Abstract
The present study reports a specific method for preparation of silver-modified anodic alumina substrates intended for biomaterial applications. Al2O3 coatings were obtained by anodization of technically pure aluminum alloy in sulfuric acid electrolyte. Silver deposition into the pores of the anodic structures was carried out employing in situ thermal reduction for different time periods. The obtained coatings were characterized using scanning electron microscopy (SEM), potentiodynamic scanning after 168 h in 3.5% NaCl solution and bioassays with human fibroblast and NIH/3T3 cell lines. The modified alumina substrates demonstrated better biocompatibility compared to the control anodic Al2O3 pads indicated by increased percent cell survival following in vitro culture with human and mouse fibroblasts. The Ag-deposition time did not affect considerably the biocompatibility of the investigated anodic layers. SEM analyses indicated that mouse NIH/3T3 cells and human fibroblasts adhere to the silver-coated alumina substrates retaining normal morphology and ability to form cell monolayer. Therefore, the present studies demonstrate that silver coating of anodic alumina substrates improves their biocompatibility and their eventual biomedical application.
1. Introduction
Nowadays, various combinations of materials are under in-depth investigations when creating medical devices. It is well known that the quintessential biomaterial has not yet been introduced into the medical practice. In this regard, surface modification of implants is considered to be the most promising method for preventing corrosion and promoting biocompatibility [1].
Aluminum (Al) is widely applicable in different technological fields owing to its mechanical properties, for example high tensile strength, good heat and electrical conductivity, low coefficients of friction and deformation, easy processing, etc. [2]. Due to its toxicity to living organisms Al has not been extensively studied as a potential material for production of medical devices [3]. However, the naturally formed in air oxide layer on aluminum and its alloys passivates the metal surface and interrupts any further interactions with the environment. There are studies stating that Al2O3 is an inert compound when introduced to biological systems [4]. Moreover, anodizing is commonly used electrochemical surface treatment resulting in a formation of anodic alumina oxide (AAO) with controllable thickness, porosity, morphology, etc. It is established that anodic films exhibit improved mechanical and anti-corrosive properties [5]. Further, these oxide layers can be additionally modified by deposition of different metals within their pores. There are various studies describing incorporation of Pb, Cd, Cr, Fe, Au, Co, Cu, Mn, Ni, Se, Ag, Te, Zn, Sn on anodized aluminum substrates [6]. At the same time the use of noble metals, such as gold and silver when creating biomaterials, is a very prospective approach to enhance bioactive and antimicrobial properties [7,8].
The increasing resistance of bacteria to commonly used antibiotics imposes extensive research activities in the field of new therapeutic agents with the aim to prevent or inhibit infectious diseases [9]. A prominent fact is that silver induces excellent inhibitory effect on different types of bacteria—P. aeruginosa, S. faecalis, S. aureus [10], M. luteus, E. coli, etc. [11], but at the same time nanosized silver can cause toxicity to liver and stem cells, human and mouse fibroblasts, as well as human carcinoma cells [12]. There are many investigations dealing with the dependency of silver biological activity and its size, shape, morphology, surface area, etc. [13]. Usually, the diameter of typical silver nanoparticles ranges from a few to dozen nm [8]. Various types of methods (physical, chemical, photochemical and biological) for their synthesis have been already reported [14]. However the resulting products in nanoform have different characteristic parameters. Therefore, they should be considered as different materials with non-identical mechanisms of action and resistance [13,15]. Due to their unique physical and chemical properties silver nanoparticles have already been used as biosensors, cosmetics, and pharmaceutical products [16], in fillers, food packaging, wastewater treatment [14], catalysis, electronics, and photonics [17], cancer treatment [18]. When deposited and interacting with a matrix, these nanostructures can reveal new operating functions. For example, such combination of porous template and incorporated nanomaterial can be useful as antibacterial drug release carrier, in orthopedics, in dentistry, and other medical fields [19]. It is worth mentioning that the increased interest in utilizing alumina coatings for biomedical applications is related to their good wear and anticorrosive properties, low cost, improved surface functionalization, etc. [20].
