Microstructure and Mechanical Properties of Ultrafine Quaternary Al-Cu-Si-Mg Eutectic Alloy
Abstract
1. Introduction
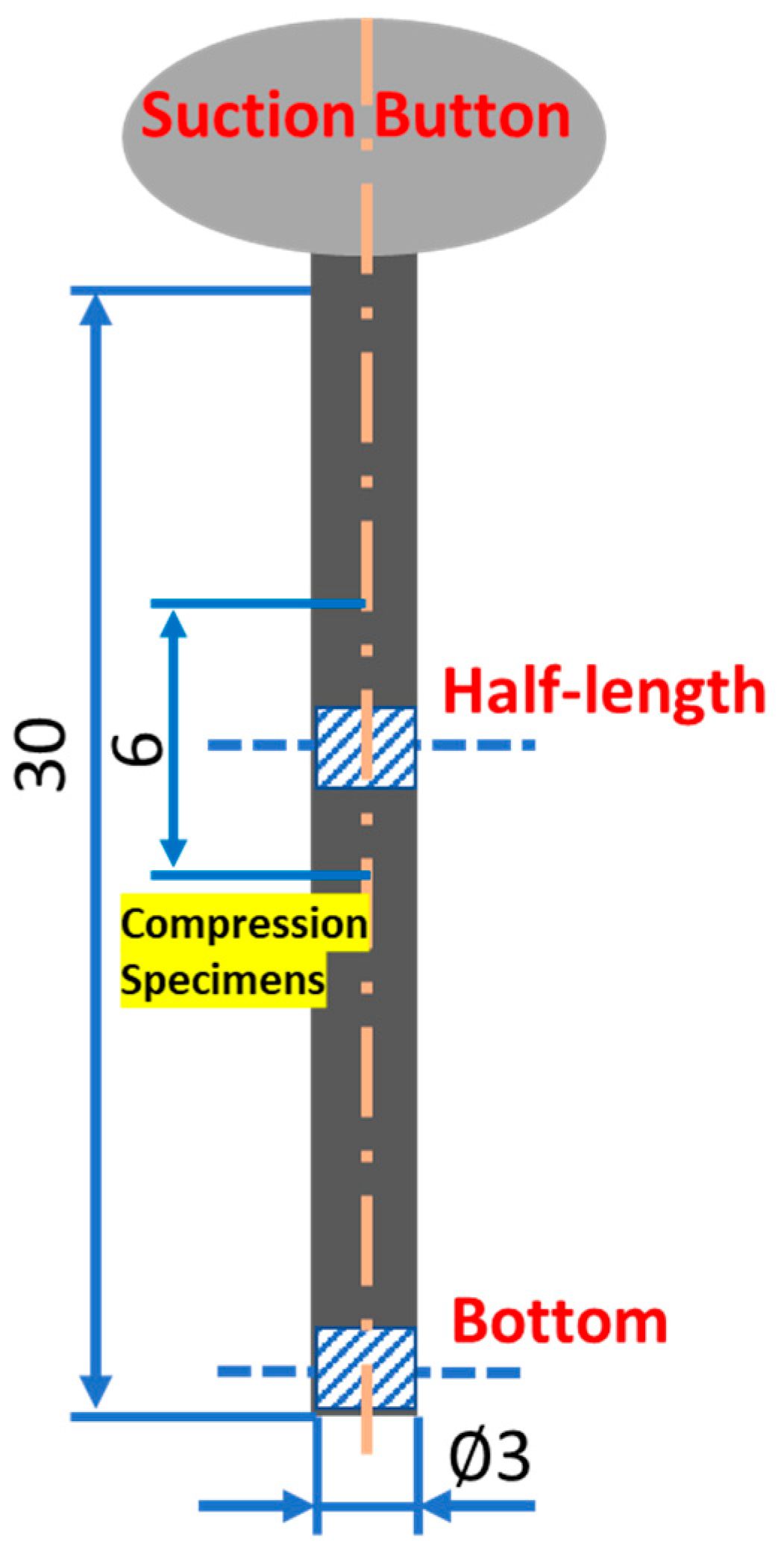
