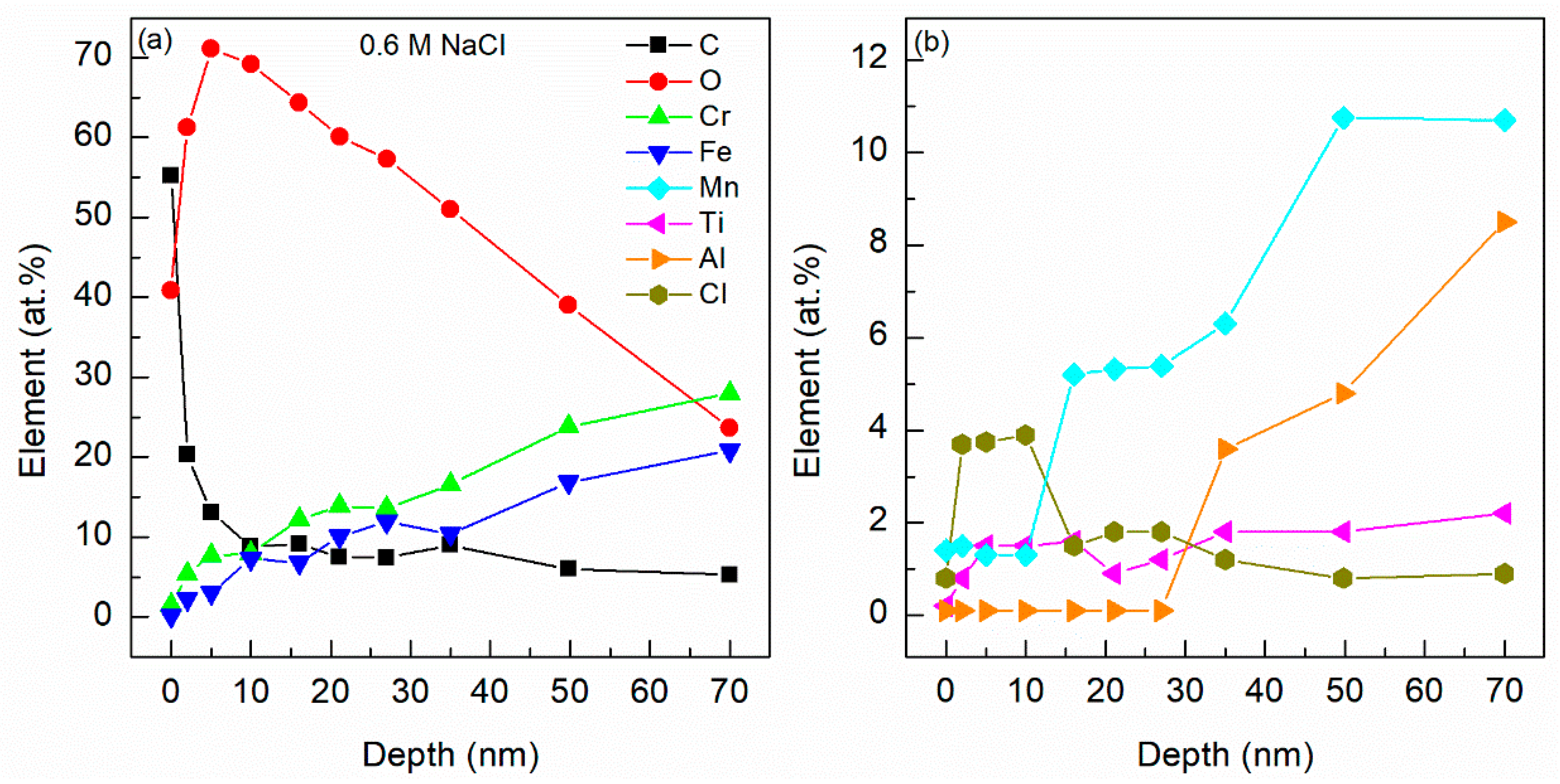
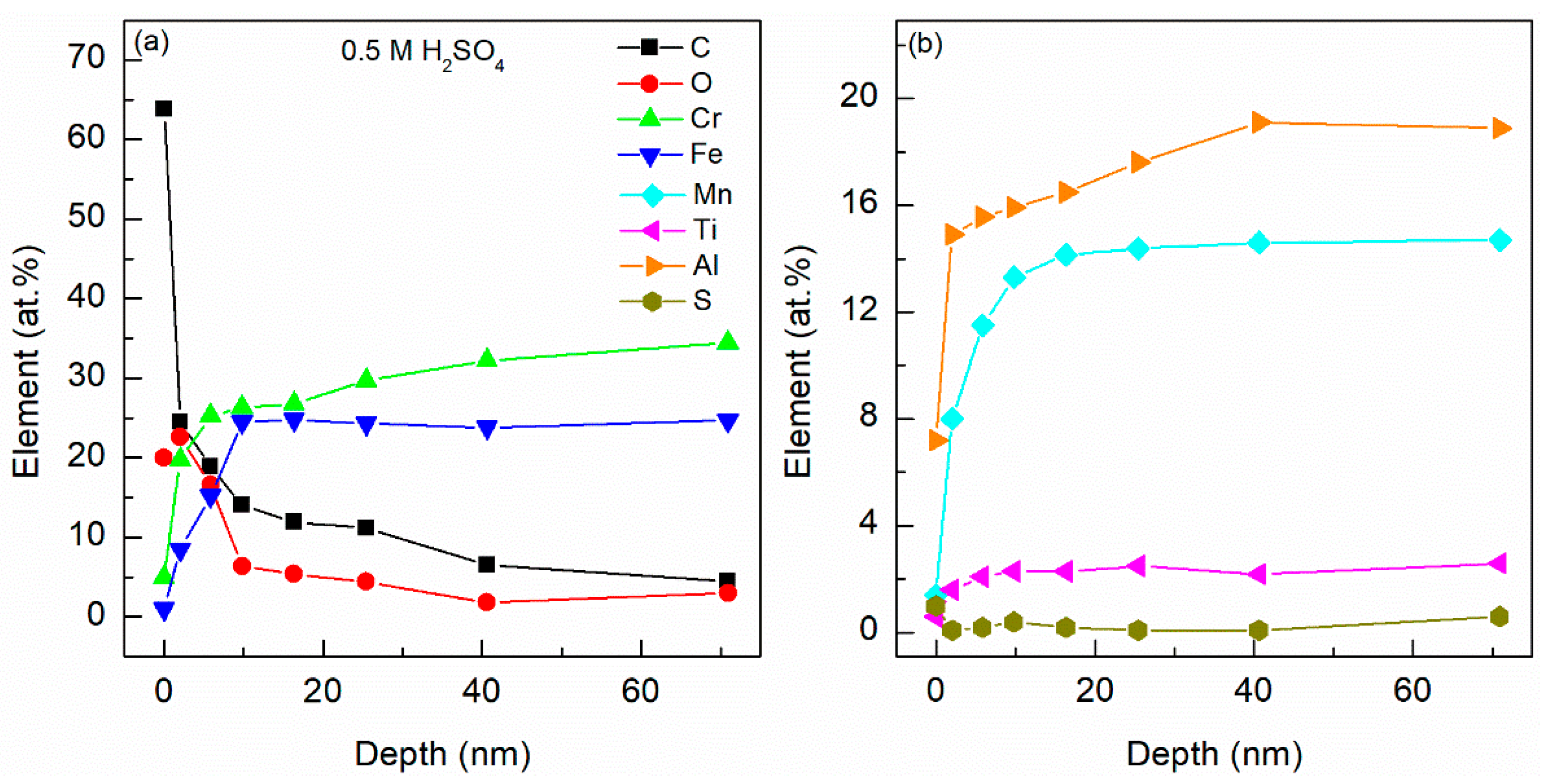
4.5. X-ray Photoelectron Spectroscopy (XPS)
4.6. Energy Dispersive X-ray Spectroscopy (EDX)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Lin, Z.; Xu, H.; Sun, Y. Studies on the microstructure and properties of AlxCoCrFeNiTi1−x high entropy alloys. J. Alloys Compd. 2018, 741, 826–833. [Google Scholar] [CrossRef]
- Liu, T.K.; Wu, Z.; Stoica, A.D.; Xie, Q.; Wu, W.; Gao, Y.F.; Bei, H.; An, K. Twinning-mediated work hardening and texture evolution in CrCoFeMnNi high entropy alloys at cryogenic temperature. Mater. Des. 2017, 131, 419–427. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Lu, C.-W.; Lu, Y.-S.; Lai, Z.-H.; Yen, H.-W.; Lee, Y.-L. Comparative corrosion behavior of Fe50Mn30Co10Cr10 dual-phase high-entropy alloy and CoCrFeMnNi high-entropy alloy in 3.5 wt% NaCl solution. J. Alloys Compd. 2020, 842, 155824. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of Al CoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Lee, C.P.; Chang, C.C.; Chen, Y.Y.; Yeh, J.W.; Shih, H.C. Effect of the aluminium content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Zhang, H.; Dong, S.Y. Phase evolution and cavitation erosion-corrosion behavior of FeCoCrAlNiTix high entropy alloy coatings on 304 stainless steel by laser surface alloying. J. Alloys Compd. 2017, 698, 761–770. [Google Scholar] [CrossRef]
- Shang, X.-L.; Wang, Z.-J.; Wu, Q.-F.; Wang, J.-C.; Li, J.-J.; Yu, J.-K. Effect of Mo addition on corrosion behavior of high-entropy alloys CoCrFeNiMox in aqueous environments. Acta Metall. Sin. 2019, 32, 41–51. [Google Scholar] [CrossRef]
- Han, L.-X.; Wang, C.-M.; Sun, H.-F. Microstructure and anticorrosion property of high-entropy alloy AlFeNiCrCoTi0.5Vx. Mater. Trans. 2016, 57, 1134–1137. [Google Scholar] [CrossRef]
- Liu, M. Effect of uniform corrosion on mechanical behavior of E690 high-strength steel lattice corrugated panel in marine environment: A finite element analysis. Mater. Res. Express 2021, 8, 66510. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. NPJ Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- ASTM G106-89; Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Qiu, Y.