In the present work, biological and anti-corrosive properties of silver-modified anodic films on the surface of technically pure aluminum alloy were investigated. For this purpose, the obtained combined layers were analyzed by scanning electron microscopy. The observations showed the presence of silver into the anodic alumina structures. The size of the deposited particles was above the nanoscale, which indicates aggregation of nanoparticles under the given conditions of in situ thermal reduction. The concentration of the silver deposits was determined using inductively coupled plasma optical emission spectroscopy (ICP-OES). The corrosion protective capabilities provided by the above mentioned modifications were studied using potentiodynamic scanning (PDS). Furthermore, in vitro biocompatibility assays with two fibroblast cell lines combined with light and scanning electron microscopy analyses were conducted to clarify the biological activity of the modified alumina substrates.
2. Materials and Methods
2.1. Anodic Alumina Oxide Preparation
All experimental procedures were performed with EN AW 1050A aluminum alloy with chemical composition corresponding to EN standard EN 573-3/485-2. The dimensions of the specimens were 20 mm × 20 mm × 0.5 mm.
In order to remove accidental dirt and some defects, metal pads with working area of 8 cm2 were pre-treated by degreasing in acetone, pickling in diluted nitric acid (1:1 v/v) and electropolishing in a mixture of concentrated perchloric acid and ethanol (1:4 v/v) at constant voltage of 10 V for 3 min. Then, the samples were anodized in 1.53 mol L−1 H2SO4 at constant current density of 15 × 10−3 A cm−2 at 20 ± 2 °C for 30 or 60 min. A two electrode cell was used with a lead plate as a counter electrode, placed symmetrically around the anode. After each operation, the samples are repeatedly washed with double distilled water and carefully dried. All solutions were prepared with analytical grade reagents.
2.2. Chemical Deposition of Silver
Anodized aluminum substrates were immersed in a solution of 5 mM AgNO3 for different time periods (3 or 6 min) at 96 ± 1 °C. The addition of a reducing agent is considered to be the start of the reaction. In the current work silver nitrate solutions were reduced by 0.1 wt.% trisodium citrate (Na3C6H5O7). Volume ratio of Na3C6H5O7 to AgNO3 was 1:10 [16]. Depending on the silver deposition time and respectively the amount of the incorporated particles, the color of the anode film changes to pale yellow.
Finally, in order to close up the pores of the Ag-containing oxide layers, the latter were soaked in boiling water at 96 ± 1 °C for 25 min. This method of simultaneously synthesis and deposition of silver nanoparticles followed by pore sealing of the layers was previously described [21].
2.3. Silver Amount Determination
The concentration of the deposited silver into the anodic films was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) using Prodigy 7 spectrometer (Teledyne Leeman Labs). For this purpose silver-modified anodic layers were dissolved in a mixture of concentrated nitric acid and water (1:1), at room temperature.
2.4. Microstructure Characterization of the Silver-Modified Structures
The as obtained Ag-doped anodic films were analyzed by scanning electron microscopy (PrismaTM E SEM by Thermo Fisher Scientific with an attachment for element analysis—EDX analyzer, Waltham, MA, USA).
2.5. Electrochemical Analysis
Potentiodynamic scanning (PDS) (performed by Universal Galvanostat/Potentiostat – AUTOLAB PG/STAT 30/2, product of Ecochemie, Kanaalweg, The Netherlands) was performed after 168 h of exposure to 3.5% NaCl solution as model corrosive medium. Ag/AgCl/3M KCl was used as a reference electrode and a platinum cylindrical mesh as a counter electrode. PDS curves were obtained from −100 to 1000 mV against open circuit potential with a voltage sweep rate of 10 mV s−1. The measurements were performed with two identical samples of each type since the electrochemical behavior of anodized EN AW 1050 alloy has already been reported with good repeatability of the results [22].