2. Materials and Methods
3. Results
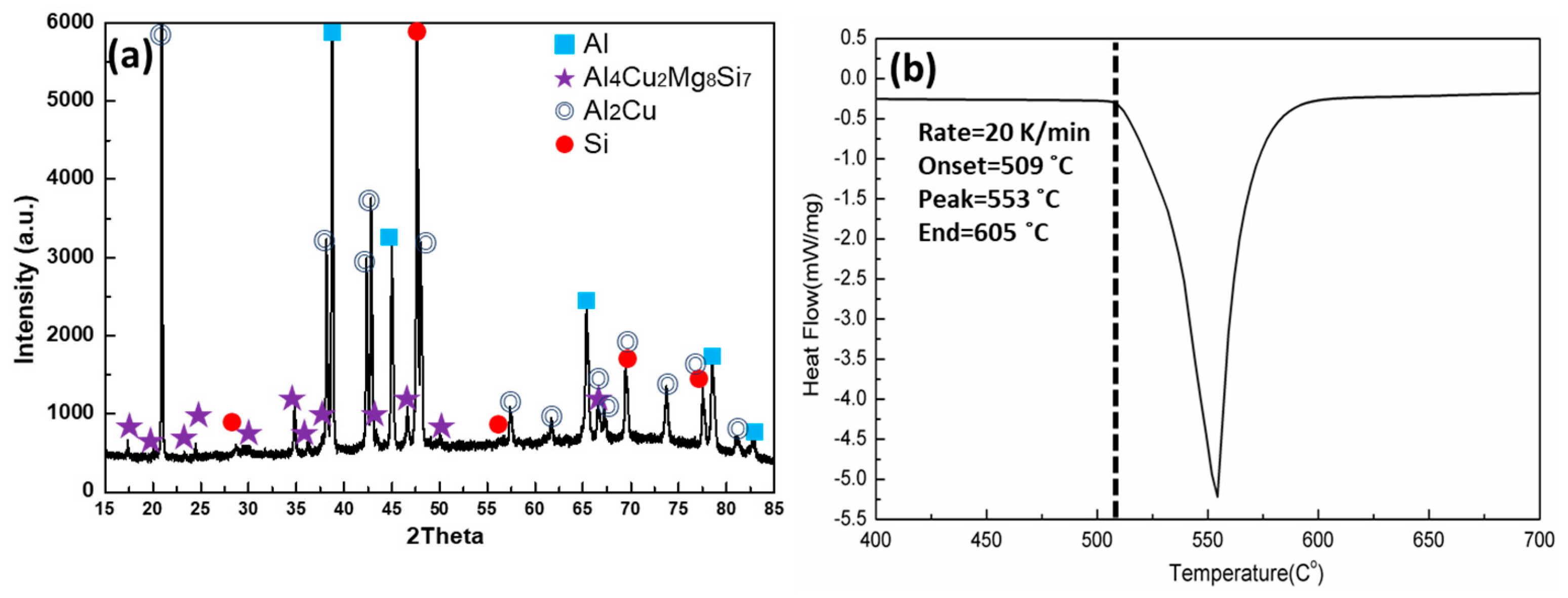
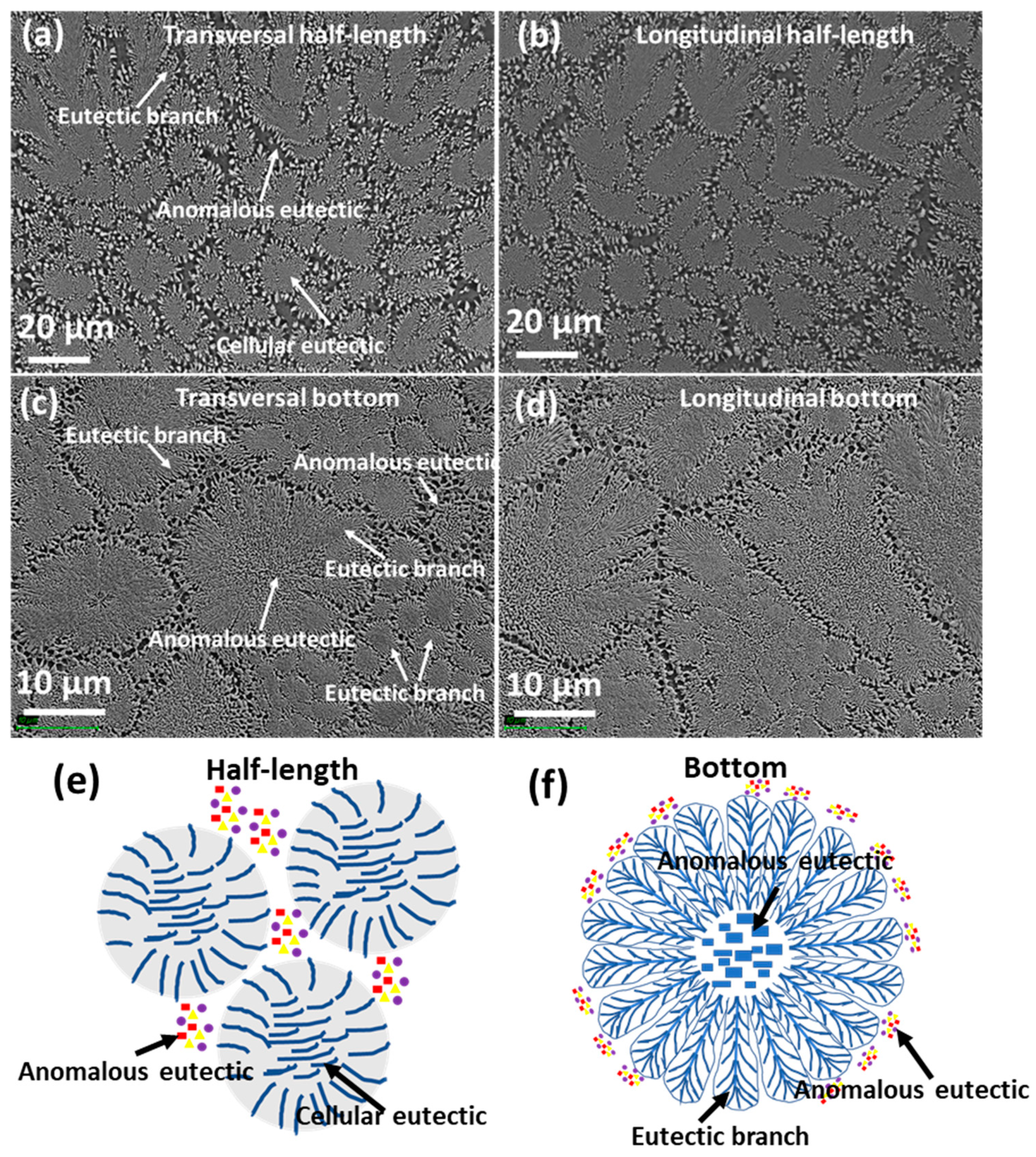
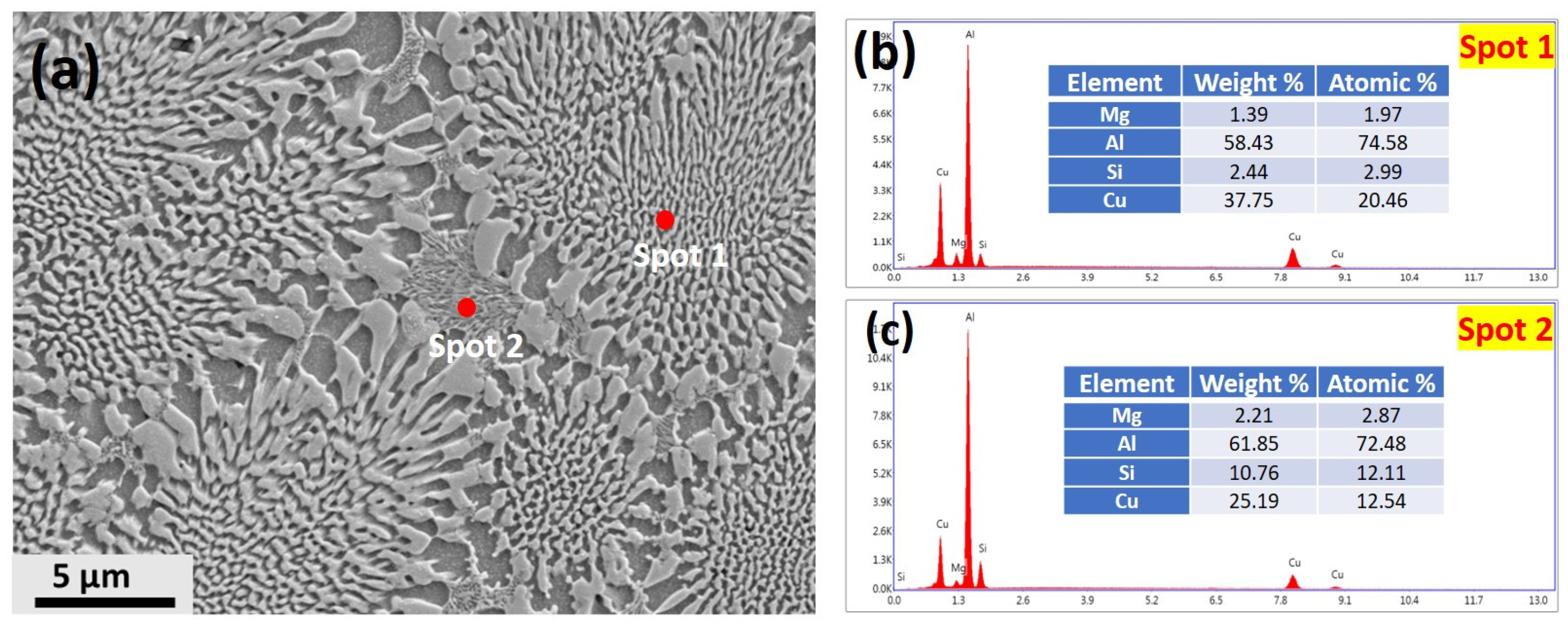
