Corrosion and Electrochemical Properties of Laser-Shock-Peening-Treated Stainless Steel AISI 304L in VVER Primary Water Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Specimens
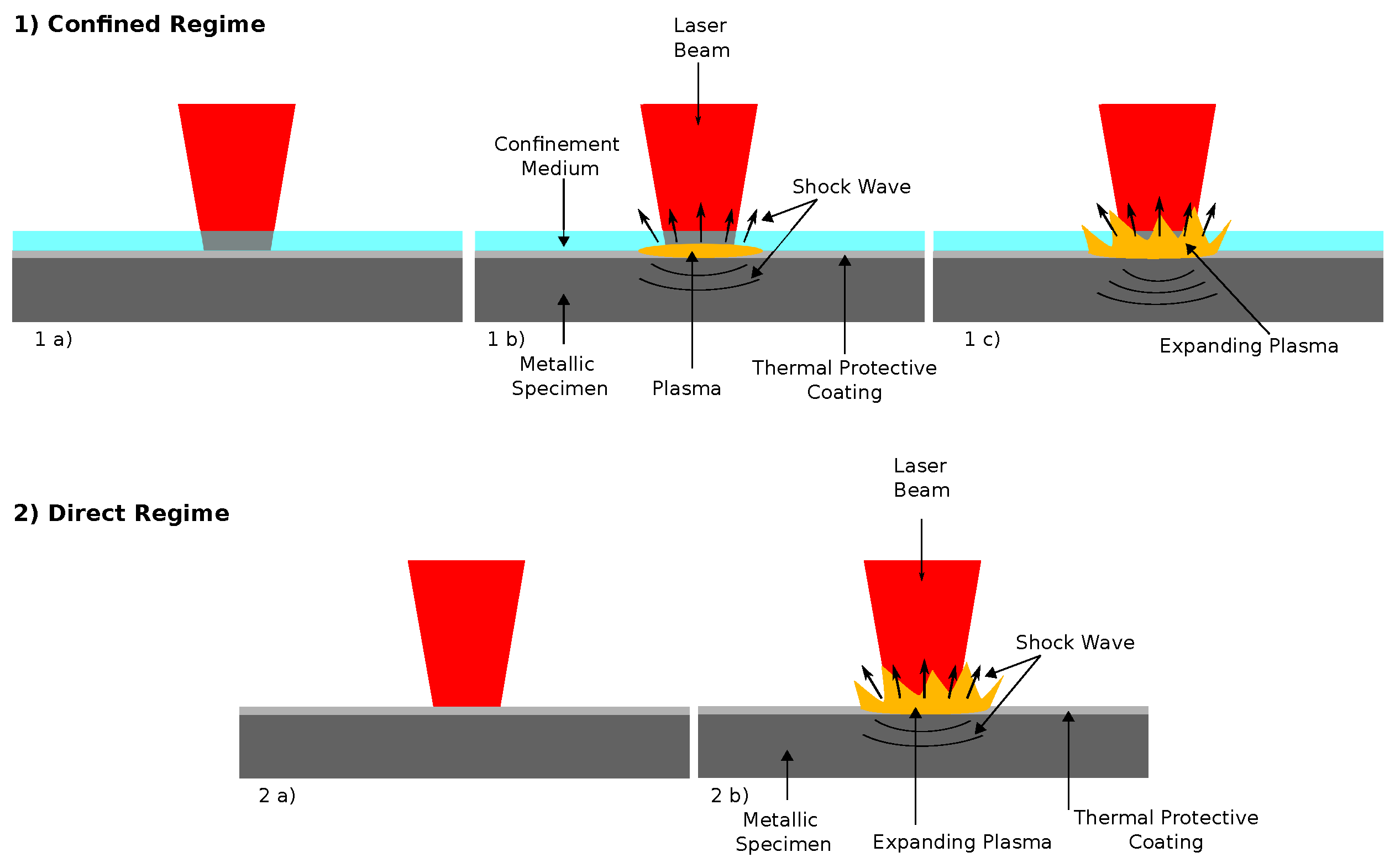
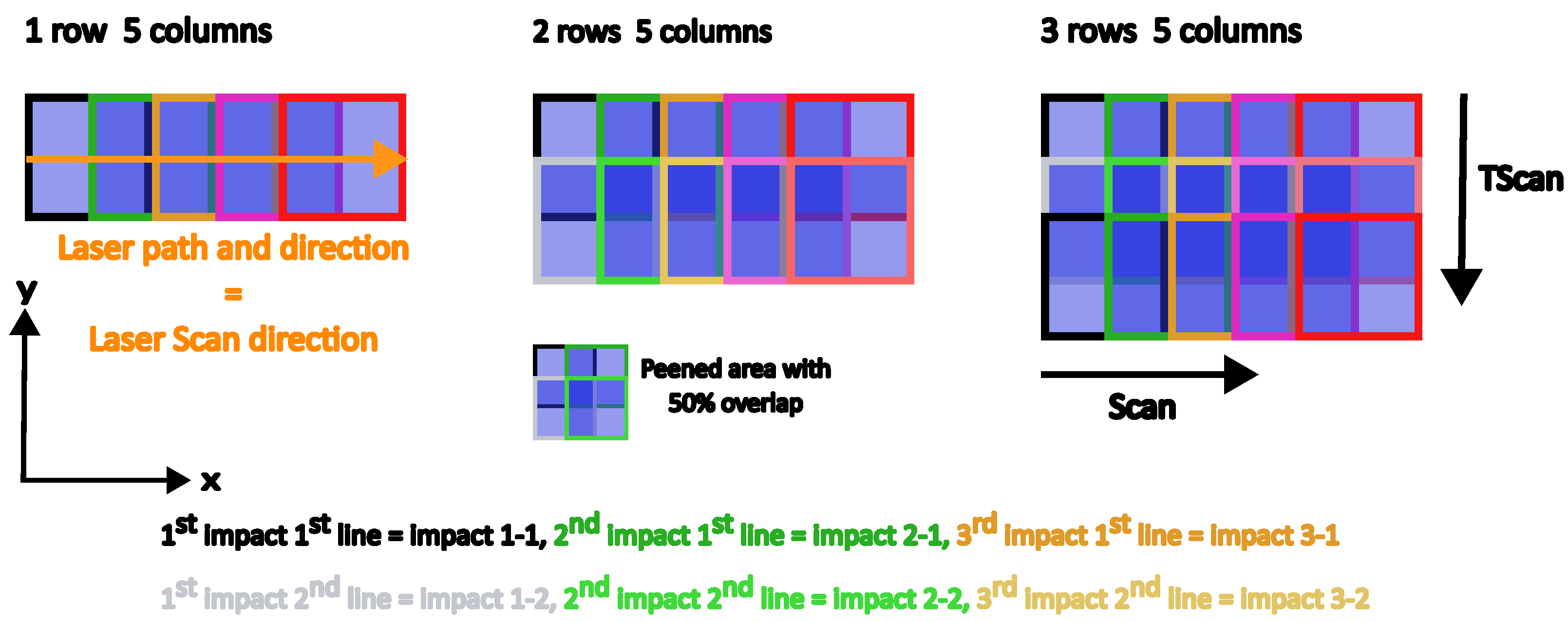
2.2. Laser Shock Peening Treatment
- Impact 1-1 (black) is covered at 50% in X-direction by impact 2-1.