2.6. Cell Lines and In Vitro Cultivation Conditions
Silver-modified alumina substrates biocompatibility was evaluated in vitro using murine and human fibroblasts: NIH/3T3 (ATCC CRL-1658)—an immortalized mouse embryonic cell line, and a finite human cell line established from preputium-derived fibroblasts. The cells were cultured in vitro in complete Dulbecco’s modified Eagles’s medium (DMEM), i.e. DMEM supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U mL−1 penicillin and 100 μg mL−1 streptomycin (all purchased from Sigma-Aldrich Inc., Merck KGaA, Darmstadt, Germany). The cultures were maintained at 37 °C, 5% CO2/atmospheric air mixture and high humidity. Before the biocompatibility evaluations the cells were grown in 75 cm2 culture flasks (TPP, Trasadingen, Switzerland) and subcultured at 80% confluency. In brief, cell monolayers were washed with Dulbecco’s modified phosphate buffered saline (DPBS) (Sigma-Aldrich Inc., Merck KGaA, Darmstadt, Germany) and detached using 0.2% trypsin/0.53 mM EDTA buffer solution. The concentration of viable cells in the resulting suspensions was determined by the Trypan blue assay [23]. A total 50 μL cell suspension were mixed with equal volume of 0.4% Trypan blue solution (Sigma-Aldrich Inc., Merck KGaA, Darmstadt, Germany), loaded on a Burker chamber (Boeco, Hamburg, Germany) and counted using Ceti Max III compound microscope (Medline scientific, Oxon, UK). Then, concentration of live cells per mL was calculated, the suspensions containing detached NIH/3T3 and human fibroblasts were diluted to appropriate concentration and new cultures were established.
2.7. Biocompatibility Evaluations
Anodized aluminum substrates and silver-doped alumina pads were placed on the bottom of separate wells in 6-well culture plates (TPP, Trasadingen, Switzerland). After that, 1 × 105 cells mL−1 NIH/3T3 and human fibroblasts (2 mL cell suspension per well) were seeded on the plates containing alumina test-substrates and cultured for 48 h. Control cells were grown for the same time period in wells containing a glass coverslip. The size of the glass substrate corresponded to the size of the analyzed alumina pads. After the end of the incubation period the test-substrates and control glass coverslips were removed from the culture plates and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution was added to the cell culture medium to a final concentration of 0.5 mg mL−1.
The MTT assay principle was initially described by Mossman [24]. In the present experiments, the MTT tests were performed in accordance to the methodology published by Edmondson et al. [25]. In brief, the cells were incubated in MTT containing medium for 2 h under standard conditions (37 °C, 5% CO2 and high humidity). After that, the cells were washed with pre-warmed (37 °C) DPBS and 1 mL dimethyl sulfoxide (DMSO) was added to each well of the culture vessels. After 15 min incubation with DMSO at 37 °C, absorbance at 570 nm was measured using Synergy-2 reader (BioTek, Winooski, VT, USA). Percent cell survival was determined based on the data for cells co-cultured with alumina substrates and control cells. All substrates were tested in duplicates.
2.8. Cell Morphology Analysis
Cellular morphology and culture monolayer integrity after 48 h co-cultivation with alumina test-substrates were evaluated using a standard inverted microscope Inverso (Medline Scientific, Oxon, UK) and Si-3000 high definition digital camera (Medline Scientific, Oxon, UK).
NIH/3T3 and human fibroblasts attached to the surface of alumina specimens were observed by scanning electron microscope (PrismaTM E SEM by Thermo Fisher Scientific). Prior to the SEM evaluations, the cells were seeded on test-substrates placed in 6-well plates (TPP, Trasadingen, Switzerland) and cultured for 48 h as described in the previous subsection. Then, alumina pads and control glass coverslips were removed from the plates and processed as described by Osahor et al. [26]. The specimens were washed twice in pre-warmed (37 °C) DPBS and fixed with 2.5% glutaraldehyde for 30 min at 37 °C. After that, the cells were immediately subjected to alcohol dehydration using 25%, 40%, 60%, 80%, 90% and 100% ethanol gradient treatment for 15 min for each concentration. The specimens were dried in a simple desiccator for 30 min.
2.9. Statistics
Statistics was calculated based on analysis of variance (ANOVA) using the StatView software version 5.0 (SAS Institute, Cary, NC, USA). Significant differences between data from control anodic alumina substrates and silver-modified samples were determined by Fisher’s PLSD method. Estimated p values lower than 0.05 were considered statistically significant.
3. Results and Discussion
All set of samples used in the current work and the corresponding treatment procedures are described in Table 1.

Table 1.
Sample groups and their abbreviation used in the manuscript.