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion characteristics of high entropy alloys. Mater. Sci. Technol. 2015, 31, 1235–1243. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.; Li, X. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Hassan, M.A.; Ghayad, I.M.; Mohamed, A.S.A.; El-Nikhaily, A.E.; Elkady, O.A. Improvement ductility and corrosion resistance of CoCrFeNi and AlCoCrFeNi HEAs by electroless copper technique. J. Mater. Res. Technol. 2021, 13, 463–485. [Google Scholar] [CrossRef]
- Shi, Y.; Mo, J.; Zhang, F.-Y.; Yang, B.; Liaw, P.K.; Zhao, Y. In-situ visualization of corrosion behavior of Al CoCrFeNi high-entropy alloys during electrochemical polarization. J. Alloys Compd. 2020, 844, 156014. [Google Scholar] [CrossRef]
- Xue, L.; Ding, Y.; Pradeep, K.G.; Case, R.; Castaneda, H.; Paredes, M. The grain size effect on corrosion property of Al2Cr5Cu5Fe53Ni35 high-entropy alloy in marine environment. Corros. Sci. 2022, 208, 110625. [Google Scholar] [CrossRef]
- Demchenko, I.N.; Melikhov, Y.; Syryanyy, Y.; Zaytseva, I.; Konstantynov, P.; Chernyshova, M. Effect of argon sputtering on XPS depth-profiling results of Si/Nb/Si. J. Electron Spectros. Relat. Phenom. 2018, 224, 17–22. [Google Scholar] [CrossRef]
- Pérez, P.; Garcés, G.; Frutos-Myro, E.; Antoranz, J.M.; Tsipas, S.; Adeva, P. Diseño y caracterización de tres aleaciones multiprincipales ligeras potencialmente candidatas a aleaciones de alta entropía. Rev. Metal. 2019, 55, 147. [Google Scholar] [CrossRef]
- Ortega, Y.; Monge, M.A.; Savoini, B.; Muñoz, A.; Pérez, P. Production and characterization of the Cr35Fe35V16.5Mo6Ti7.5 high entropy alloy. Nucl. Mater. Energy 2022, 30, 101148. [Google Scholar] [CrossRef]
- Liu, M.; Li, J. In-situ raman characterization of initial corrosion behavior of copper in neutral 3.5% (wt.) NaCl solution. Materials 2019, 12, 2164. [Google Scholar] [CrossRef] [PubMed]
- Lins, V.F.C.; Freitas, M.A.; e Silva, E.M.P. Corrosion resistance study of Fe–Mn–Al–C alloys using immersion and potentiostatic tests. Appl. Surf. Sci. 2005, 250, 124–134. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; de Oliveira, M.C.L.; Antunes, R.A. A review on corrosion of high entropy alloys: Exploring the interplay between corrosion properties, alloy composition, passive film stability and materials selection. Mater. Res. 2022, 25. [Google Scholar] [CrossRef]
