Abstract
The melting temperature and viscosity of magnesium reduction slag were calculated by using Factsage thermodynamic software. The composition range of the magnesium-slag-based dephosphorizing agent was analyzed by drawing a multiphase diagram of the slag system. The Box–Behnken high-temperature dephosphorization experiment was designed to study the effect of different composition of magnesium-slag-based dephosphorizers on the dephosphorization rate of the steelmaking process. The results show that magnesium slag can be used as a slag-forming agent for smelting low-silicon hot metal to promote slagging, and the effect of each factor on the phosphorus removal rate is ranked, and the results are ω(Fe2O3) > basicity > ω(Al2O3): ω(Al2O3) has no significant effect on the rate of phosphorus removal. When the basicity was 2.8, ω(Fe2O3) was 25.94%, ω(Al2O3) was 6.73%, and ω(MgO) was 6%, the dephosphorization rate reached a maximum of 96.7%, and the error was experimentally verified to be 2.6% from the predicted value, indicating that the model can be optimized to determine the best magnesium-slag-based dephosphorization agent and has a good prediction of dephosphorization effect.
1. Introduction
Magnesium reduction slag (referred to as magnesium slag) is a by-product of the smelting of metallic magnesium by the Pidgeon method, with an annual output of more than 5 million tons [1]. It contains a high content of CaO and SiO2, and some Fe2O3, Al2O3 and MgO, which are secondary resources that can be used.
At present, the resource utilization of magnesium slag is mainly concentrated in the preparation of building materials [2,3,4,5,6], industrial desulfurization [7,8,9], agricultural fertilizers [10,11] and mine filling [12,13]. In terms of building materials, magnesium slag is used to prepare concrete: when the content of magnesium slag is 30%, the durability and long-term performance of concrete are improved [6]. Magnesium slag is used as a hot metal desulfurizer, and the desulfurization efficiency can reach more than 80% in industrial desulfurization [8]. In terms of agricultural fertilizers, magnesium slag is used as a raw material to prepare a silicon–potassium fertilizer, which has achieved a good application effect on calcareous cinnamon soil in northern China [11]. The modified magnesium slag is prepared into a cementitious material, which can be used to replace cement in mine filling [12]. However, the above utilization methods all have problems such as low consumption of magnesium slag, high-processing costs and a low comprehensive utilization rate. Therefore, it is very meaningful to study the resource utilization of magnesium slag.
The composition structure of magnesium slag is similar to that of steel-making slag. Steelmaking lime consumption can be reduced by CaO in magnesium slag and furnace-lining erosion can be reduced by MgO. SiO2, Al2O3 and Fe2O3 are conducive to the formation of a multi-component composite slag system, and they reduce the melting point of the slag system. The oxidizing property of slag can also be improved by Fe2O3, which is beneficial to dephosphorization in the early stage of smelting. Especially for low-silicon molten iron or semi-steel after vanadium extraction and dephosphorization operations, the low acidic fraction of slag during smelting makes it difficult for lime to be melted and the dephosphorization efficiency is deteriorated [14]. At present, by adding slag-forming materials such as river sand, silica, ferrosilicon, glass and bauxite to steelmaking enterprises, the structure of the steel-dephosphorization slag system is improved, lime melting is promoted, and the efficiency of dephosphorization is increased [15,16,17,18].
In this paper, the composition range of the magnesium-slag-based dephosphorization agent was determined by studying the composition and phase structure of magnesium slag, combined with Factsage thermodynamic software to draw a multi-component slag system phase diagram. The Box–Behnken (part of responsive surface design) high-temperature dephosphorization test was designed by Design-Expert software (for design of experiments (DOE)) to analyze the influence of magnesium slag components on the dephosphorization efficiency of low-silicon molten iron, and to provide a theoretical basis and technical support for magnesium slag as a steelmaking slag material.
2. Physical Properties of Magnesium Slag
2.1. Phase Analysis and Composition
The magnesium slag used in this paper were obtained from enterprise A and enterprise B, which are by-products of the Pidgeon process (silicon-thermal reduction process) magnesium metal. The main components are shown in Table 1.

Table 1.
Composition of magnesium slag (mass fraction %).