It is considered that cell growth, cytotoxicity, genotoxicity and biocompatibility depend on the morphological characteristics such as size, shape, surface charge, concentration, distribution of particles, exposure time and cell type [27,28]. In order to determine the concentration of deposited Ag particles into the anodized alumina layers, all samples were submitted to induction coupled plasma optical emission spectroscopy (ICP-OES) and the acquired quantitative data are graphically represented in Figure 1. As expected, the Ag amount is proportional to the silver deposition time. It was also found that the concentration of Ag is higher at the anodic films formed in the acid electrolyte for 60 min.
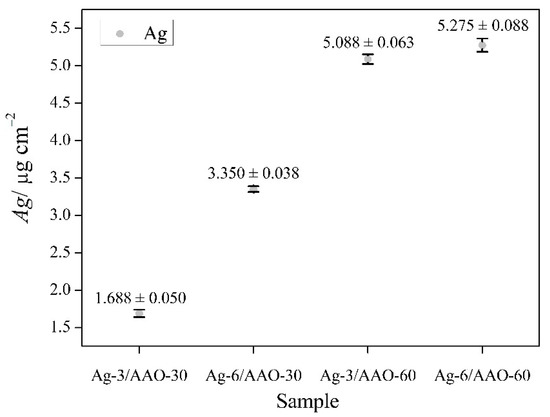
Figure 1.
Correlation between the deposited Ag content, silvering time and anodization time periods. The mean value of Ag content (μg cm−2) and standard deviation (SD) of it are presented at the figure. All analysis were performed three times (n = 3).
The images in Figure 2 show potentiodynamic polarization curves of different metal pads of chosen sample groups. As it can be seen, the bare aluminum coupons suffer uniform corrosion attack. At the same time, the curves of anodized samples (with or without silver deposits) have almost horizontal branches, revealing good passivation properties by the thick alumina layers. In Table 2 the obtained experimental results of the PDS analysis are summarized. The corrosion potentials of all samples underwent anodic oxidation are shifted to more positive values. This fact is consequence of the modifications of the surface chemical composition, as proposed in Kozhukharov et al. [22]. Significant difference of the polarization resistance and corrosion current density values was observed only between the untreated aluminum coupons and all AAO samples. The presence of silver particles in these layers does not significantly change the barrier ability of the combined films.
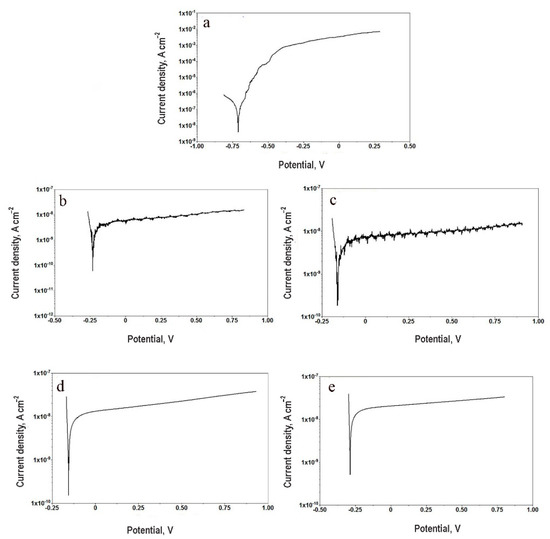
Figure 2.
Potentiodynamic curves acquired after 168 h for (a) AA coupons, (b) AAO-30, (c) AAO-60, (d) Ag-6/AAO-30, (e) Ag-6/AAO-60.

Table 2.
A summary of PDS data results, acquired from Tafel slope analysis.
3.1. Scanning Electron Microscope Observations
In order to examine the morphological futures of the silver doped alumina films systematic SEM observations of all types of silver modified probes were performed. The topological and compositional characteristics of similar anodic alumina coatings are investigated in detail. The presence of equally distributed pits and cavities on the anodized specimens has already been described [29]. These imperfections of the alumina films are formed on sites of heavier intermetallic inclusions, which are typical contaminants for the technically pure aluminum alloy. On Figure 3 micrographs of Ag-doped anodic oxide surfaces at magnification ×20,000 are presented. As it can be seen, the performance of the anodic processes at these conditions results in the formation of Al2O3 with irregular distribution and structure of its pores. More interesting is the fact that depending on the silver deposition time and the following sealing procedure the final Ag/AAO morphology is unique. Silver particles with different size (over nanoscale) are not evenly distributed on the alumina surfaces. The chemical compositions of specimens were investigated by the attachment for element analysis—EDX analyzer.