- Impact 2-1 (dark green) is covered at 50% in X-direction by the impact 3-1.
- Impact 1-2 (grey) covers the impacts 1-1 and 2-1 at 50% in Y-direction, and so forth.
2.3. Roughness Measurement
2.4. XRD Residual Stress Measurement
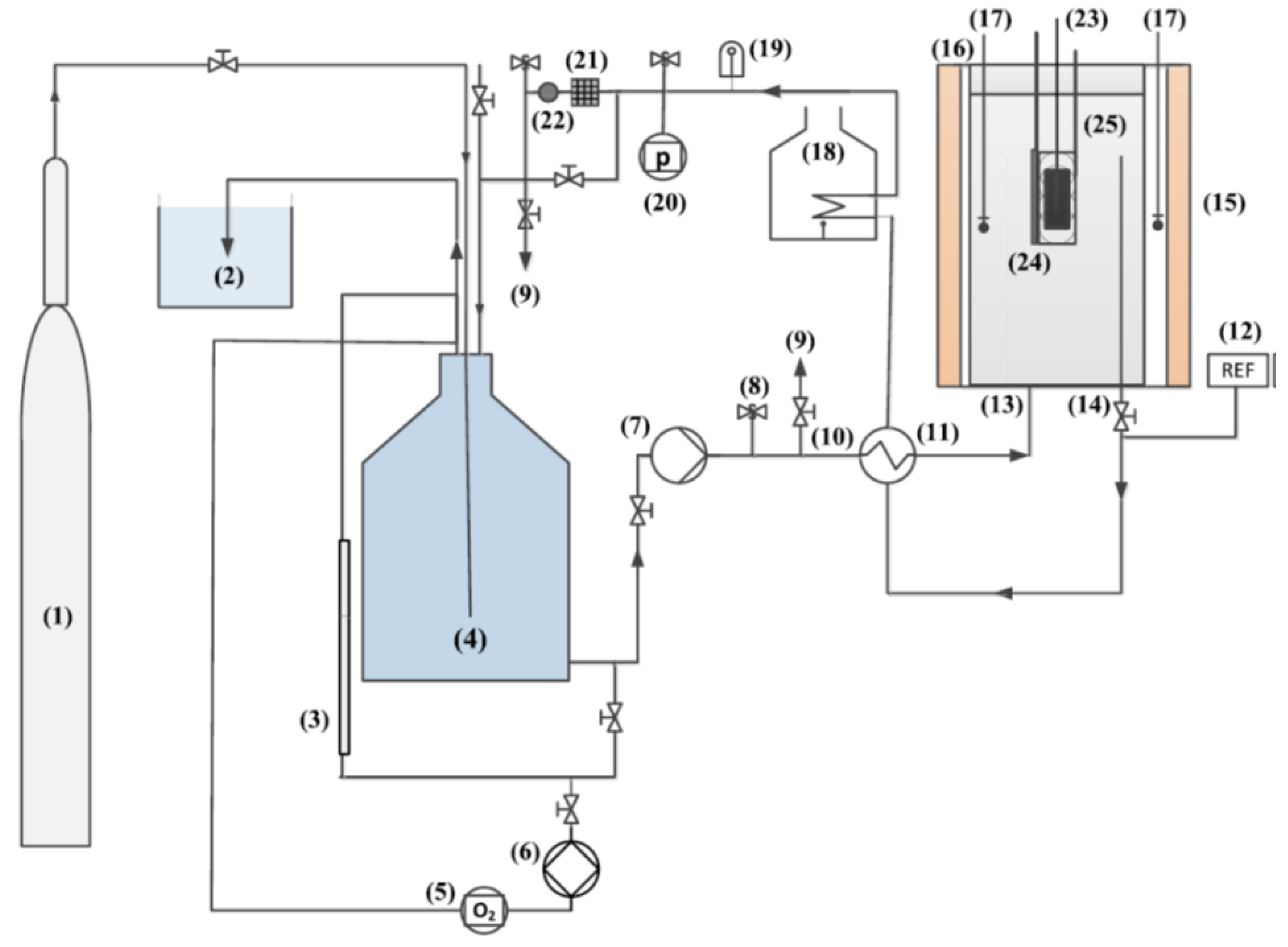
2.5. Electrochemical Impedance Spectroscopy (EIS) in Autoclave
3. Results and Discussion
3.1. Residual Stress and Surface Roughness Measurements
3.2. Estimation of the Roughness Factor by EIS
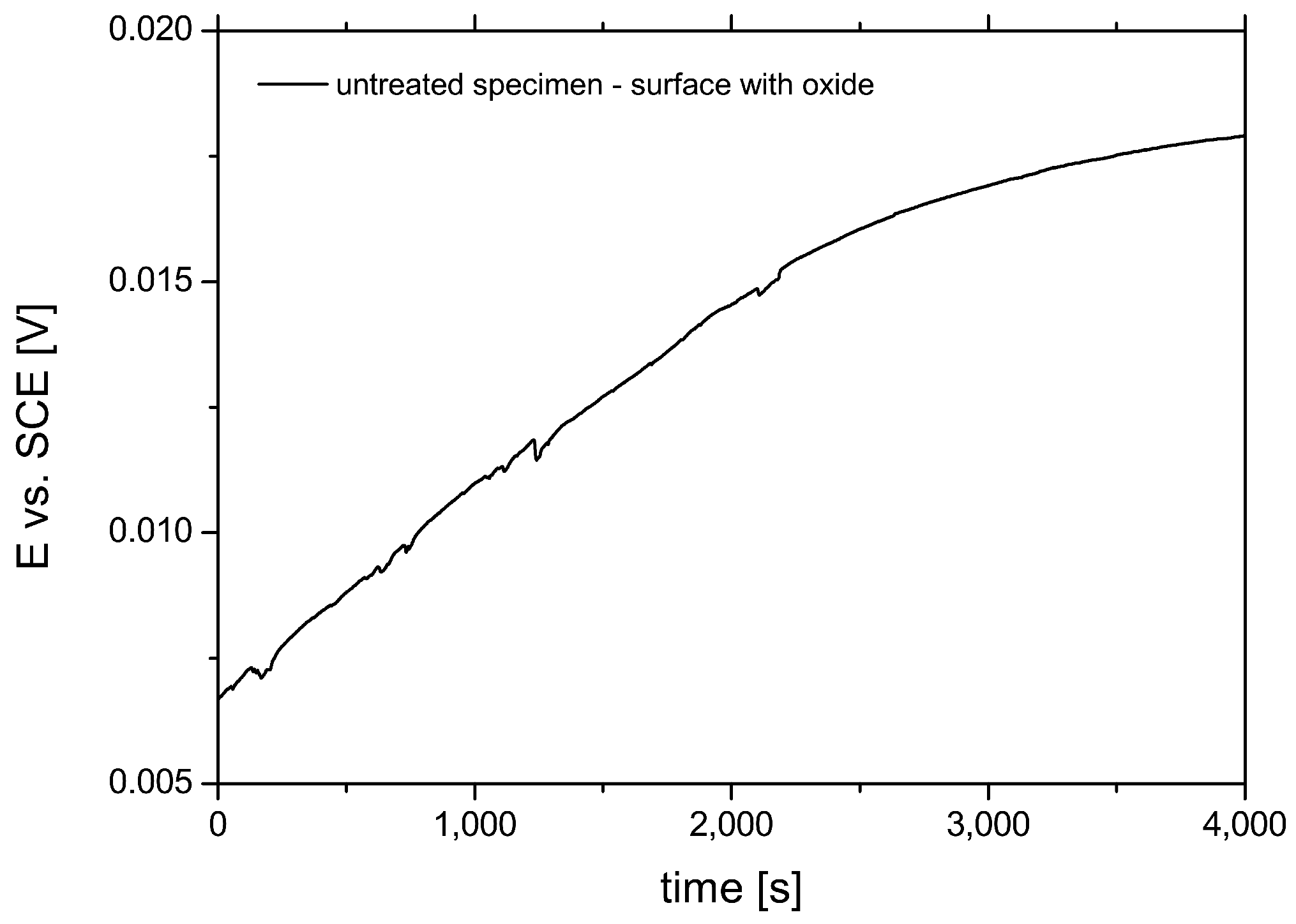
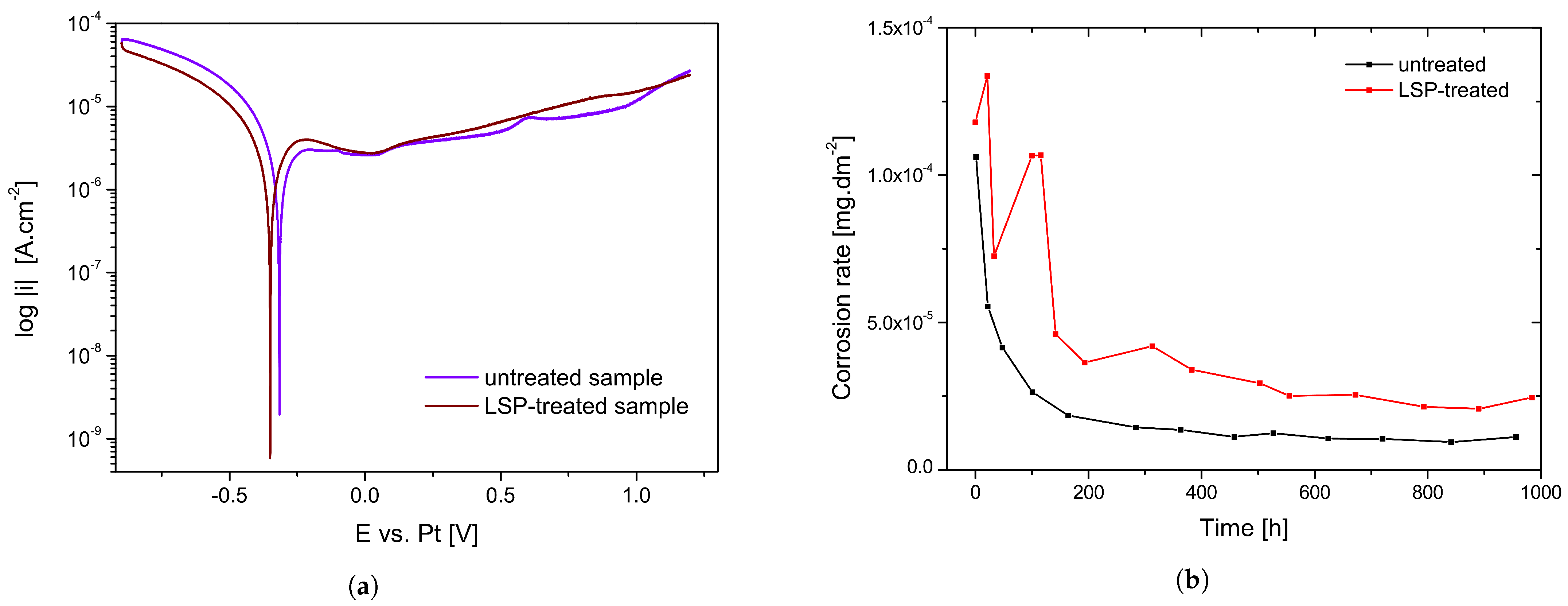
3.3. Corrosion Potential Measurements
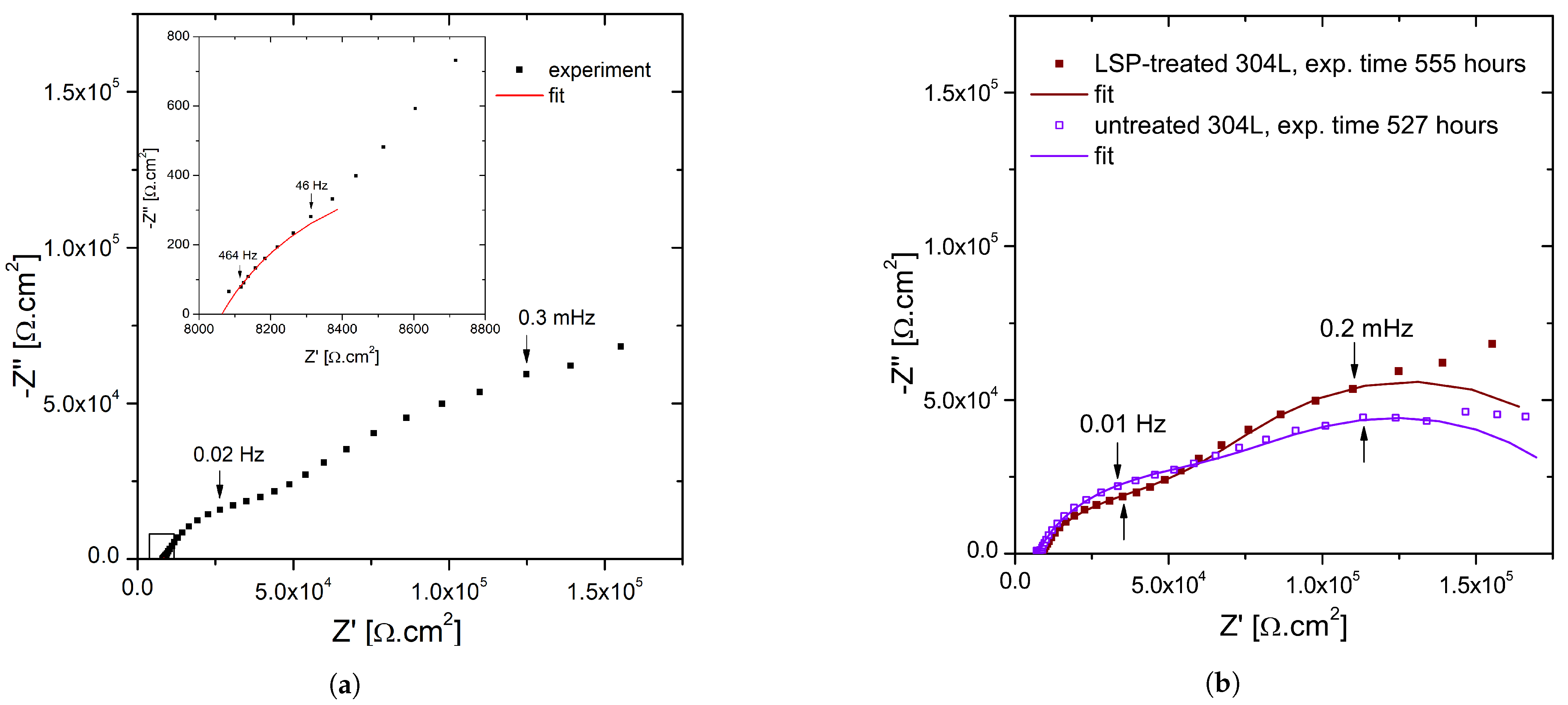
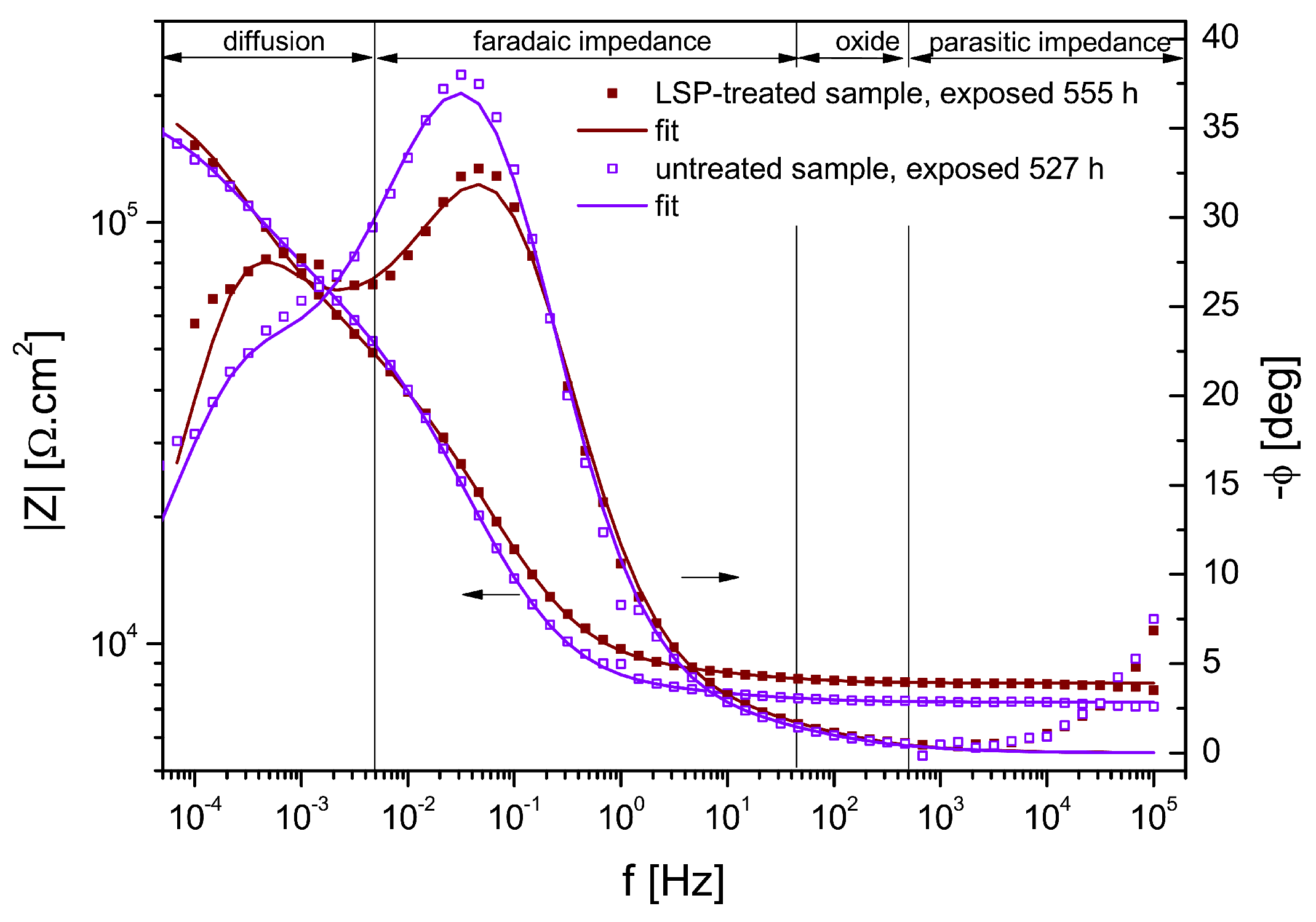
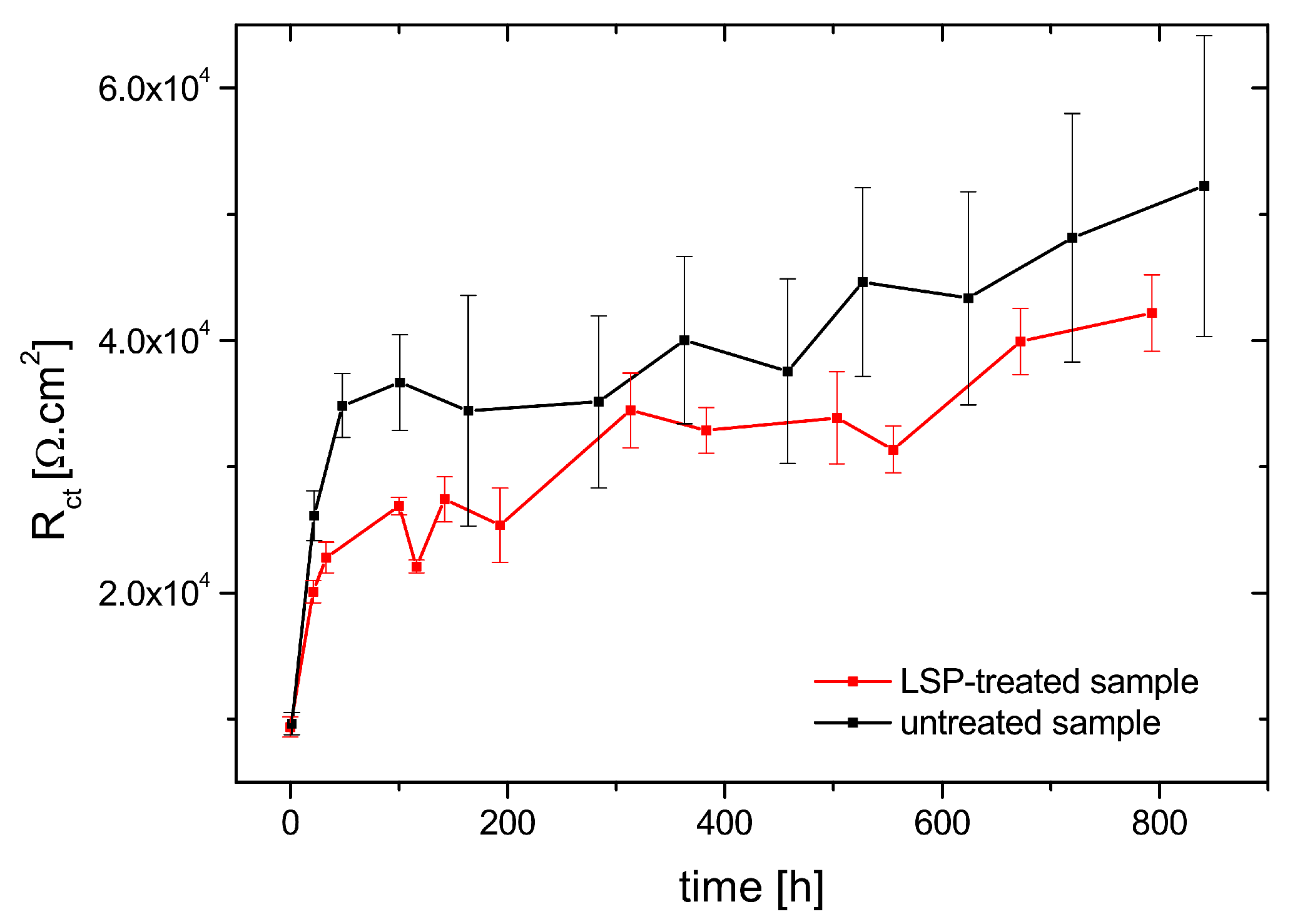
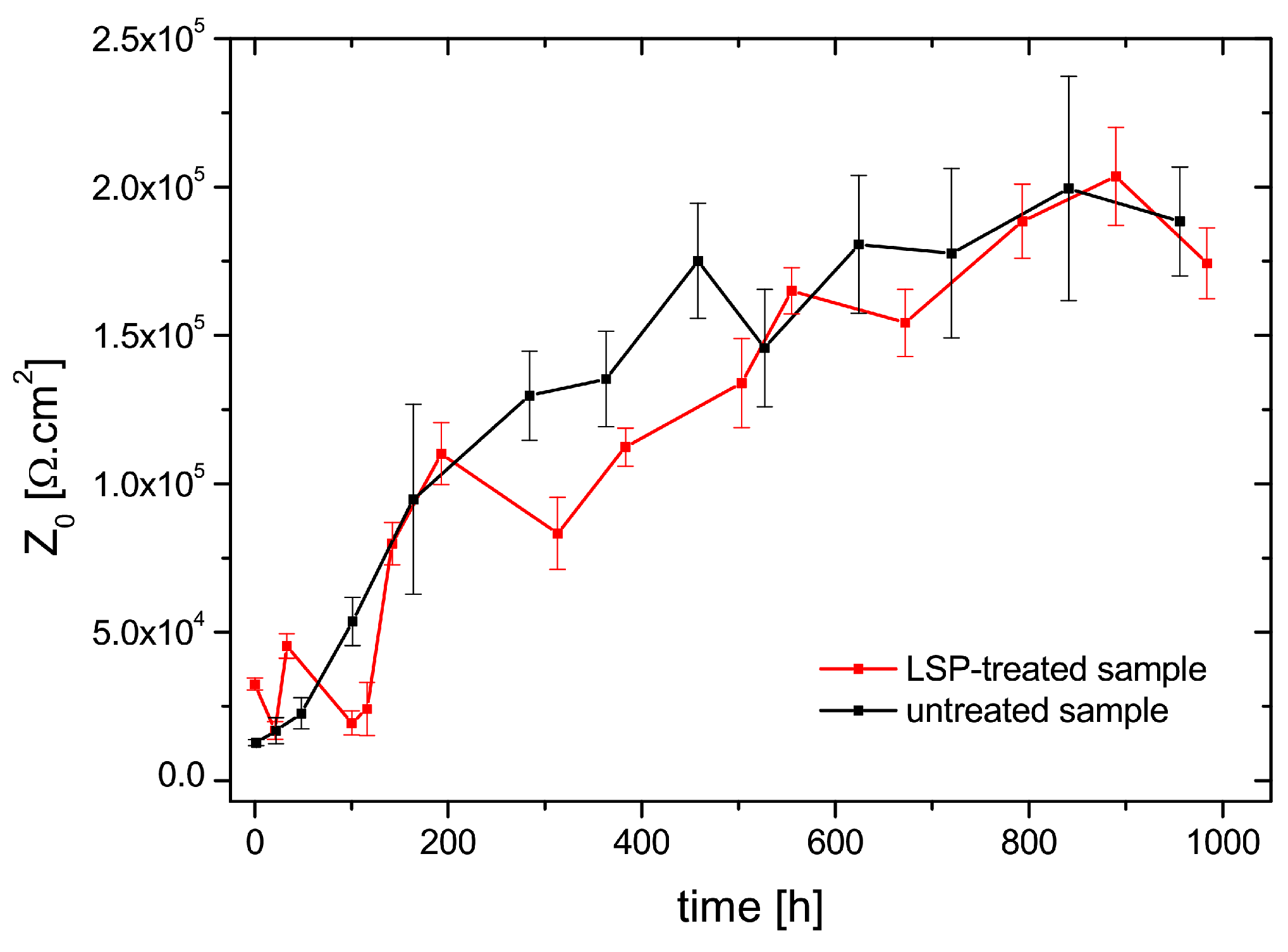
3.4. Electrochemical Impedance Spectroscopy Measurements
3.5. Surface Analysis with FEG-SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LSP | Laser Shock Peening |
| EIS | Electrochemical Impedance Spectroscopy |
| RS | residual stress |
| XRD | X-ray diffractometer |
| Scan | laser scan direction |
| TScan | transverse laser scan direction |
| A | electrode surface |
| a | activity of diffusing species |
| c | concentration of diffusing species |
| effective capacitance | |
| effective capacitance for untreated specimen | |
| effective capacitance for diamond polished specimen | |
| effective capacitance for LSP-treated specimen | |
| equilibrium concentration of the diffusing species | |
| CPE | constant phase element |
| constant phase element of the oxide layer | |
| constant phase element of the double layer | |
| chemical diffusion coefficient | |
| FLW | Finite Length Warburg |
| F | Faraday constant |
| corrosion potential | |
| j | imaginary unit |
| l | thickness of the diffusion layer |