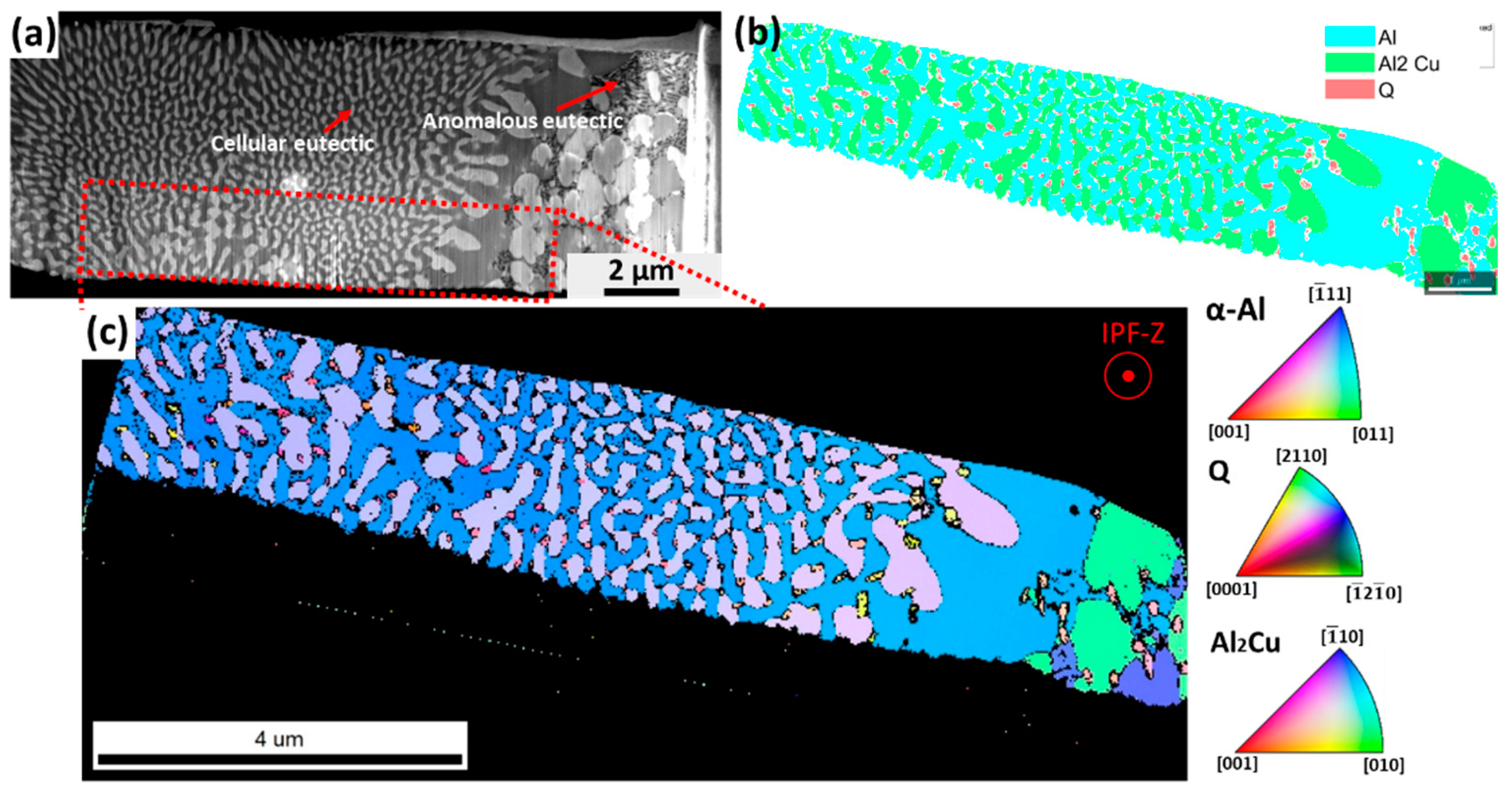
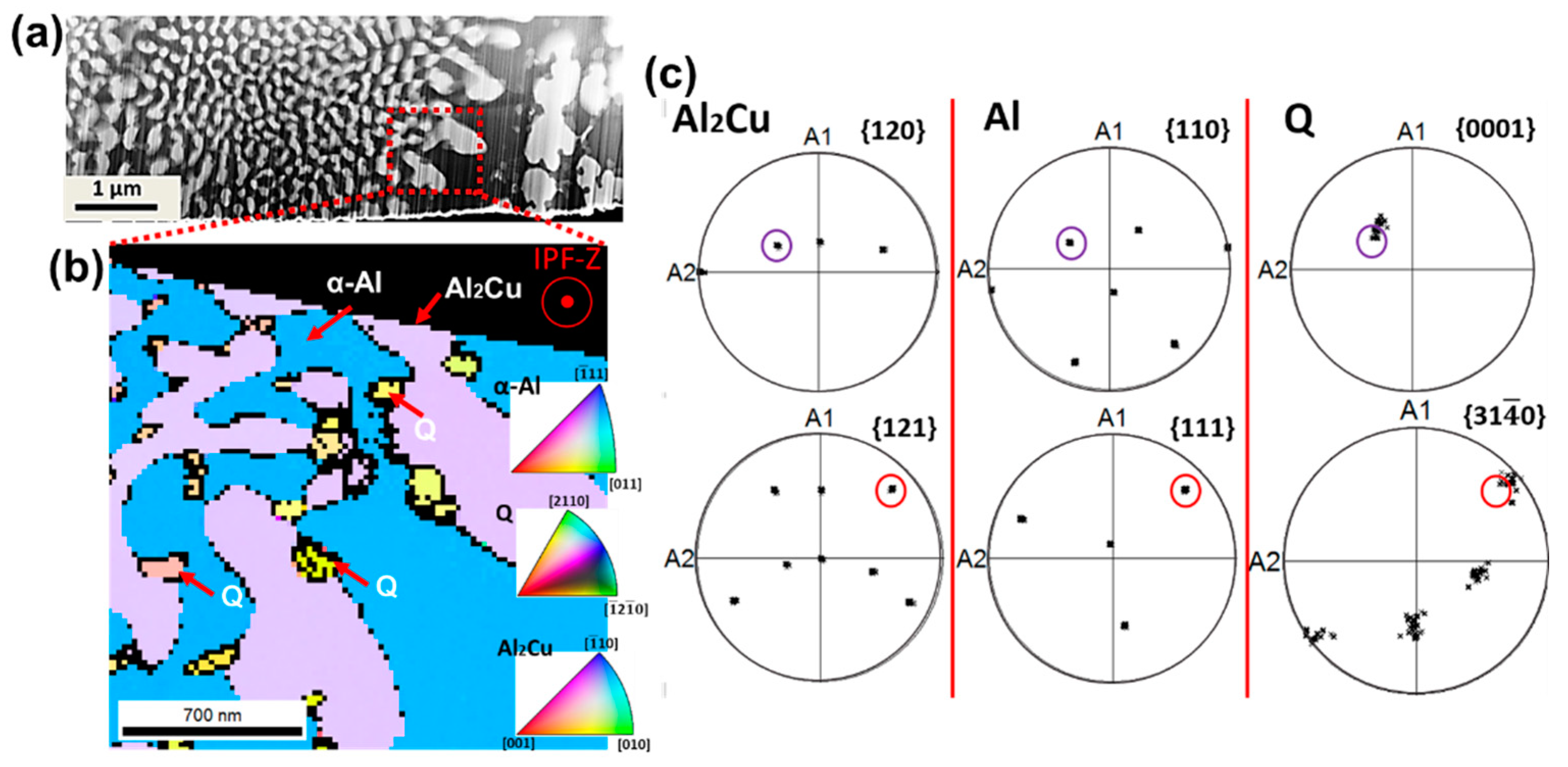
3.1. Microstructure
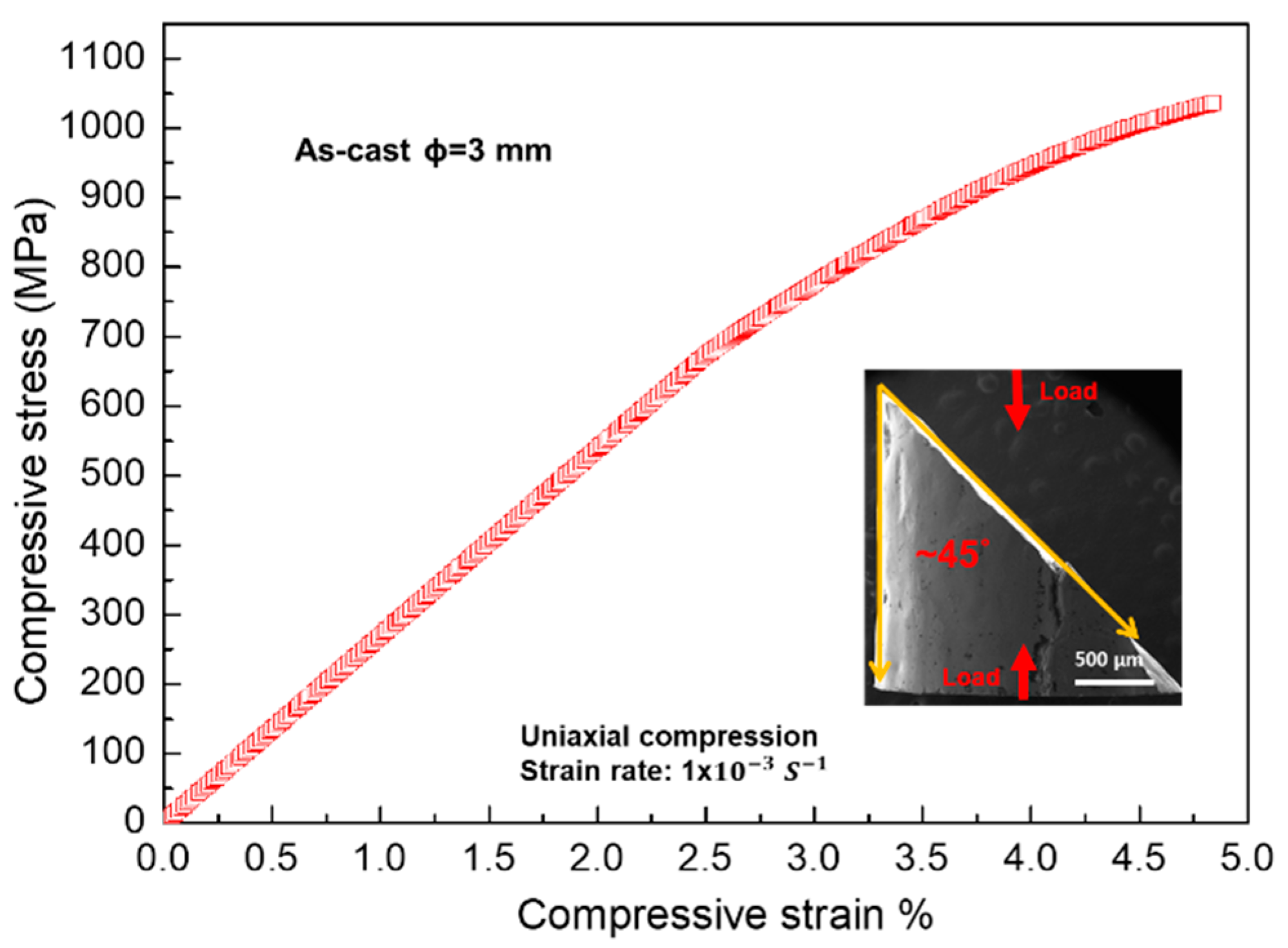
3.2. Mechanical Properties
4. Discussion