- Zendejas Medina, L.; Tavares da Costa, M.V.; Paschalidou, E.M.; Lindwall, G.; Riekehr, L.; Korvela, M.; Fritze, S.; Kolozsvári, S.; Gamstedt, E.K.; Nyholm, L.; et al. Enhancing corrosion resistance, hardness, and crack resistance in magnetron sputtered high entropy CoCrFeMnNi coatings by adding carbon. Mater. Des. 2021, 205, 109711. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, B.; Yuan, Y.; Guo, S.; Wei, G. Study on corrosion behavior and mechanism of CoCrFeMnNi HEA interfered by AC current in simulated alkaline soil environment. J. Electroanal. Chem. 2021, 882, 115026. [Google Scholar] [CrossRef]
- Muftah, W.; Allport, J.; Vishnyakov, V. Corrosion performance and mechanical properties of FeCrSiNb amorphous equiatomic HEA thin film. Surf. Coat. Technol. 2021, 422, 127486. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Zhang, M.; Wang, X.; Gong, P.; Zhang, J.; Liu, J. Effect of the grain size on the corrosion behavior of CoCrFeMnNi HEAs in a 0.5 M H2SO4 solution. J. Alloys Compd. 2021, 858, 157712. [Google Scholar] [CrossRef]
- Bertolini, L. Steel corrosion and service life of reinforced concrete structures. Struct. Infrastruct. Eng. 2008, 4, 123–137. [Google Scholar] [CrossRef]
- Bautista, A.; Alvarez, S.M.; Paredes, E.C.; Velasco, F.; Guzman, S. Corrugated stainless steels embedded in carbonated mortars with and without chlorides: 9-Year corrosion results. Constr. Build. Mater. 2015, 95, 186–196. [Google Scholar] [CrossRef]
- Zeng, Y.; Kang, L.; Wu, Y.; Wan, S.; Liao, B.; Li, N.; Guo, X. Melamine modified carbon dots as high effective corrosion inhibitor for Q235 carbon steel in neutral 3.5 wt% NaCl solution. J. Mol. Liq. 2022, 349, 118108. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Gorsse, S.; Nguyen, M.H.; Senkov, O.N.; Miracle, D.B. Database on the mechanical properties of high entropy alloys and complex concentrated alloys. Data Br. 2018, 21, 2664–2678. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Lu, Z.; Yu, L. Effects of Y2O3/Ti/Zr addition on microstructure and hardness of ODS-CoCrFeNi HEAs produced by mechanical alloying and spark plasma sintering. J. Alloys Compd. 2021, 861, 157940. [Google Scholar] [CrossRef]