The physical phase analysis of magnesium slag A and magnesium slag B were carried out by XRD (X-ray diffraction), and the experimental results were analyzed by HighScore Plus software. The physical analysis of magnesium slag A and B were obtained as shown in Figure 1, showing the phase analysis of magnesium slag A and B. The main forms of magnesium slag were Ca2SiO4, MgO, Al2O3 and Fe2O3, where the crystalline phases of Ca2SiO4 wer β-Ca2SiO4 and γ-Ca2SiO4, respectively. The phase difference of magnesium slag A and B were mainly reflected in the difference of β-Ca2SiO4 content between them. The increase in the phase β-Ca2SiO4 can make magnesium slag have a higher hydration activity and porosity [19]. In order to ensure the good physical and chemical properties of the magnesium slag used in the experiment, magnesium slag A was selected as the experimental raw material.

Figure 1.
Phase analysis of magnesium slag: (a) magnesium slag A, (b) magnesium slag B.
2.2. Melting Temperature and Viscosity of Magnesium Slag
The magnesium-slag-melting temperature was measured by the CQKJ-II-type slag melting temperature characteristic tester. After the equipment reached the upper temperature limit of 1600 °C, the magnesium slag did not show obvious melting characteristics. Factsage 7.1 thermodynamic software was used to conduct the thermodynamic analysis of magnesium slag, and it was calculated that the initial melting temperature of magnesium slag was 1259 °C, while the complete melting temperature was 2008 °C. Figure 2 shows the change of phase of magnesium slag with the temperature, and Figure 3 shows the variation in viscosity and temperature of magnesium slag. When the temperature was raised from 1000 °C, the mass fraction of Ca3MgSi2O8 and melilite in magnesium slag was gradually reduced, and the mass fraction of dicalcium silicate (Ca2SiO4) was slowly increased before the liquid phase was generated. When the temperature reached 1259 °C, the liquid phase in the system began to be generated. At this time, as the temperature increased, the mass fraction of dicalcium silicate (Ca2SiO4) was gradually reduced, and the slag viscosity also showed a downward trend. When the temperature was raised to 1350~1450 °C, the viscosity of magnesium slag was still at a high level.
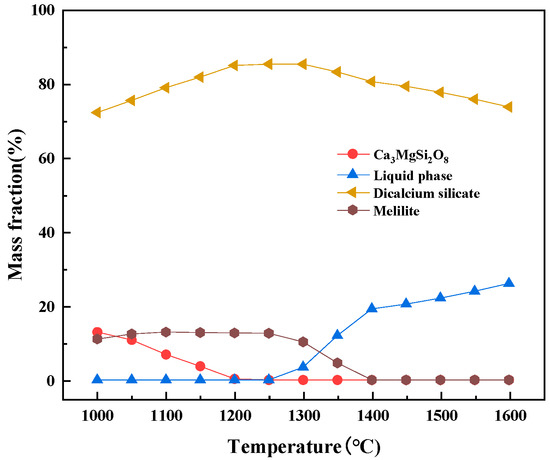
Figure 2.
Phase change of magnesium slag.
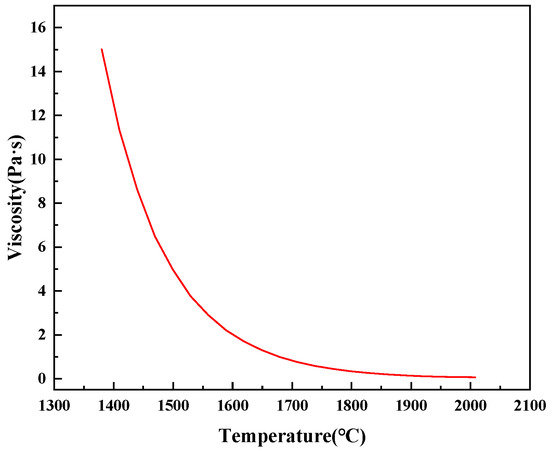
Figure 3.
Visco-temperature curve of magnesium slag.
The pseudo-pentad phase diagram of CaO–SiO2–Fe2O3–MgO–Al2O3 is shown in Figure 4; point A is the composition point of magnesium slag A. When the ω(MgO) and ω(Al2O3) in magnesium slag were kept unchanged, the basicity was adjusted by 1.5–3.5, and ω(Fe2O3) was adjusted to 10–30%, the smelting conditions were still not satisfied. Therefore, the mass fractions of ω(MgO) and ω(Al2O3) needed to be adjusted simultaneously to improve the slag system structure to fulfill the smelting requirements.