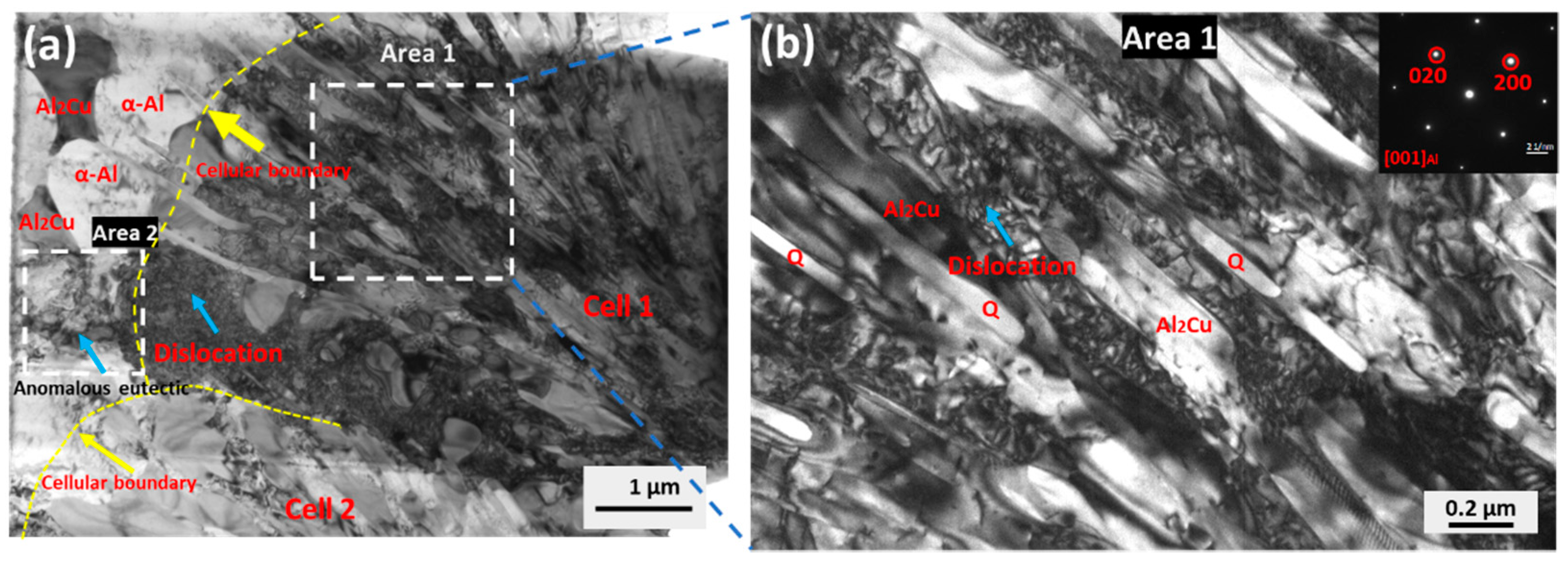
4.1. Microstructure Evolution
4.2. Relationship between the Microstructure and the Mechanical Properties
5. Conclusions
- The quaternary eutectic alloy solidifies with four distinct solid phases (α-Al, Si, Al2Cu and Al4Cu2Mg8Si7 (Q)), and one endothermic DSC peak with a melting point of 509 °C.