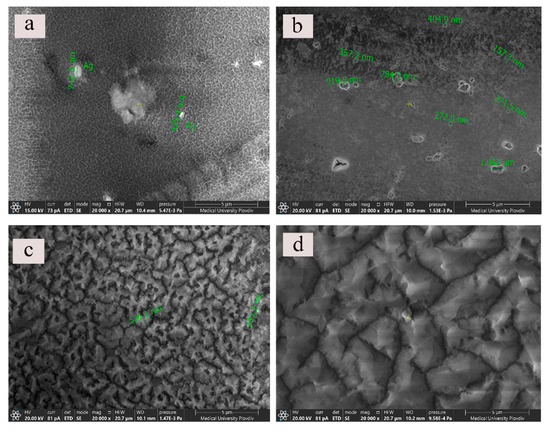
Figure 3.
SEM micrographs of (a) Ag-6/AAO-30, (b) Ag-6/AAO-30, (c) Ag-3/AAO-60 (d) Ag-6/AAO-60 at ×20,000 magnification.
In Figure 3a it can be seen the irregular porous structure of the oxide, individual silver particles and agglomerates. In Figure 3b, probably as a result of the longer silvering time (6 min), the Ag particles are located on the edges of the pits and cavities of the alumina. In addition, the oxide film looks less porous. Presumably, the extended silver deposition time in a combination with the sealing treatment of the sample results in the production of crystalline hydrate phase which fills the pores of the oxide film. Hydrothermal sealing of anodized aluminum at temperatures above 95 °C is previously reported to promote hydration of porous oxides and barrier layers, producing boehmite (γ-AlO(OH)) [30]. More obvious this can be seen in Figure 3c where the anodic film formation is extended to 60 min. In Figure 3d (60 min anodization and 6 min silver deposition time) the surface morphology of the alumina layer is substantially different. There is a total lack of the porous skeleton of the Al2O3 probably due to the filling of the pore mouths with hydrated products like boehmite, Al2(SO4)3 [30].
It should be pointed out that for better detection of the silver particles with the EDX analyzer detailed observations of the top-view of the surfaces at different magnifications were performed. In Figure 4 a micrograph of a specimen corresponding to Ag-6/AAO-60 at ×35,000 is presented. It can be seen that silver particles are incorporated in the hydrated porous structure.
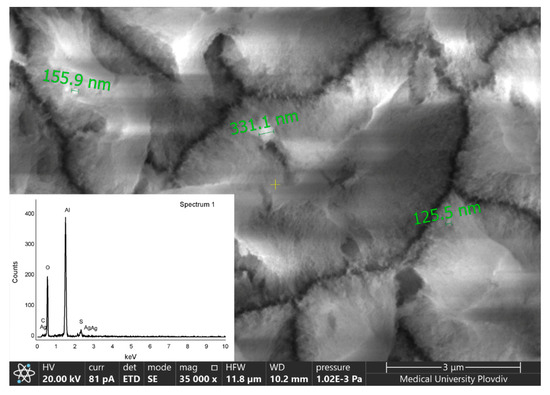
Figure 4.
SEM image of Ag-6/AAO-60 specimen at magnification ×35,000.
