Mechanical Properties and Microstructure Evolution of Mg-Gd Alloy during Aging Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
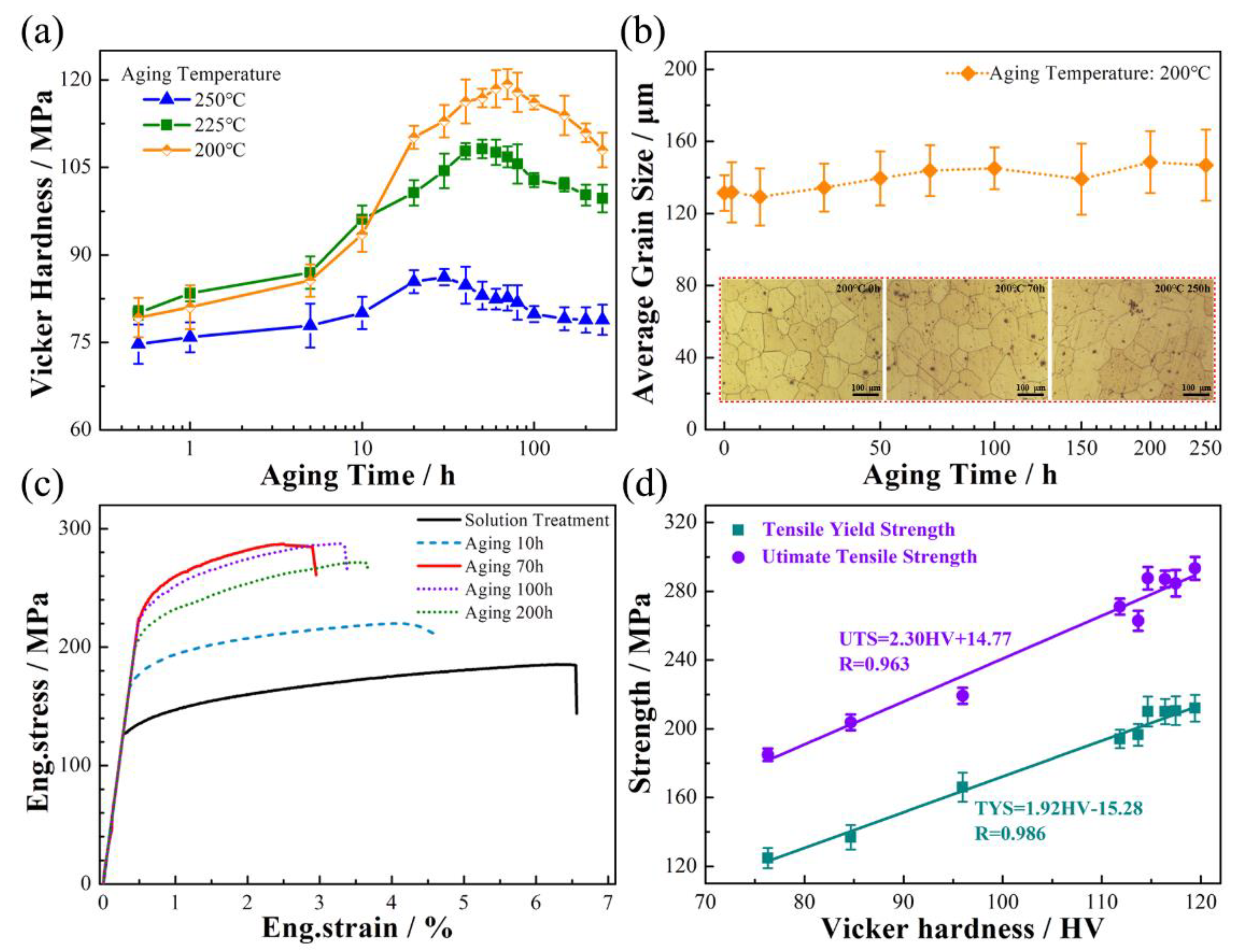
- Significant improvement of mechanical properties is achieved by aging treatment, which has a linear correlation with hardness. An optimized aging treatment that perform at 200 °C for 70 h improves the hardness to 120 HV from the initial 72 HV, and the UTS is increased from 170 MPa to 300 MPa;
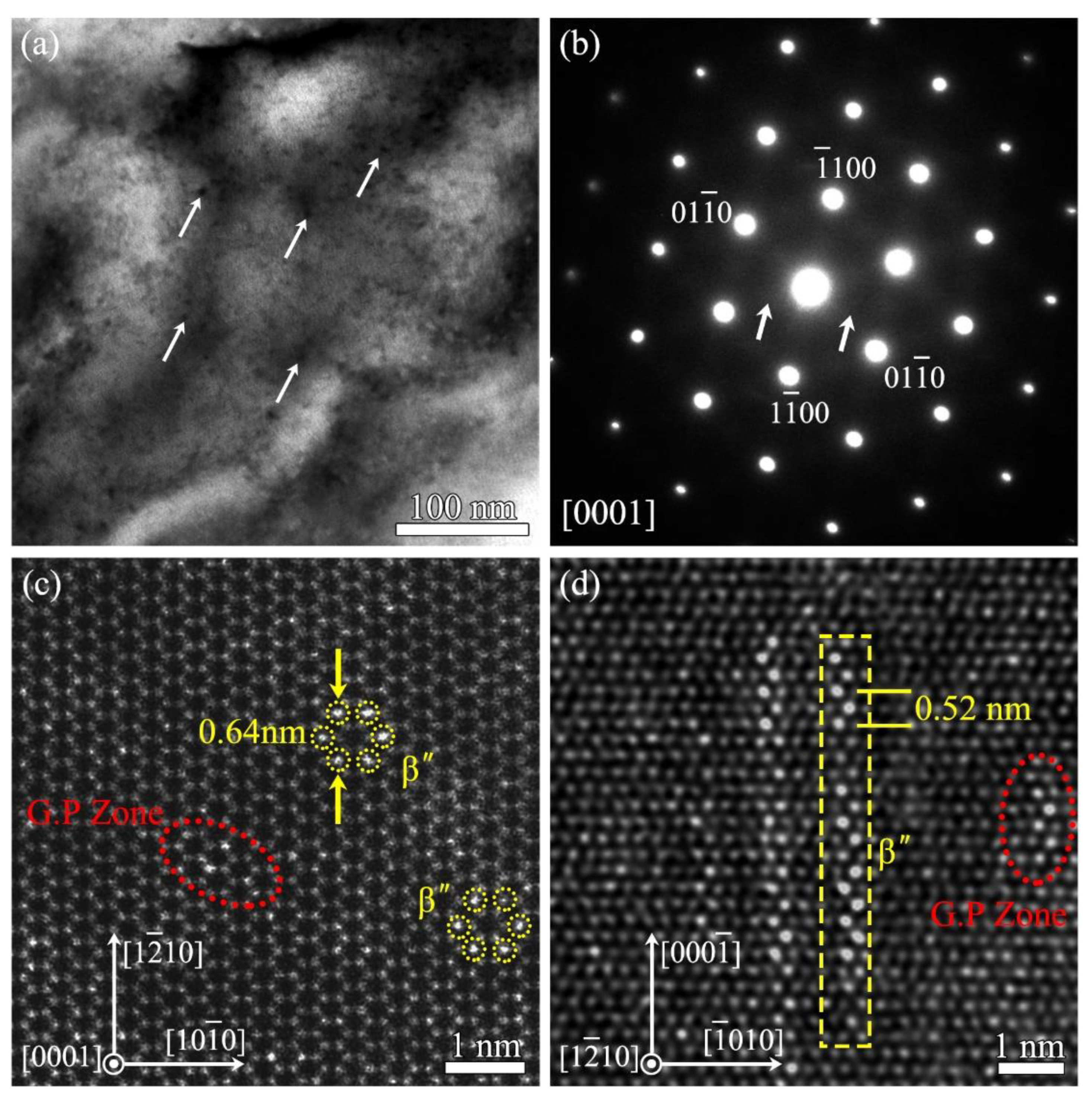