| n | CPE exponent |
| Q | CPE coefficient |
| R universal gas constant | roughness factor for as-received specimen |
| roughness factor for LSP-treated specimen | |
| resistance of the environment | |
| faradaic resistance of the double layer | |
| polarization resistance | |
| resistance of the oxide layer | |
| resistance of charge transfer | |
| T temperature z | charge of mobile ion |
| fractal FLW impedance | |
| Warburg coefficient | |
| cathodic Tafel slope | |
| characteristic time constant | |
| angular frequency | |
| impedance of the CPE |
References
- Clauer, A.H. Laser Shock Peening, the Path to Production. Metals 2019, 9, 626. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Lu, J.; Dai, F.; Feng, A.; Luo, K.; Zhong, J.; Wang, Q.; Luo, M.; Qi, H. Effects of laser shock processing on electrochemical corrosion resistance of ANSI 304 stainless steel weldments after cavitation erosion. Corros. Sci. 2013, 66, 5–13. [Google Scholar] [CrossRef]
- Telang, A.; Gill, A.S.; Kumar, M.; Teysseyre, S.; Qian, D.; Mannava, S.R.; Vasudevan, V.K. Iterative thermomechanical processing of alloy 600 for improved resistance to corrosion and stress corrosion cracking. Acta Mater. 2016, 113, 180–193. [Google Scholar] [CrossRef]
- Peyre, P.; Fabbro, R.; Merrien, P.; Lieurade, H. Laser shock processing of aluminium alloys. Application to high cycle fatigue behaviour. Mater. Sci. Eng. A 1996, 210, 102–113. [Google Scholar] [CrossRef]
- Sano, Y.; Mukai, N.; Okazaki, K.; Obata, M. Residual stress improvement in metal surface by underwater laser irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1997, 121, 432–436. [Google Scholar] [CrossRef]