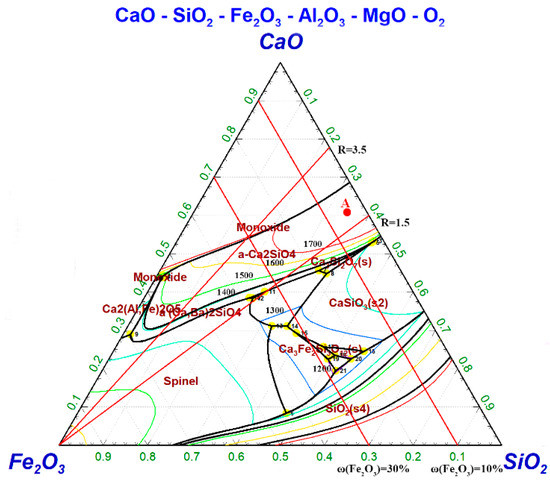
Figure 4.
Pseudo-pentad phase diagram of CaO–SiO2–Fe2O3–2.5%MgO–1%Al2O3.
3. Effect of Different Components on the Liquid-Phase Region
Figure 5 shows the ternary phase diagram of CaO–SiO2–Fe2O3 drawn by Factsage software. When ω(Fe2O3) was between 10% and 30%, the phase changed from CaSiO3 to 3CaO–2SiO2 as the basicity was increased from 1.0 to 1.5, and when the basicity was raised to 3.0, the main phase was Ca2SiO4. With the increasing basicity, the complete melting temperature of the slag system was gradually raised, and the area of the liquid-phase zone was gradually reduced. The structure of the slag system needed to be improved by Al2O3 and MgO, and the melting point was also lowered.
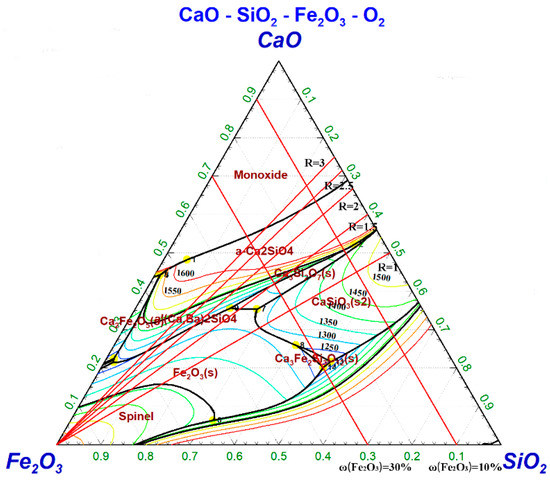
Figure 5.
Ternary phase diagram of CaO–SiO2–Fe2O3.
3.1. Effect of MgO on the Liquid Phase Region
Figure 6 shows the effect of MgO on the liquid-phase zone of the CaO–SiO2–Fe2O3 slag system at 1450 °C. When the MgO content increased from 1% to 3%, the area of the liquid phase did not change significantly, and when it continued to increase to 9%, the area of the liquid phase was rapidly reduced. The excessive MgO content resulted in the presence of a large number of unmelted MgO particles in the slag, which affected the melting temperature and viscosity of the slag and was not conducive to the melting of the slag [20]. When the MgO content was 6%, the slag system still had high oxidizing properties, and the liquidus line tended to the high SiO2 region, and the slag with low basicity in the early stage of smelting was accelerated to melt. Additionally, the MgO content in slag should be kept in a reasonable range to avoid serious erosion of the furnace lining due to the low MgO content, in which the life of the converter will be affected. Therefore, the MgO content in the magnesium-slag-based dephosphorization agent was set to 6% in this study.
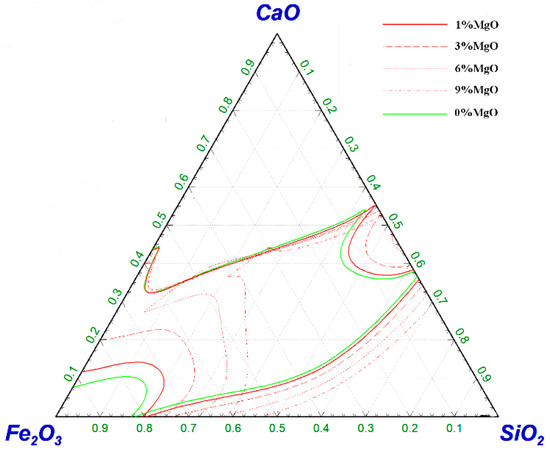
Figure 6.