- The as-cast microstructure of the quaternary eutectic alloy consists of cellular eutectic and fine anomalous eutectic in the intercellular regions. Both eutectic microstructures exhibit four solid phases (α-Al, Si, Al2Cu and Al4Cu2Mg8Si7 (Q)). A cellular eutectic with surrounding branches was found in the fastest cooled part of the sample.
- A well-defined orientation relationship between the α-Al, Al2Cu and Q phases was found in the centres of the cells, with and . However, at the cellular boundaries, decoupled growth of Q phase was also found.
- The alloy shows an excellent compressive yield strength of 835 ± 35MPa, with a moderate compressive fracture strain of 4.7 ± 1.1%. The propagation of cracks along cell boundaries is the main factor leading to the catastrophic failure of the sample.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mortensen, A.; Jin, I. Solidification processing of metal matrix composites. Int. Mater. Rev. 1992, 37, 101–128. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Lipsitt, H.A.; Jackson, M.R.; Reeder, W.J.; Sutliff, J.A. Solidification processing of high temperature intermetallic eutectic-based alloys. Mater. Sci. Eng. A 1995, 192–193, 534–543. [Google Scholar] [CrossRef]
- Dahle, A.K.; Nogita, K.; McDonald, S.D.; Dinnis, C.; Lu, L. Eutectic modification and microstructure development in Al-Si Alloys. Mater. Sci. Eng. A 2005, 413–414, 243–248. [Google Scholar] [CrossRef]
- Tiwary, C.S.; Pandey, P.; Sarkar, S.; Das, R.; Samal, S.; Biswas, K.; Chattopadhyay, K. Five decades of research on the development of eutectic as engineering materials. Prog. Mater. Sci. 2022, 123, 100793. [Google Scholar] [CrossRef]
- Winegard, W.C.; Majka, S.; Thall, B.M.; Chalmers, B. Eutectic solidification in metals. Can. J. Chem. 1951, 29, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R. Eutectic solidification. Mater. Sci. Eng. 1984, 65, 85–92. [Google Scholar] [CrossRef]
- Zener, C. Kinetics of the decomposition of austenite. Trans. Am. Inst. Min. Metall. Eng. 1946, 167, 550–595. [Google Scholar]
- Hunt, J.; Chilton, J. Rod transition in binary eutectics. J. Inst. Met. 1963, 92, 338. [Google Scholar]
- Jackson, K.A.; Hunt, J.D. Lamellar and Rod Eutectic Growth. In Dynamics of Curved Fronts; Elsevier: New York, NY, USA, 1988; pp. 363–376. ISBN 9780080925233. [Google Scholar]
- Trivedi, R.; Magnin, P.; Kurz, W. Theory of eutectic growth under rapid solidification conditions. Acta Metall. 1987, 35, 971–980. [Google Scholar] [CrossRef]
- Zimmermann, M.; Carrard, M.; Kurz, W. Rapid solidification of Al-Cu eutectic alloy by laser remelting. Acta Metall. 1989, 37, 3305–3313. [Google Scholar] [CrossRef]
- Mullis, A.M.; Clopet, C.R. On the origin of anomalous eutectic growth from undercooled melts: Why re-melting is not a plausible explanation. Acta Mater. 2018, 145, 186–195. [Google Scholar] [CrossRef]
- Li, J.F.; Jie, W.Q.; Zhao, S.; Zhou, Y.H. Structural evidence for the transition from coupled to decoupled growth in the solidification of undercooled Ni-Sn eutectic melt. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2007, 38, 1806–1816. [Google Scholar] [CrossRef]
- Goetzinger, R.; Barth, M.; Herlach, D.M. Mechanism of formation of the anomalous eutectic structure in rapidly solidified Ni-Si, Co-Sb and Ni-Al-Ti. Acta Mater. 1998, 46, 1647–1655. [Google Scholar] [CrossRef]
- Lin, X.; Cao, Y.Q.; Wang, Z.T.; Cao, J.; Wang, L.L.; Huang, W.D. Regular eutectic and anomalous eutectic growth behavior in laser remelting of Ni-30wt%Sn alloys. Acta Mater. 2017, 126, 210–220. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, H.J.; Kim, J.T.; Hong, S.H.; Park, G.H.; Park, J.M.; Suh, J.Y.; Kim, K.B. Influence of Nb on microstructure and mechanical properties of Ti-Sn ultrafine eutectic alloy. Met. Mater. Int. 2017, 23, 20–25. [Google Scholar] [CrossRef]
- Kang, J.L.; Xu, W.; Wei, X.X.; Ferry, M.; Li, J.F. Solidification behavior of Co-Sn eutectic alloy with Nb addition. J. Alloys Compd. 2017, 695, 1498–1504. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Liu, L.; Zhou, Y. Solidification of undercooled Ag-Cu eutectic alloy with the Sb addition. J. Alloys Compd. 2009, 478, 252–256. [Google Scholar] [CrossRef]
- Pandey, P.; Kashyap, S.; Tiwary, C.S.; Chattopadhyay, K. Development of High-Strength High-Temperature Cast Al-Ni-Cr Alloys Through Evolution of a Novel Composite Eutectic Structure. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 5940–5950. [Google Scholar] [CrossRef]