- Ambartsu, R.; Basov, N.; Boiko, V.; Zuev, V.; Krokhin, O.; Kryukov, P.; Senatski, Y.; Stoilov, Y. Heating of matter by focused laser radiation. Sov. J. Exp. Theor. Phys. 1965, 21, 1061–1064. [Google Scholar]
- Mora, P. Theoretical model of absorption of laser light by a plasma. Phys. Fluids 1982, 25, 1051. [Google Scholar] [CrossRef]
- Scius-Bertrand, M.; Videau, L.; Rondepierre, A.; Lescoute, E.; Rouchausse, Y.; Kaufman, J.; Rostohar, D.; Brajer, J.; Berthe, L. Laser induced plasma characterization in direct and water confined regimes: New advances in experimental studies and numerical modelling. J. Phys. D Appl. Phys. 2021, 54, 055204. [Google Scholar] [CrossRef]
- Peyre, P.; Fabbro, R. Laser shock processing: A review of the physics and applications. Opt. Quantum Electron. 1995, 27, 1213–1229. [Google Scholar]
- Fabbro, R.; Fournier, J.; Ballard, P.; Devaux, D.; Virmont, J. Physical study of laser-produced plasma in confined geometry. J. Appl. Phys. 1990, 68, 775–784. [Google Scholar] [CrossRef]
- Ballard, P.; Fournier, J.; Fabbro, R.; Frelat, J. Residual stress induced by laser-shocks. J. Phys. IV 1991, 01, C3-487–C3-494. [Google Scholar] [CrossRef]
- Fairand, B.P.; Clauer, A.H. Laser generation of high-amplitude stress waves in materials. J. Appl. Phys. 1979, 50, 1497–1502. [Google Scholar] [CrossRef]
- Geng, Y.; Dong, X.; Wang, K.; Yan, X.; Duan, W.; Fan, Z.; Wang, W.; Mei, X. Effect of microstructure evolution and phase precipitations on hot corrosion behavior of IN718 alloy subjected to multiple laser shock peening. Surf. Coat. Technol. 2019, 370, 244–254. [Google Scholar] [CrossRef]
- Rai, A.K.; Biswal, R.; Gupta, R.K.; Rai, S.K.; Singh, R.; Goutam, U.K.; Ranganathan, K.; Ganesh, P.; Kaul, R.; Bindra, K.S. Enhancement of oxidation resistance of modified P91 grade ferritic-martensitic steel by surface modification using laser shock peening. Appl. Surf. Sci. 2019, 495, 143611. [Google Scholar] [CrossRef]