Effect of ω(MgO) on the liquid-phase region of CaO–SiO2–Fe2O3 slag system at 1450 °C.
3.2. Effect of Al2O3 on the Liquid-Phase Region
Figure 7 shows the effect of Al2O3 on the liquid-phase zone of the CaO–SiO2–Fe2O3 slag system at 1450 °C. With the increase in Al2O3 content, the liquid-phase line gradually tended to the high CaO region, and the dense Ca2SiO4 outer layer in the slag was loosened by Al2O3, which was conducive to the diffusion of Fe2O3 and SiO2 into the lime [21], and the melting of the slag was accelerated. When the Al2O3 content was in the range of 1–12%, the slag system had a wide range of basicity and a high oxidizability, which was consistent with the composition of slag in the pre-phosphate removal stage.
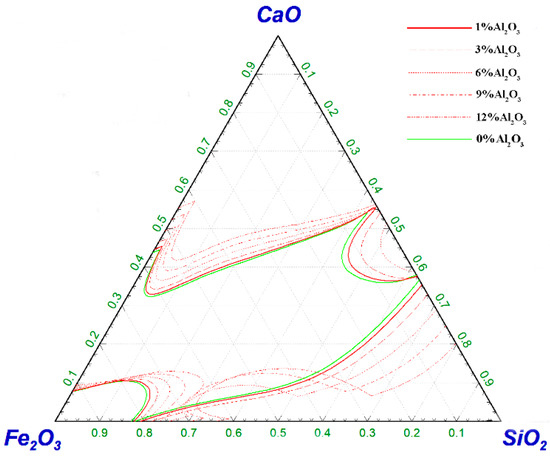
Figure 7.
Effect of ω(Al2O3) on the liquid-phase region of CaO–SiO2–Fe2O3 slag system at 1450 °C.
The pseudo-quinary phase diagram of CaO–SiO2–Fe2O3–Al2O3–6%MgO is shown in Figure 8. The increase in Al2O3 content led to a reduction in the liquid-phase region, but it was conducive to the melting of high-basicity slag, and the minimum content of Fe2O3 in the liquid-phase region was still greater than 30%. This met the dephosphorization conditions for steelmaking. At this time, the basicity, melting temperature and oxidizability of the slag system could be satisfied for the dephosphorization conditions of steelmaking.
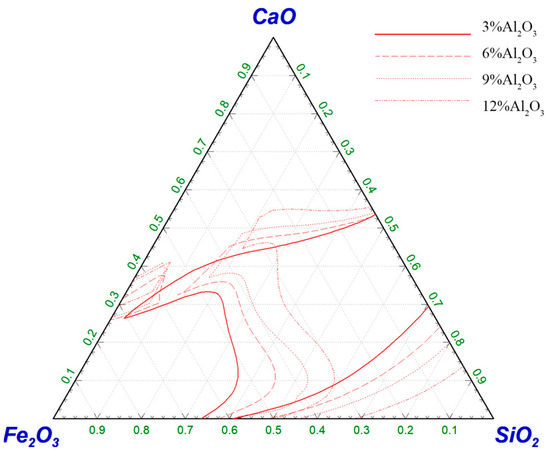
Figure 8.
Effect of ω(Al2O3) on the liquid-phase region of CaO–SiO2–Fe2O3–Al2O3–6%MgO slag system at 1450 °C.
Based on the above research, the composition and content of the magnesium-slag-based dephosphorization agent studied in this paper were determined as shown in Table 2.

Table 2.
Composition range of magnesium-slag-based dephosphorization agent (mass fraction %).
4. High-Temperature Experiment of Magnesium Slag for Dephosphorization in Steelmaking Process
4.1. Experimental Scheme
The experimental design was carried out using the Box–Behnken module of the Design-Expert software, as shown in Table 3, and three factors (basicity, ω(Fe2O3%) and ω(Al2O3%)) were selected as independent variables to design a three-factor, three-level experiment with the response value of the dephosphorization rate and response curves used to determine the optimal dephosphorizer composition.

Table 3.
Box–Behnken analysis factors and levels.
4.2. Experimental Materials and Methods
The magnesium slag used in the experiments was obtained from enterprise A. The low-silicon hot metal used was taken from the semi-steel of a steel company after vanadium extraction, and its composition is shown in Table 4. The other oxides used in the preparation of the magnesium slag were all analytical pure reagents.