- Ocak, B.C.; Goller, G. Investigation the effect of FeNiCoCrMo HEA addition on properties of B4C ceramic prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2021, 41, 6290–6301. [Google Scholar] [CrossRef]
- Xiao, J.K.; Tan, H.; Chen, J.; Martini, A.; Zhang, C. Effect of carbon content on microstructure, hardness and wear resistance of CoCrFeMnNiCx high-entropy alloys. J. Alloys Compd. 2020, 847, 156533. [Google Scholar] [CrossRef]
- Mishra, R.K.; Shahi, R.R. A novel low-density semi-hard magnetic Al20Fe20Mg20Ni20Ti20 high entropy alloy. J. Magn. Magn. Mater. 2020, 516, 167342. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef]
- Negahdar, L.; Zeng, F.; Palkovits, S.; Broicher, C.; Palkovits, R. Mechanistic aspects of the electrocatalytic oxygen evolution reaction over Ni−Co oxides. ChemElectroChem 2019, 6, 5588–5595. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, T.; Chen, H.; Liao, B.; Guo, X. Kapok leaves extract and synergistic iodide as novel effective corrosion inhibitors for Q235 carbon steel in H2SO4 medium. Ind. Crops Prod. 2022, 178, 114649. [Google Scholar] [CrossRef]
- Bastidas, D.M. Interpretation of impedance data for porous electrodes and diffusion processes. Corrosion 2007, 63, 515–521. [Google Scholar] [CrossRef]
- Bastidas, D.M. High temperature corrosion of metallic interconnects in solid oxide fuel cells. Rev. Metal. 2006, 42, 425–443. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Luo, H.; Zou, S.; Chen, Y.-H.; Li, Z.; Du, C.; Li, X. Influence of carbon on the corrosion behaviour of interstitial equiatomic CoCrFeMnNi high-entropy alloys in a chlorinated concrete solution. Corros. Sci. 2020, 163, 108287. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; Antunes, R.A. Electronic properties of the passive films formed on CoCrFeNi and CoCrFeNiAl high entropy alloys in sodium chloride solution. J. Mater. Res. Technol. 2020, 9, 13879–13892. [Google Scholar] [CrossRef]