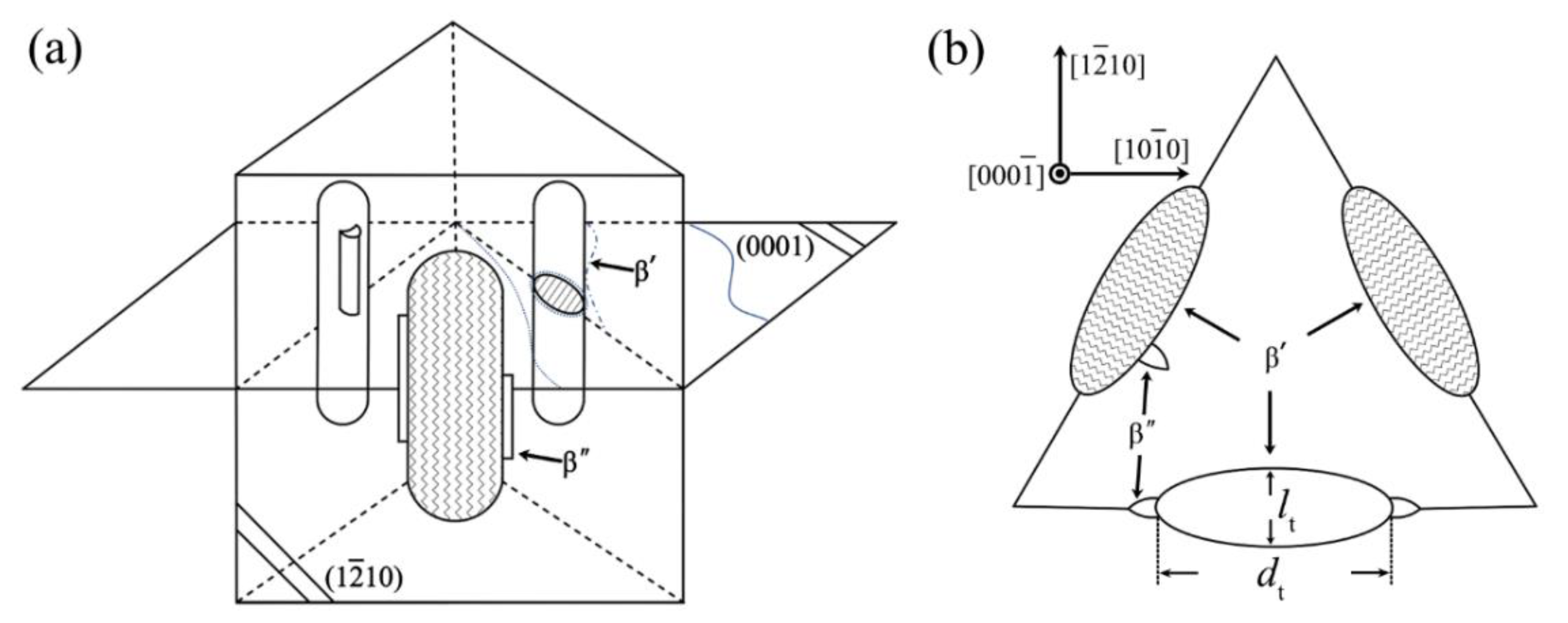
- Most precipitations nucleated at early stage of aging treatment are G.P. Zone and β” phases. The morphology of β” phase is a thin column, with a D019 crystal structure (hexagon, a = 0.640 nm and c = 0.520 nm). The orientation relationship of β” phase and matrix is [0001]β”//[0001]α, (110)β”//(110)α;
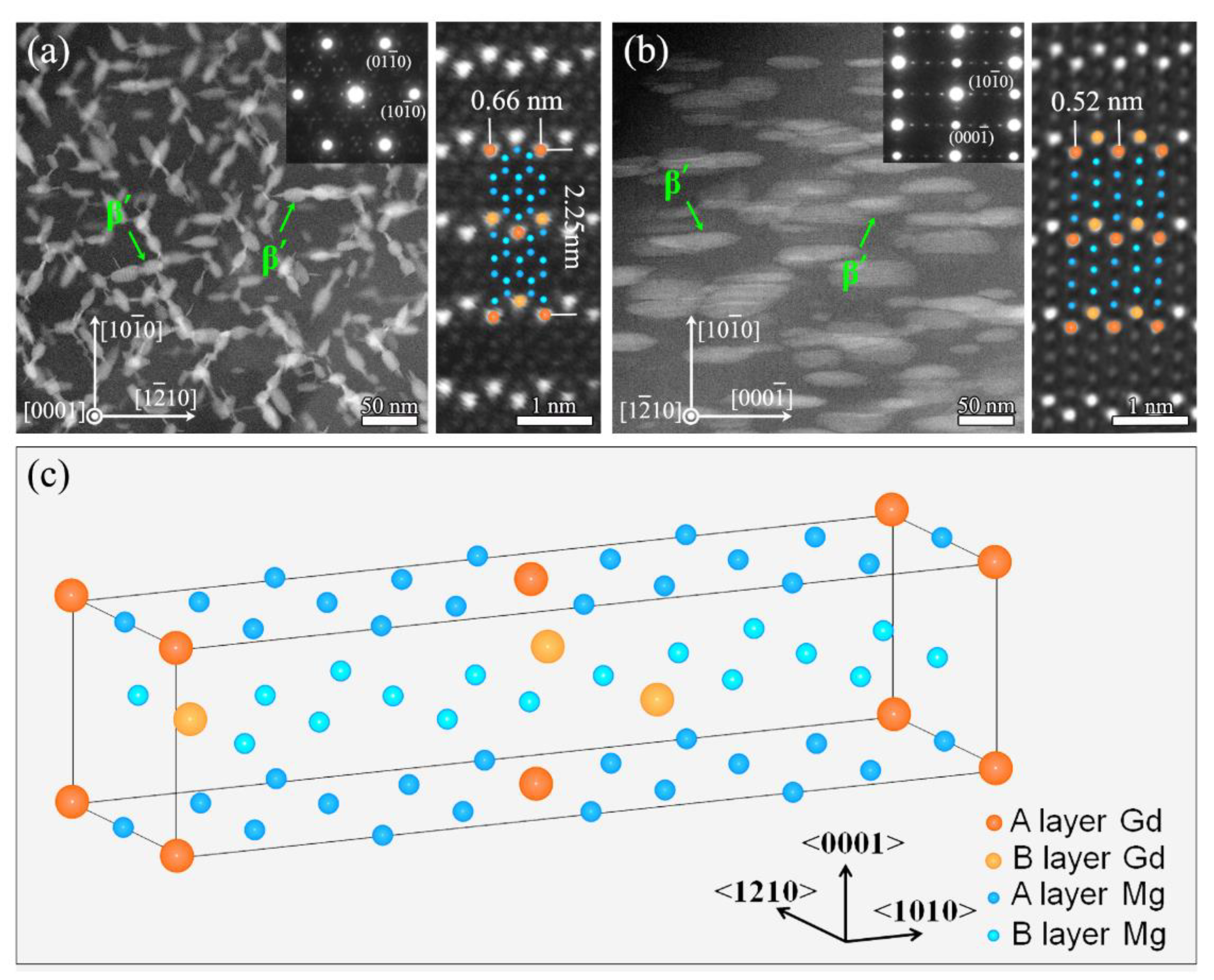
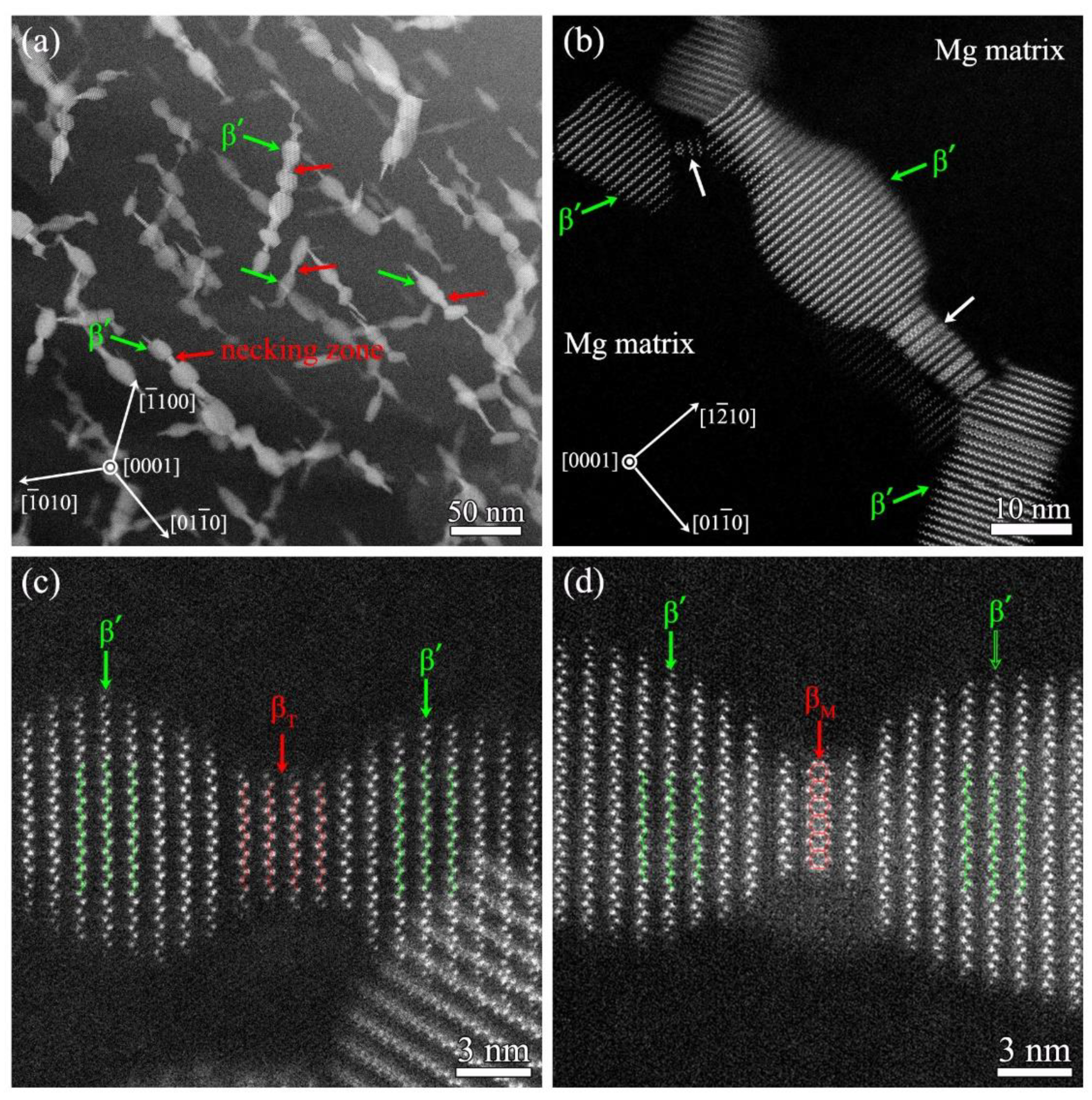
- At the stage of peak aging, a high density of β’ phase is formed and dispersed in