- Trdan, U.; Skarba, M.; Grum, J. Laser shock peening effect on the dislocation transitions and grain refinement of Al–Mg–Si alloy. Mater. Charact. 2014, 97, 57–68. [Google Scholar] [CrossRef]
- Wang, C.; Luo, K.; Bu, X.; Su, Y.; Cai, J.; Zhang, Q.; Lu, J. Laser shock peening-induced surface gradient stress distribution and extension mechanism in corrosion fatigue life of AISI 420 stainless steel. Corros. Sci. 2020, 177, 109027. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, C.; Ling, X. Effects of laser shock processing on corrosion resistance of AISI 304 stainless steel in acid chloride solution. J. Alloys Compd. 2017, 723, 237–242. [Google Scholar] [CrossRef]
- Lu, J.; Han, B.; Cui, C.; Li, C.; Luo, K. Electrochemical and pitting corrosion resistance of AISI 4145 steel subjected to massive laser shock peening treatment with different coverage layers. Opt. Laser Technol. 2017, 88, 250–262. [Google Scholar] [CrossRef]
- Chukwuike, V.; Echem, O.; Prabhakaran, S.; AnandKumar, S.; Barik, R. Laser shock peening (LSP): Electrochemical and hydrodynamic investigation of corrosion protection pre-treatment for a copper surface in 3.5 % NaCl medium. Corros. Sci. 2021, 179, 109156. [Google Scholar] [CrossRef]
- Peyre, P.; Scherpereel, X.; Berthe, L.; Carboni, C.; Fabbro, R.; Béranger, G.; Lemaitre, C. Surface modifications induced in 316L steel by laser peening and shot-peening. Influence on pitting corrosion resistance. Mater. Sci. Eng. A 2000, 280, 294–302. [Google Scholar] [CrossRef]
- John, M.; Kalvala, P.R.; Misra, M.; Menezes, P.L. Peening Techniques for Surface Modification: Processes, Properties, and Applications. Materials 2021, 14, 3841. [Google Scholar] [CrossRef] [PubMed]