- Hakiki, N.B.; Boudin, S.; Rondot, B.; Da Cunha Belo, M. The electronic structure of passive films formed on stainless steels. Corros. Sci. 1995, 37, 1809–1822. [Google Scholar] [CrossRef]
- Fajardo, S.; Bastidas, D.M.; Criado, M.; Bastidas, J.M. Electrochemical study on the corrosion behaviour of a new low-nickel stainless steel in carbonated alkaline solution in the presence of chlorides. Electrochim. Acta 2014, 129, 160–170. [Google Scholar] [CrossRef]
- Deepa, P.; Padmalatha, R. Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arab. J. Chem. 2017, 10, S2234–S2244. [Google Scholar] [CrossRef]
- Zhang, J.; Klasky, M.; Letellier, B.C. The aluminum chemistry and corrosion in alkaline solutions. J. Nucl. Mater. 2009, 384, 175–189. [Google Scholar] [CrossRef]
- Martin, U.; Bosch, J.; Ress, J.; Bastidas, D.M. Long-term stability and electronic properties of passive film of lean-duplex stainless steel reinforcements in chloride containing mortar. Constr. Build. Mater. 2021, 291, 123319. [Google Scholar] [CrossRef]



| Element | Cr | Mn | Fe | Al | Ti |
|---|---|---|---|---|---|
| Content (at.%) | 30 | 30 | 25 | 10 | 5 |
| Content (wt.%) | 30.5 | 32.2 | 27.3 | 5.3 | 4.7 |
| Region | Cr | Mn | Fe | Al | Ti |
|---|---|---|---|---|---|
| Average | 31.5 | 28.9 | 25.6 | 8.0 | 6.0 |
| Matrix | 38.1 | 25.8 | 23.6 | 8.3 | 4.2 |
| Secondary phase | 24.3 | 29.6 | 28.8 | 6.1 | 11.1 |
| Electrolyte Solution | Ecorr mVSCE | icorr A/cm2 | βa mV/dec | βc mV/dec | B mV | αa | αc |
|---|---|---|---|---|---|---|---|
| 2.5 M NaOH | −331 | 1.61 × 10−5 | 592 | –601 | 129 | 0.165 | 0.121 |
| 0.6 M NaCl | −198 | 4.10 × 10−7 | 549 | –573 | 121 | 0.047 | 0.045 |
| 0.5 M H2SO4 | −173 | 1.36 × 10−8 | 719 | –820 | 161 | 0.036 | 0.031 |
| HEA | Electrolyte Solution | Ecorr (mVSCE) | icorr (A/cm2) | ǀZǀ (Ω cm2) | Ref. |
|---|---|---|---|---|---|
| Al10Cr30Fe25Mn30Ti5 | 2.5 M NaOH | −331 | 1.61 × 10−5 | 3.21 × 103 | This work |
| 0.6 M NaCl | −198 | 4.10 × 10−7 | 1.26 × 104 | ||
| 0.5 M H2SO4 | −173 | 1.36 × 10−8 | 2.25 × 105 | ||
| Cr18Mn18Fe21Co21Ni22 | 0.05 M H2SO4 | −320 | 8.0 × 10−6 | 1.25 × 105 | [25] |
| Cr19.6Ni20.2Co21.3Mn20.5Fe18.4 | 0.4 M Na2CO3 1 M NaHCO3 | −250 | 3.0 × 10−6 | 1.0 × 105 | [26] |
| CoCrFeNi | 0.6 M NaCl | −130 | 2.11 × 10−6 | 1.2 × 104 | [16] |