3.2. Biocompatibility of Silver-Modified Alumina Substrates
NIH/3T3 cells and human dermal fibroblasts were co-cultured for 48 h with silver-doped alumina pads and control anodized alumina specimens without silver modification. After the end of the incubation period cell survival was determined by MTT assays. Figure 5 represents the obtained results. In accordance with our previous findings [27] NIH/3T3 cells demonstrated higher sensitivity to anodic alumina and silver-modified alumina substrates compared to human fibroblasts. The two specimens with anodized for 30 or 60 min alumina film showed the lowest cell survival level. A statistically significant difference was present between anodized alumina samples and the silver-doped substrates when co-cultured with the mouse fibroblast cell line (Figure 5a,b). These results demonstrate that the generated silver layers on AAO-30 and AAO-60 substrates improved their biocompatibility when tested with NIH/3T3 cells. The same tendency was demonstrated by human fibroblasts (Figure 5c,d) confirming the biocompatibility of silver-doped samples. The cells were not affected significantly by co-cultivation with AAO-30 and AAO-60 samples—the calculated cell survival level was approximately 90%. However, when co-cultured with silver-modified alumina substrates, human fibroblasts survival was higher, even similar to the control cells co-cultured with a glass substrate. These results suggest that silver modification enhances fibroblast cell survival rates when co-cultured with anodic alumina pads. It could be assumed that these positive effects are induced by changes in the substrate surface. Silver particles incorporate in the porous hydrated structure of the pads (Figure 4) and thus, affect their roughness and possibly improve cellular adhesion and survival. Additional experiments are needed to confirm this hypothesis. AAO-30 and AAO-60 specimens doped with silver for 3 and 6 min did not show significant difference in survival of both NIH/3T3 and human fibroblasts. Although SEM analyses indicated surface differences between AAO-30 and AAO-60 specimens doped with silver for 3 and 6 min cell survival levels did not show significant variance. It could be suggested that silver modification eliminates sites of intermetallic inclusions/imperfections of anodic alumina substrates independent of time for deposition (3 or 6 min), which leads to improved cell survival.
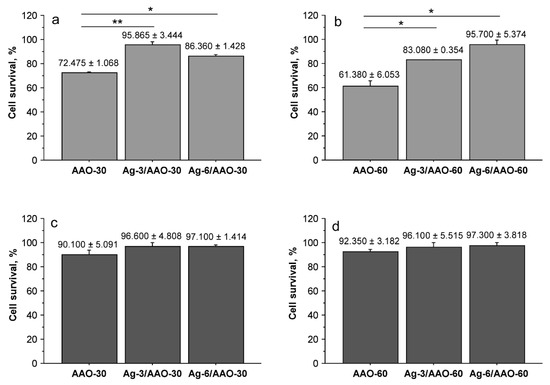
Figure 5.
Cell survival levels determined after 48 h in vitro culture of NIH/3T3 cells (a), (b) and human fibroblasts (c), (d) with anodized alumina and silver-modified alumina substrates. All samples were tested in duplicates (n = 2). Figure bars represent mean ± standard error of the mean and ± SD is indicated for each sample. * Indicates p < 0.05, ** p < 0.01.
To support the data from MTT assays, light microscopy observations were performed. As shown on Figure 6 NIH/3T3 cells (Figure 6a) and human fibroblasts (Figure 6b) retained normal morphology, as well as ability to proliferate and form integral monolayer during co-culture with silver-modified alumina specimens. These findings confirm the high cell survival rates detected by the colorimetric assays. In addition, we were interested to find whether the cells are able to attach to the alumina substrates. Therefore, SEM analyses were performed. They revealed that NIH/3T3 and human fibroblasts adhere to both AAO (data not shown) and silver-doped AAO pads (Figure 6c,d). The cells displayed typical morphology that could be observed only after characteristic reorganization of the cytoskeleton during the experiment, formation of specific cellular protrusions and focal adhesions to the substrate followed by stable attachment. Moreover, SEM pictures showed individual rounded cells with discrete contact to the substrate. Generally, they were located above the plane of the layer formed by the majority of attached cells with characteristic morphology. Such position and the spherical form of the cells indicate a proliferative phenotype. The presence of mitotic cells on the silver-modified substrates proves further the biocompatibility of the tested materials. These findings show that both mouse and human fibroblasts are able to attach to the test-substrates. Moreover, as shown on Figure 6e,f the cells develop and proliferate giving rise to a stable monolayer on the surface of silver-modified alumina specimens.