- De Wilde, J.; Froyen, L.; Rex, S. Coupled two-phase [α(Al) + θ(Al2Cu)] planar growth and destabilisation along the univariant eutectic reaction in Al-Cu-Ag alloys. Scr. Mater. 2004, 51, 533–538. [Google Scholar] [CrossRef]
- Song, G.A.; Han, J.H.; Lim, K.R.; Kim, T.E.; Park, J.M.; Kim, D.H.; Park, J.Y.; Seo, Y.; Kim, K.B. Optimization of mechanical properties of Ti-Fe-Sn alloys by controlling heterogeneous eutectic structure. Intermetallics 2012, 23, 27–31. [Google Scholar] [CrossRef]
- Genau, A.L.; Ratke, L. Crystal orientation and morphology in Al-Ag-Cu ternary eutectic. IOP Conf. Ser. Mater. Sci. Eng. 2011, 27, 012032. [Google Scholar] [CrossRef]
- Tiwary, C.S.; Kashyap, S.; Chattopadhyay, K. Development of alloys with high strength at elevated temperatures by tuning the bimodal microstructure in the Al-Cu-Ni eutectic system. Scr. Mater. 2014, 93, 20–23. [Google Scholar] [CrossRef]
- Sprenger, H.; Richter, H.; Nickl, J.J. Unidirectional solidification of Ni-Mo-Al eutectic alloys. J. Mater. Sci. 1976, 11, 2075–2081. [Google Scholar] [CrossRef]
- Lee, C.H.; Hong, S.H.; Kim, J.T.; Park, H.J.; Song, G.A.; Park, J.M.; Suh, J.Y.; Seo, Y.; Qian, M.; Kim, K.B. Chemical heterogeneity-induced plasticity in Ti-Fe-Bi ultrafine eutectic alloys. Mater. Des. 2014, 60, 363–367. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, S.W.; Hong, S.H.; Park, H.J.; Park, J.Y.; Lee, N.; Seo, Y.; Wang, W.M.; Park, J.M.; Kim, K.B. Understanding the relationship between microstructure and mechanical properties of Al-Cu-Si ultrafine eutectic composites. Mater. Des. 2016, 92, 1038–1045. [Google Scholar] [CrossRef]
- Hötzer, J.; Steinmetz, P.; Dennstedt, A.; Genau, A.; Kellner, M.; Sargin, I.; Nestler, B. Influence of growth velocity variations on the pattern formation during the directional solidification of ternary eutectic Al-Ag-Cu. Acta Mater. 2017, 136, 335–346. [Google Scholar] [CrossRef]
- Park, E.M.; Lee, C.H.; Park, J.M.; Han, J.H.; Song, G.A.; Kim, J.T.; Hong, S.H.; Park, J.Y.; Seo, Y.; Lee, N.S.; et al. Heterogeneous duplex structured Ti-Sn-Mo alloys with high strength and large plastic deformability. J. Alloys Compd. 2013, 574, 546–551. [Google Scholar] [CrossRef]
- Chanda, B.; Potnis, G.; Jana, P.P.; Das, J. A review on nano-/ultrafine advanced eutectic alloys. J. Alloys Compd. 2020, 827, 154226. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Tikhonovsky, M.A.; Salishchev, G.A. Tensile properties of the Cr-Fe-Ni-Mn non-equiatomic multicomponent alloys with different Cr contents. Mater. Des. 2015, 87, 60–65. [Google Scholar] [CrossRef]
- Eckert, H.; He, G.; Das, J.; Loser, W. Nanostructured composites in multicomponent alloy systems. Mater. Trans. 2003, 44, 1999–2006. [Google Scholar] [CrossRef][Green Version]
- Mondol’fo, L. Aluminum Alloys-Structure and Properties; Butterworths and Co., Ltd.: London, UK, 1976.
- Kayg, Y.; Maras, N. Directional solidification of Al-Cu-Si-Mg quaternary eutectic alloy. J. Alloys Compd. 2017, 721, 764–771. [Google Scholar] [CrossRef]
- Chang, I.; Cai, Q. From simple binary to complex multicomponent eutectic alloys. Prog. Mater. Sci. 2022, 123, 100779. [Google Scholar] [CrossRef]
- Li; Kuribayashi Nucleation-controlled microstructures and anomalous eutectic formation in undercooled Co-Sn and Ni-Si eutectic melts. Metall. Mater. Trans. A 2003, 34, 2999–3008. [CrossRef]
- Zhao, S.; Li, J.F.; Liu, L.; Zhou, Y.H. Cellular growth of lamellar eutectics in undercooled Ag-Cu alloy. Mater. Charact. 2009, 60, 519–524. [Google Scholar] [CrossRef]
- Mullins, W.W.; Sekerka, R.F. Stability of a planar interface during solidification of a dilute binary alloy. J. Appl. Phys. 1964, 35, 444–451. [Google Scholar] [CrossRef]
- Cantor, B.; Chadwick, G.A.A. Crystallography of Al-Al3Ni, Al-Al2Cu and Al-ζ(AlAg) eutectics during nucleation and the early stages of growth. J. Cryst. Growth 1975, 30, 101–108. [Google Scholar] [CrossRef]
- Ruggiero, M.A.; Rutter, J.W. Origin of microstructure in the 332 K eutectic of the Bi-In-Sn system. Mater. Sci. Technol. 1997, 13, 5–11. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Eckert, J.; Löser, W.; Dhindaw, B.K.; Schultz, L. Cooling Rate Evaluation for Bulk Amorphous Alloys from Eutectic Microstructures in Casting Processes. Mater. Trans. 2002, 43, 1670–1675. [Google Scholar] [CrossRef]
- Pawlik, P.; Pawlik, K.; Przybł, A. Investigation of the cooling rate in the suction casting process. Rev. Adv. Mater. Sci. 2008, 18, 81–84. [Google Scholar]