Table 4.
Composition of semi-steel (mass fraction%).
As is shown in Table 4, the silicon mass fraction of the low-silicon hot metal used was 0.004%, and the SiO2 required for steelmaking slag coul not be produced by iron oxidation; rather, it came from the magnesium slag.
- (1)
- Preparation of dephosphorization agent:
(1) The components were prepared in accordance with their respective ratios of the phosphorus removal agent required for the experiment and were fully mixed, weighing 100g into the corundum crucible;
(2) The dephosphorization agent was pre-melted in a high-temperature box furnace and the temperature was raised to 1400 °C, and then kept for 30 min. After the sample was completely melted, the heating was stopped, and the temperature was naturally cooled;
(3) The cooled dephosphorized pre-melted slag was crushed to 200 mesh by the crusher.
- (2)
- Dephosphorization experiment:
(1) A total of 200 g of semi-steel and 20 g of dephosphorized slag were weighed, mixed uniformly and placed in a corundum crucible, and were then put into a high-temperature box furnace to start heating up;
(2) The semi-steel melting point was calculated to be 1140 °C; therefore, the experimental temperature was set to 1450 °C, which ensured the complete melting of semi-steel and the maintenance of a continuous temperature for six hours. It also ensured the complete reaction of the slag-steel interface;
(3) After the sample was cooled to room temperature, the slag and iron were separated, and the phosphorus content in the steel was analyzed by ICP.
4.3. Experimental Results and Analysis
4.3.1. Analysis of Model-Fitting Degree
The experimental data in Table 5 were fitted by Design-Expert with a quadratic multiple regression, which resulted in a quadratic multiple regression model of D (dephosphorization rate) versus A (Basicity), B (ω(Fe2O3)) and C (ω(Al2O3)) represented by:

Table 5.
Response surface experimental design and results.
The model determination coefficient R2 was 0.9866, and the fitting degree was relatively high. The experimental variance analysis results are shown in Table 5.
As shown in Table 6, the model was very significant with p < 0.0001, and the lack-of-fit model was greater than 0.05, indicating that the quadratic polynomial regression equation was well fitted.

Table 6.
Box–Behnken test ANOVA results.
According to the various p values, the ranking results of the influence of various factors on the dephosphorization rate were ω(Fe2O3) > basicity > ω(Al2O3), and the effect of ω(Al2O3) on the dephosphorization efficiency was not significant. In the quadratic term, A2 and B2 had significant effects on the dephosphorization rate, while C2 had no significant effect on it, indicating that the relationship between the experimental factors and the response values were not a simple linear relationship. The interaction between basicity and ω(Fe2O3) had the largest F value compared with the other two, and p was less than 0.05, indicating that its interaction had a more significant effect on the dephosphorization rate. The p values of AC and BC were greater than 0.05, but the F value of AC was larger than that of BC, so the effect of AC interaction on the dephosphorization rate was greater than that of BC.
4.3.2. Interaction Analysis among Various Factors
The normal probability distribution of the residual dephosphorization rate is shown in Figure 9, and the comparison between the measured value and the predicted value is shown in Figure 10. The normal probability-distribution points of the residuals were located on or immediately on both sides of the straight line: the measured and predicted values were in good agreement, and the correlation between them was high. It showed that the selected model could be used to guide the composition optimization of the magnesium slag dephosphorization agent.
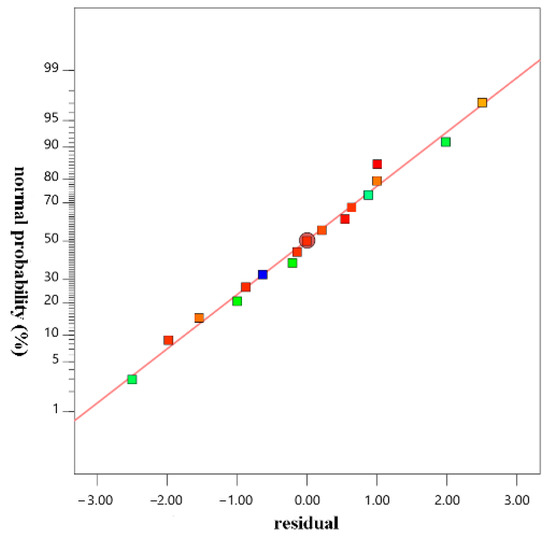
Figure 9.
Normal Distribution Plot of Residuals.