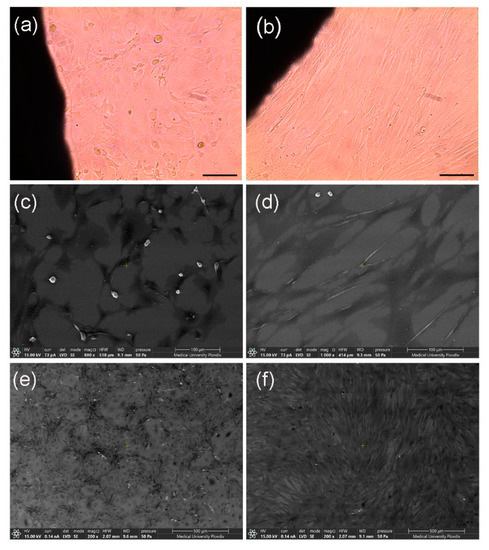
Figure 6.
Light (a,b) and scanning electron microscopy (c–f) pictures of NIH/3T3 (a,c,e) and human fibroblasts (b,d,f) co-cultured with silver-modified alumina substrates.
4. Conclusions
The present work demonstrates the biocompatibility of silver-modified anodic alumina pads based on in vitro assays with mouse NIH/3T3 and human fibroblasts. The mouse cell cultures demonstrated higher sensitivity to all anodic layers. However, when co-cultured with silver-modified AAO films, the cell survival levels of the two cell lines were sufficient. The concentration of the deposited silver particles did not affect these levels. The cell ability to attach to the surface of the samples was proven by scanning electron microscopy. SEM analyses were also performed in order to examine the morphological characteristics of the silver-modified layers. Depending on the Ag deposition time and the hydrothermal sealing procedure the resulting morphology of each specimen type was unique. Finally, regardless the structural differences all AAO layers (underwent or not silver modification) demonstrated good barrier ability in potentiodynamic polarization studies.
Author Contributions
Conceptualization, D.K., N.M. and T.B.; Formal analysis, D.K., T.B. and N.Z.; Funding acquisition, D.K. and N.M.; Investigation, D.K., T.B., N.Z. and N.M.; Methodology, D.K., N.M., R.M., B.D. and T.B.; Project administration, T.B. and D.K.; Software, D.K.; Supervision, D.K., N.M., T.B., R.M. and B.D.; Validation, D.K. and N.M.; Visualization, D.K. and N.M.; Writing—original draft, B.D., R.M., D.K. and T.B.; Writing—review & editing, D.K., B.D., R.M., T.B. and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Medical University of Plovdiv for financial support.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. All data are in the form of tables and figures.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical Implants: Corrosion and Its Prevention-A Review. Recent Patents Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Polmear, I.; StJohn, D.; Nie, J.-F.; Qian, M. (Eds.) Wrought Aluminium Alloys. In Light Alloys; Butterworth-Heinemann: Oxford, UK, 2017; pp. 157–263. [Google Scholar] [CrossRef]
- Langley, A.; Dameron, C.T. Modern Metal Implant Toxicity and Anaesthesia. Australas. Anaesth. 2015, 57–65. [Google Scholar] [CrossRef]
- Christel, P.; Meunier, A.; Dorlot, J.M.; Crolet, J.M.; Witvoet, J.; Sedel, L.; Boutin, P. Biomechanical Compatibility and Design of Ceramic Implants for Orthopedic Surgery. Ann. N. Y. Acad. Sci. 1988, 523, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. Role of Intermetallics in Corrosion of Aluminum Alloys. Smart Corrosion Protection. In Intermetallic Matrix Composites; Mitra, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 425–462. [Google Scholar] [CrossRef]
- Tomassi, P.; Nanostructures, Z.B. Aluminum Anodic Oxide AAO as a Template for Formation of Metal Nanostructures. In Electroplating of Nanostructures; Mahmood, A., Ed.; InTech: London, UK, 2015; pp. 75–102. [Google Scholar] [CrossRef]
- Darouiche, R.O. State-Of-The-Art Clinical Anti-Infective Efficacy of Silver-Coated Medical Prostheses. Epidemiology 2011, 29, 1371–1377. [Google Scholar] [CrossRef] [Green Version]