- Siddaiah, A.; Mao, B.; Liao, Y.; Menezes, P.L. Surface characterization and tribological performance of laser shock peened steel surfaces. Surf. Coat. Technol. 2018, 351, 188–197. [Google Scholar] [CrossRef]
- Maleki, E.; Unal, O.; Guagliano, M.; Bagherifard, S. The effects of shot peening, laser shock peening and ultrasonic nanocrystal surface modification on the fatigue strength of Inconel 718. Mater. Sci. Eng. A 2021, 810, 141029. [Google Scholar] [CrossRef]
- Gujba, A.; Medraj, M. Laser Peening Process and Its Impact on Materials Properties in Comparison with Shot Peening and Ultrasonic Impact Peening. Materials 2014, 7, 7925–7974. [Google Scholar] [CrossRef]
- Lu, J.; Qi, H.; Luo, K.; Luo, M.; Cheng, X. Corrosion behaviour of AISI 304 stainless steel subjected to massive laser shock peening impacts with different pulse energies. Corros. Sci. 2014, 80, 53–59. [Google Scholar] [CrossRef]
- Guzonas, D.; Cook, W. Cycle chemistry and its effect on materials in a supercritical water-cooled reactor: A synthesis of current understanding. Corros. Sci. 2012, 65, 48–66. [Google Scholar] [CrossRef]
- Penttilä, S.; Toivonen, A.; Li, J.; Zheng, W.; Novotny, R. Effect of surface modification on the corrosion resistance of austenitic stainless steel 316L in supercritical water conditions. J. Supercrit. Fluids 2013, 81, 157–163. [Google Scholar] [CrossRef]
- Lozano-Perez, S.; Kruska, K.; Iyengar, I.; Terachi, T.; Yamada, T. The role of cold work and applied stress on surface oxidation of 304 stainless steel. Corros. Sci. 2012, 56, 78–85. [Google Scholar] [CrossRef]