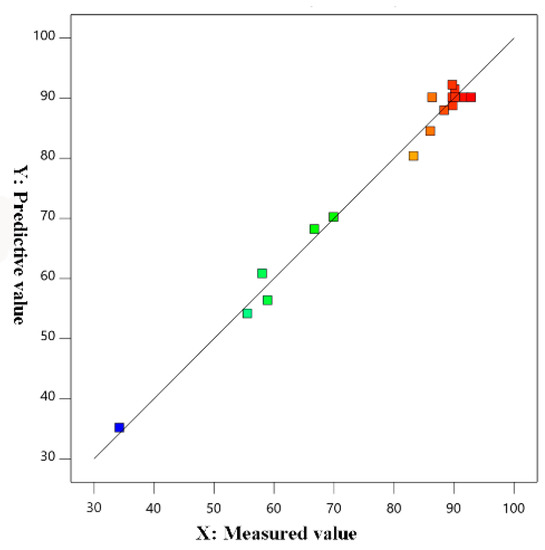
Figure 10.
Comparison of actual and predicted values.
Figure 11 shows the contour map and 3D response surface of the interaction between the two factors, and the relationship between the factors and the response value is visualized by the 3D coordinate system. The greater the degree of distortion of the response surface, the more significant the corresponding factors had on the response value, and a darker color in the area of the figure indicates that the result is more significant and the dephosphorization rate is higher.
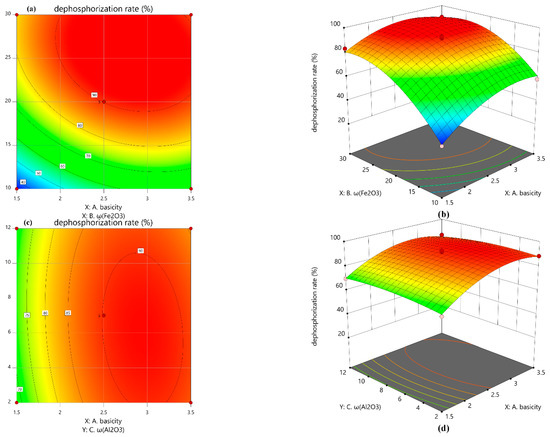
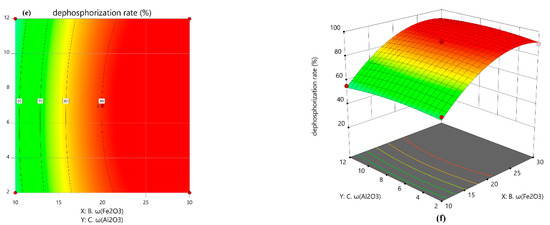
Figure 11.
Contour plot and 3D response surface plot of each two-factor interaction. (a) contour plot of basicity and ω(Fe2O3); (b) 3D response surface plot of basicity and ω(Fe2O3); (c) contour plot of basicity and ω(Al2O3); (d) 3D response surface plot of basicity and ω(Al 2O3); (e) contour plot of ω(Fe2O3) and ω(Al2O3); (f) 3D response surface plot of ω(Fe2O3) and ω(Al2O3). It can be intuitively seen from the response surface diagram: the distortion of the response surface (b) was the most obvious, that was, the interaction between basicity and ω(Fe2O3) had the most significant effect on the dephosphorization rate. With the increase in basicity and Fe2O3 content, the rate of phosphorus removal was rapidly increased to reach the peak after a decreasing trend. A further increase in basicity led to slagging difficulties, which was not conducive to phosphorus removal.
Al2O3 did not directly participate in the dephosphorization reaction, but it reacted with high-melting-point compounds to reduce the melting point of the slag, improve the fluidity of the slag, and promote dephosphorization. However, the high content of Al2O3 causes the basicity and ω(Fe2O3) to be reduced, and the phosphorus content in Ca2SiO4 will also be reduced [22], resulting in a lower rate of phosphorus removal. Therefore, the interaction between basicity and ω(Al2O3), ω(Fe2O3) and ω(Al2O3) had no significant effect on the rate of phosphorus removal, so the distortion of the response surfaces of (c) and (e) were low. It could also be seen from the contour plots of (d) and (f) that the dephosphorization rate was less affected by the change in Al2O3 content.