- Aina, V.; Cerrato, G.; Martra, G.; Bergandi, L.; Costamagna, C.; Ghigo, D.; Malavasi, G.; Lusvardi, G.; Menabue, L. Gold-Containing Bioactive Glasses: A Solid-State Synthesis to Produce Alternative Biomaterials for Bone Implantations. J. R. Soc. Interface 2013, 10, 20121040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, A.R.; Mecsas, J.; Moir, D.T. Beyond Antibiotics: New Therapeutic Approaches for Bacterial Infections. Clin. Infect. Dis. 2016, 63, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Chi, G.J.; Yao, S.W.; Fan, J.; Zhang, W.G.; Wang, H.Z. Antibacterial Activity of Anodized Aluminum with Deposited Silver. Surf. Coatings Technol. 2002, 157, 162–165. [Google Scholar] [CrossRef]
- Jagminas, A.; Žalnėravičius, R.; Rėza, A.; Paškevičius, A.; Selskienė, A. Design, Optical and Antimicrobial Properties of Extremely Thin Alumina Films Colored with Silver Nanospecies. Dalt. Trans. 2015, 44, 4512–4519. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, A.; Kang, I.-K. Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review. Adv. Mater. Sci. Eng. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and Antibacterial Activity of Silver Nanoparticles with Different Sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Rashid, M.U.; Bhuiyan, M.K.H.; Quayum, M.E. Synthesis of Silver Nano Particles (Ag-NPs) and Their Uses for Quantitative Analysis of Vitamin C Tablets. Dhaka Univ. J. Pharm. Sci. 2013, 12, 29–33. [Google Scholar] [CrossRef]
- Guzmán, M.G.; Dille, J.; Godet, S. Synthesis of Silver Nanoparticles by Chemical Reduction Method and Their Antibacterial Activity. Int. J. Mater. Metall. Eng. 2008, 2, 91–98. [Google Scholar] [CrossRef]
- Kulkarni, N.; Muddapur, U. Biosynthesis of Metal Nanoparticles: A Review. J. Nanotechnol. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Thorat, S.; Diaspro, A.; Scarpellini, A.; Povia, M.; Salerno, M. Comparative Study of Loading of Anodic Porous Alumina with Silver Nanoparticles Using Different Methods. Materials 2013, 6, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toccafondi, C.; Dante, S.; Reverberi, A.P.; Salerno, M. Biomedical Applications of Anodic Porous Alumina. Curr. Nanosci. 2015, 11, 572–580. [Google Scholar] [CrossRef]
- Pornnumpa, N.; Jariyaboon, M. Antibacterial and Corrosion Resistance Properties of Anodized AA6061 Aluminum Alloy. Eng. J. 2019, 23, 171–181. [Google Scholar] [CrossRef]
- Kozhukharov, S.; Girginov, C.; Kiradzhiyska, D.; Tsanev, A.; Avdeev, G. Evaluation of the Electrochemical Performance of Ag Containing AAO Layers after Extended Exposure to a Model Corrosive Medium. J. Electrochem. Sci. Eng. 2020, 10, 317–334. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Edmondson, J.M.; Armstrong, L.S.; Martinez, A.O. A Rapid and Simple MTT-Based Spectrophotometric Assay for Determining Drug Sensitivity in Monolayer Cultures. J. Tissue Cult. Methods 1988, 11, 15–17. [Google Scholar] [CrossRef]
- Osahor, A.; Deekonda, K.; Lee, C.-W.; Sim, E.U.-H.; Radu, A.; Narayanan, K. Rapid Preparation of Adherent Mammalian Cells for Basic Scanning Electron Microscopy (SEM) Analysis. Anal. Biochem. 2017, 534, 46–48. [Google Scholar] [CrossRef]
- Kiradzhiyska, D.; Batsalova, T.; Dzhambazov, B.; Mancheva, R. In Vitro Biocompatibility Evaluation of Anodic Alumina Substrates with Electrochemically Embedded Silver. Rev. Chim. 2020, 71, 81–88. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [Green Version]
- Kozhukharov, S.; Girginov, C.; Tsanev, A.; Petrova, M. Elucidation of the Anodization and Silver Incorporation Impact on the Surface Properties of AA1050 Aluminum Alloy. J. Electrochem. Soc. 2019, 166, C231. [Google Scholar] [CrossRef]
- Ofoegbu, S.U.; Fernandes, F.A.O.; Pereira, A.B. The Sealing Step in Aluminum Anodizing: A Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).