- Abbaschian, R.; Lipschutz, M.D. Eutectic solidification processing via bulk melt undercooling. Mater. Sci. Eng. A 1997, 226–228, 13–21. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.; Yeon, S.M.; Aranas, C.; Choi, K.; Yoon, J.; Yang, S.W.; Lee, H. Selective compositional range exclusion via directed energy deposition to produce a defect-free Inconel 718/SS 316L functionally graded material. Addit. Manuf. 2021, 47, 102288. [Google Scholar] [CrossRef]
- Trivedi, R.; Kurz, W. Morphological stability of a planar interface under rapid solidification conditions. Acta Metall. 1986, 34, 1663–1670. [Google Scholar] [CrossRef]
- Li, Q.; Beckermann, C. Evolution of the sidebranch structure in free dendritic growth. Acta Mater. 1999, 47, 2345–2356. [Google Scholar] [CrossRef]
- Wei, X.X.; Lin, X.; Xu, W.; Huang, Q.S.; Ferry, M.; Li, J.F.; Zhou, Y.H. Remelting-induced anomalous eutectic formation during solidification of deeply undercooled eutectic alloy melts. Acta Mater. 2015, 95, 44–56. [Google Scholar] [CrossRef]
- Cantor, B.; Chadwick, G.A. The tensile deformation of unidirectionally solidified AI-Al3Ni and Al-Al2Cu eutectics. J. Mater. Sci. 1975, 10, 578–588. [Google Scholar] [CrossRef]
- Nabarro, F.R.N.; Duesbery, M.S.; Hirth, J.P. Dislocations in Solids; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1979; ISBN 9780444850041. [Google Scholar]
- Bramfitt, B.L. The Effect of Carbide and Nitride Additions on the Heterogeneous Nucleation Behavior of Liquid Iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Kurz, W.; Trivedi, R. Eutectic growth under rapid solidification conditions. Metall. Trans. A 1991, 22, 3051–3057. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, K.B.; Kim, D.H.; Mattern, N.; Li, R.; Liu, G.; Eckert, J. Multi-phase Al-based ultrafine composite with multi-scale microstructure. Intermetallics 2010, 18, 1829–1833. [Google Scholar] [CrossRef]
- Lei, Q.; Ramakrishnan, B.P.; Wang, S.; Wang, Y.; Mazumder, J.; Misra, A. Structural refinement and nanomechanical response of laser remelted Al-Al2Cu lamellar eutectic. Mater. Sci. Eng. A 2017, 706, 115–125. [Google Scholar] [CrossRef]
- Cantor, B.; May, G.J.; Chadwick, G.A. The tensile fracture behaviour of the aligned Al-Al3Ni and Al-CuAl2 eutectics at various temperatures. J. Mater. Sci. 1973, 8, 830–838. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, D.H.; Kim, K.B.; Kim, W.T. Deformation-induced rotational eutectic colonies containing length-scale heterogeneity in an ultrafine eutectic Fe83Ti7Zr6B4 alloy. Appl. Phys. Lett. 2007, 91, 131907. [Google Scholar] [CrossRef]
- Kim, J.T.; Hong, S.H.; Park, J.M.; Eckert, J.; Kim, K.B. Microstructure and mechanical properties of hierarchical multi-phase composites based on Al-Ni-type intermetallic compounds in the Al-Ni-Cu-Si alloy system. J. Alloys Compd. 2018, 749, 205–210. [Google Scholar] [CrossRef]




| Alloy Composition /Atomic Percent | Sample Size /mm | Yield Strength /MPa | Fracture Strength /MPa | Plastic Strain /% |
|---|---|---|---|---|
| Al83Cu17 [51] | ∅1 | ~1000 | ~1200 | ~2 |
| Al81Cu13Si6 [26] | ∅3 | ~773 | ~1000 | ~8 |
| Al81Ni13Si6 [55] | ∅3 | 502 | 556 | 5.2 |
| Al81Ni5Cu8Si6 [55] | ∅3 | 598 | 773 | 14.8 |
| Al88Cu10.5Ni1.5 [23] | ∅3 | 800 | 1000 | 9 |
| Al96.9Ni3.1 [19] | ∅3 | ~210 | ~350 | ~20 |
| Al95.4Ni4Cr0.6 [19] | ∅3 | ~380 | ~800 | ~35 |
| Al77.1Cu13.6Mg3Si6.3 (current work) | ∅3 | ~835 | ~1036 | ~4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Q.; Cantor, B.; Tong, V.S.; Wang, F.; Mendis, C.L.; Chang, I.T.H.; Fan, Z. Microstructure and Mechanical Properties of Ultrafine Quaternary Al-Cu-Si-Mg Eutectic Alloy. Metals 2022, 12, 7. https://doi.org/10.3390/met12010007
Cai Q, Cantor B, Tong VS, Wang F, Mendis CL, Chang ITH, Fan Z. Microstructure and Mechanical Properties of Ultrafine Quaternary Al-Cu-Si-Mg Eutectic Alloy. Metals. 2022; 12(1):7. https://doi.org/10.3390/met12010007
Chicago/Turabian StyleCai, Qing, Brian Cantor, Vivian S. Tong, Feng Wang, Chamini L. Mendis, Isaac T.H. Chang, and Zhongyun Fan. 2022. "Microstructure and Mechanical Properties of Ultrafine Quaternary Al-Cu-Si-Mg Eutectic Alloy" Metals 12, no. 1: 7. https://doi.org/10.3390/met12010007
APA StyleCai, Q., Cantor, B., Tong, V. S., Wang, F., Mendis, C. L., Chang, I. T. H., & Fan, Z. (2022). Microstructure and Mechanical Properties of Ultrafine Quaternary Al-Cu-Si-Mg Eutectic Alloy. Metals, 12(1), 7. https://doi.org/10.3390/met12010007