4.3.3. Optimization Results
Based on the influence of the interaction among the factors on the dephosphorization rate, it was observed that the rate of phosphorus removal increased and then decreased under the influence of the interaction among the factors. Therefore, there was an optimal ratio between basicity, ω(Al2O3) and ω(Fe2O3), which maximizes the dephosphorization rate. The optimal ratios among the three were obtained by simulations conducted by Design-Expert as the basicity was 2.8, ω(Fe2O3) was 25.94% and ω(Al2O3) was 6.73%, at which the maximum phosphorus removal rate of 96.7% was achieved.
In order to test the accuracy of the response surface model, three groups of experimental verifications were carried out using the optimized magnesium-slag-based dephosphorization agent, and the results are shown in Table 7.

Table 7.
Optimization experimental results.
As can be seen from Table 7, in the optimized experimental results, the dephosphorization rates were 94.1%, 93.5% and 94.7%. Additionally, the experimental repeatability was good, and the error between the results and the predicted results was 2.6%. This model was shown to effectively optimize and determine the optimal composition of magnesium-slag-based dephosphorization agents.
5. Conclusions
(1) Magnesium slag can be used as a slag-making material for smelting low-silicon hot metal in the steelmaking process, as well as reducing the consumption of steelmaking lime. The slag structure was improved by the SiO2, Al2O3, Fe2O3, etc. brought in by it, and the efficiency of phosphorus removal in the pre-smelting stage was improved.
(2) The multi-component slag system phase diagram was drawn by Factsage thermodynamic software, and a reasonable composition range of the magnesium-slag-based dephosphorization agent was designed: CaO is 30~64%; SiO2 is 10~33%; Fe2O3 is 10~30%; Al2O3 is 2~12%; and MgO is 6%.
(3) The Box–Behnken response surface analysis showed that the effects of the model factors on the phosphorus removal rate were in the following order: ω(Fe2O3) > basicity > ω(Al2O3). Additionally, the effect of ω(Al2O3) on the rate of phosphorus removal was not significant, while the interaction between basicity and ω(Fe2O3) had a significant effect on the rate of phosphorus removal.
(4) When the basicity was 2.8, ω(Fe2O3) was 25.94%, ω(Al2O3) was 6.73% and ω(MgO) was 6%, the maximum phosphorus removal rate was 96.7%, and the error of the predicted value was experimentally verified to be 2.6%, indicating that the model can be optimized to determine the best magnesium-slag-based dephosphorization agent, and that it has a good prediction of dephosphorization effect.
Author Contributions
Conceptualization, M.L. and K.X. (Kun Xie); methodology, M.L. and K.X. (Kun Xie); software, K.X. (Kun Xie) and K.X. (Kui Xue); validation, Z.Z., K.X. (Kun Xie) and K.X. (Kui Xue); formal analysis, Z.Z.; investigation, K.X. (Kui Xue); resources, K.X. (Kun Xie) and K.X. (Kui Xue); data curation, K.X. (Kun Xie) and M.L.; writing—original draft preparation, K.X. (Kun Xie) and M.L.; writing—review and editing, K.X. (Kun Xie) and M.L.; visualization, Z.Z.; supervision, Z.Z. and M.L.; project administration, Z.Z. and M.L.; funding acquisition, Z.Z. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Joint Fund Project of Shaanxi (Grant No. 2021JLM-32) and Scientific Research Plan Project of Shaanxi Education Department (Grant No. 22JC044).