- Rostohar, D.; Arnoult, X.; Böhm, M.; Brajer, J.; Kaufman, J.; Zulić, S.; Mocek, T. HILASE center: Development of new-generation lasers for laser shock peening. In Proceedings of the Advanced Laser Processing and Manufacturing II; Liu, J., Hong, M., Xiao, R., Eds.; SPIE: Beijing, China, 2018; p. 31. [Google Scholar] [CrossRef]
- Boukamp, B.A. Derivation of a Distribution Function of Relaxation Times for the (fractal) Finite Length Warburg. Electrochim. Acta 2017, 252, 154–163. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Xu, A.; Fekete, B.; Macdonald, D.D. The electrochemical properties of alloy 690 in simulated pressurized water reactor primary water: Effect of temperature. J. Nucl. Mater. 2019, 518, 305–315. [Google Scholar] [CrossRef]
- Maekawa, T.; Kagawa, M.; Nakajima, N. Corrosion Behaviors of Stainless Steel in High-Temperature Water and Superheated Steam. Trans. Jpn. Inst. Met. 1968, 9, 130–136. [Google Scholar] [CrossRef]
- Ziemniak, S.; Hanson, M. Corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros. Sci. 2002, 44, 2209–2230. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Hanson, M.; Sander, P.C. Electropolishing effects on corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros. Sci. 2008, 50, 2465–2477. [Google Scholar] [CrossRef]





| C | Si | Mn | P | S | Cr | Ni | N | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.024 | 0.491 | 1.468 | 0.220 | 0.0075 | 18.296 | 11.8 | 0.0436 | Balance |
| Radiation source | CrKα1 |
| Smoothing method | Savitzky-Golay |
| ψ angles | 0 °C to 60 °C |
| Aperture size | 1 mm |
| Bragg’s angle | 128.8 °C |
| Young’s modulus | 180 MPa |
| Rs [Ω cm2] | R1 [Ω cm2] | Q [Ω−1 sn cm−2] | n | Ceff [F cm−2] | |
|---|---|---|---|---|---|
| polished sample | 81.1 | 68,685 | 6.29 × 10−6 | 0.896 | 2.6 × 10−6 |