| Cu 5 wt.% + AlCoCrFeNi | −197 | 12.97 × 10−6 | 1.7 × 103 | ||
| FeCrSiNb thin film | 0.6 M NaCl | +60 | 8.66 × 10−10 | – | [27] |
| 0.6 M H2SO4 | +360 | 1.22 × 10−8 | – | ||
| Co21Cr19Fe20Mn19Ni20 | 0.5 M H2SO4 | −366 | 57.9 × 10−6 | 1.0 × 103 | [28] |
| Electrolyte Solution | Rs | Rfilm | Yfilm | nfilm | Rct | Ydl | ndl | Ceff,film | χ2 (*) |
|---|---|---|---|---|---|---|---|---|---|
| Ω cm2 | kΩ cm2 | µS/cm2 snfilm | kΩ cm2 | µS/cm2 sndl | F/cm2 | ||||
| 2.5 M NaOH | 3.91 | 0.52 | 3.65 | 0.82 | 5.35 | 987.62 | 0.79 | 8.36 × 10−5 | 2.69 × 10−3 |
| 0.6 M NaCl | 13.44 | 1.53 | 3.29 | 0.85 | 11.39 | 35.01 | 0.81 | 6.56 × 10−5 | 1.91 × 10−3 |
| 0.5 M H2SO4 | 2.48 | 2.45 | 1.12 | 0.93 | 494.28 | 9.28 | 0.74 | 1.54 × 10−5 | 1.45 × 10−3 |
| HEA | Hardness (HV) | Density (g/cm3) | Ref. |
|---|---|---|---|
| Al10Cr30Fe25Mn30Ti5 | 660 | 6.84 | This work |
| CoCrFeNi | 676 | – | [34] |
| FeNiCoCrMo | 1000 | 8.86 | [35] |
| CoCrFeNi | 225 | 8.34 | [16] |
| Cu 5 wt.% AlCoCrFeNi | 400 | 7.21 | |
| FeCrSiNb thin film | 1530 | – | [27] |
| CoCrFeMnNiC0.6 | 566.4 | – | [36] |
| Co21Cr19Fe20Mn19Ni20 | 3000 | – | [28] |
| Al20Fe20Mg20Ni20Ti20 | 94.5 | 4.64 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, U.; Ress, J.; Pérez, P.; Adeva, P.; Bastidas, D.M. Corrosion Behavior of Al10Cr30Fe25Mn30Ti5 High-Entropy Alloy: Microstructural, Electrochemical, and Surface Analysis. Metals 2022, 12, 1736. https://doi.org/10.3390/met12101736
Martin U, Ress J, Pérez P, Adeva P, Bastidas DM. Corrosion Behavior of Al10Cr30Fe25Mn30Ti5 High-Entropy Alloy: Microstructural, Electrochemical, and Surface Analysis. Metals. 2022; 12(10):1736. https://doi.org/10.3390/met12101736
Chicago/Turabian StyleMartin, Ulises, Jacob Ress, Pablo Pérez, Paloma Adeva, and David M. Bastidas. 2022. "Corrosion Behavior of Al10Cr30Fe25Mn30Ti5 High-Entropy Alloy: Microstructural, Electrochemical, and Surface Analysis" Metals 12, no. 10: 1736. https://doi.org/10.3390/met12101736
APA StyleMartin, U., Ress, J., Pérez, P., Adeva, P., & Bastidas, D. M. (2022). Corrosion Behavior of Al10Cr30Fe25Mn30Ti5 High-Entropy Alloy: Microstructural, Electrochemical, and Surface Analysis. Metals, 12(10), 1736. https://doi.org/10.3390/met12101736