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, L.; Han, F.; Liu, G. Comprehensive Utilization of Magnesium Slag by Pidgeon Process; Open Access; Springer: Singapore, 2021; pp. 68–120. [Google Scholar]
- Cui, Z.; Li, S.; Zhang, C.; Chen, D. Drying shrinkage characteristics of magnesium slag composite admixture concrete. J. Sichuang Univ. 2016, 48, 207–212. [Google Scholar]
- Peng, X.; Wang, K.; Li, J.; Yu, Z.; Wang, S. Hydraulic potential stimulation and bricks preparation of magnesium slag. J. Chongqing Univ. 2013, 36, 48–52+58. [Google Scholar]
- Tang, Y.; Li, L.; Wang, C.; Mao, W.; Feng, L. New progress in reutilization of magnesium slag. Mod. Chem. Ind. 2020, 40, 63–67. [Google Scholar]
- Li, H.; Huang, Y.; Yang, X.; Jiang, Z.; Yang, Z. Approach to the management of magnesium slag via the production of portland cement clinker. J. Mater. Cycles Waste Manag. 2018, 20, 1701–1709. [Google Scholar] [CrossRef]
- Ji, G.; Peng, X.; Wang, S.; Hu, C.; Ran, P.; Sun, K.; Zeng, L. Influence of magnesium slag as a mineral admixture on the performance of concrete. Constr. Build. Mater. 2021, 295, 123619. [Google Scholar] [CrossRef]
- Feng, L.; Han, F.; Qiao, X.; Zhen, X.; Jin, Y.; Fan, B. Experimental study on wet desulfurization performance of magnesium slag. Environ. Pollut. Control 2018, 40, 1112–1115+1121. [Google Scholar]
- Xu, X.; Chen, Y.; Yu, J. Industrial test of desulfurization of hot metal by magnesium slag. Light Met. 2017, 1, 42–44. [Google Scholar]
- Fan, B.; Jia, L.; Han, F.; Huo, R.; Yao, Y.; Qiao, X.; Zhao, C.; Jin, Y. Study on magnesium slag desulfurizer modified by additives in quenching hydration. J. Mater. Cycles Waste Manag. 2019, 21, 1211–1233. [Google Scholar] [CrossRef]
- Xia, D.; Ren, L.; Chen, L. Study of Ca-Mg-S-Si fertilizer produced by magnesium slag. Adv. Mater. Res. 2011, 347–353, 3166–3170. [Google Scholar] [CrossRef]
- Fan, Y.; Qin, W.; Chen, Z.; Cheng, F. A silicon–potash fertilizer prepared from magnesium slag and how it can improve soil fertility and agronomic performance. Soil Sci. Plant Nutr. 2019, 65, 274–280. [Google Scholar] [CrossRef]
- Liu, L.; Ruan, S.; Fang, Z.; Hou, D.; Zhang, B.; Sun, W. Modification of magnesium slag and its application in the field of mine filling. J. China Coal Soc. 2021, 46, 1–15. [Google Scholar]
- Liu, L.; Ruan, S.; Qi, C.; Zhang, B.; Tu, B.; Yang, Q.; Song, K.I.-I.L. Co-disposal of magnesium slag and high-calcium fly ash as cementitious materials in backfill. J. Clean. Prod. 2021, 279, 123684. [Google Scholar] [CrossRef]
- Diao, J.; Gu, W.; Liu, L.; Tan, W.; Li, H.; Xie, B. Slag formation path in converter smelting process of semi-steel containing chromium. Ironmak. Steelmak. 2020, 47, 1078–1083. [Google Scholar] [CrossRef]
- Yang, W.; Wu, W.; Wang, M.; Shi, H.; Wang, T. Study on steel making with hot metal containing low silicon in large converter. Iron Steel 2005, 8, 22–25. [Google Scholar]
- Yang, W.; Wu, W.; Wang, M.; Wang, Y.; Chen, Y.; Yu, Z. High Efficient Blow of low-silicon hot metal in large converter. J. Iron Steel Res. 2006, 12, 10–14+26. [Google Scholar]
- Chen, J.; Zeng, J.; Chen, L.; Ge, W.; Yang, S. Development and application of thermal compensation process of Pangang. J. Iron Steel Res. 2020, 32, 618–625. [Google Scholar]
- Antsiferov, A.A.; Khaidukov, V.P.; Rogotovskii, A.N.; Borisov, A.V.; Bocharov, A.V. Processing of low-silicon hot metal with slag formation by ferruginous lime. Steel Transl. 2015, 45, 507–510. [Google Scholar] [CrossRef]
- Li, Y.; Ge, T.; Cheng, F. Effect of different treatment methods on the physico-chemical properties of magnesium slag (MS). Inorg. Chem. Ind. 2016, 48, 52–55. [Google Scholar] [CrossRef]
- Li, W.; Sun, Q.; Wang, C.; Yu, S. Control practice on MgO in slag of BOF. Iron Steel 2011, 46, 40–44. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, M.; Li, J.; Qiu, S.; Wang, S.; Ning, S. Thermodynamics of dephosphorization capacity of containing TiO2 and Al2O3 of CaO-based semi-steel slag. Iron Steel 2018, 53, 27–37. [Google Scholar]
- Deo, B.; Halder, J.; Snoeijer, B.; Overbosch, A.; Boom, R. Effect of MgO and Al2O3 variations in oxygen steelmaking (BOF) slag on slag morphology and phosphorus distribution. Ironmak. Steelmak. 2005, 32, 54–60. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).