| untreated sample | 81.7 | 23,312 | 2.09 × 10−5 | 0.868 | 7.9 × 10−6 |
| LSP-treated | 81.2 | 8361 | 3.33 × 10−5 | 0.843 | 1.1 × 10−5 |
| Specimen Designation | [mg dm−2] |
|---|---|
| untreated specimen | 56.65 |
| LSP-treated specimen | 137.53 |
| pickled polished specimen at 300 °C deaerated demineralized water [32] | 7.8 |
| abraded specimen at 300 °C deaerated demineralized water [32] | 17.8 |
| machined specimen at 260 °C deionized water hydrogenated [33] | 39.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnoult, X.; Arnoult-Růžičková, M.; Maňák, J.; Viani, A.; Brajer, J.; Arrigoni, M.; Kolman, R.; Macák, J. Corrosion and Electrochemical Properties of Laser-Shock-Peening-Treated Stainless Steel AISI 304L in VVER Primary Water Environment. Metals 2022, 12, 1702. https://doi.org/10.3390/met12101702
Arnoult X, Arnoult-Růžičková M, Maňák J, Viani A, Brajer J, Arrigoni M, Kolman R, Macák J. Corrosion and Electrochemical Properties of Laser-Shock-Peening-Treated Stainless Steel AISI 304L in VVER Primary Water Environment. Metals. 2022; 12(10):1702. https://doi.org/10.3390/met12101702
Chicago/Turabian StyleArnoult, Xavier, Mariana Arnoult-Růžičková, Jan Maňák, Alberto Viani, Jan Brajer, Michel Arrigoni, Radek Kolman, and Jan Macák. 2022. "Corrosion and Electrochemical Properties of Laser-Shock-Peening-Treated Stainless Steel AISI 304L in VVER Primary Water Environment" Metals 12, no. 10: 1702. https://doi.org/10.3390/met12101702
APA StyleArnoult, X., Arnoult-Růžičková, M., Maňák, J., Viani, A., Brajer, J., Arrigoni, M., Kolman, R., & Macák, J. (2022). Corrosion and Electrochemical Properties of Laser-Shock-Peening-Treated Stainless Steel AISI 304L in VVER Primary Water Environment. Metals, 12(10), 1702. https://doi.org/10.3390/met12101702