grain interior. The three-dimensional structure of β’ phase is an elliptic cylinder. The structure of β’ phase is base-centered orthorhombic (b.c.o) with lattice parameters of a = 0.66 nm, b = 2.25 nm and c = 0.52 nm. According to the unit cell, its composition is confirmed as Mg7Gd;
- The enhanced strength of Mg-10Gd alloy is mainly attributed to the type of precipitations and their densities. It is found that nucleation of precipitation occurs with only 0.5 h of aging. Owing to the continuously increasing growth of the β’ phase during 10–70 h of aging, the strength is enhanced significantly, though this comes at the expense of ductility. The phase transformation of β’ phase occurs at the over-aging stage, which transforms into the intermediate transition phases, including βT and βM phases, leading to a slight decrease of strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joost, W.J.; Krajewski, P.E. Towards magnesium alloys for high-volume automotive applications. Scripta Mater. 2017, 128, 107–112. [Google Scholar] [CrossRef]
- Kiani, M.; Gandikota, I.; Rais-Rohani, M.; Motoyama, K. Design of lightweight magnesium car body structure under crash and vibration constraints. J. Magnes. Alloy. 2014, 2, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Pan, H.C.; Xie, D.S.; Li, J.R.; Xie, H.B.; Huang, Q.Y.; Yang, Q.S.; Qin, G.W. Development of novel lightweight and cost-effective Mg-Ce-Al wrought alloy with high strength. Mater. Res. Lett. 2021, 9, 329–335. [Google Scholar] [CrossRef]
- Wei, K.; Xiao, L.R.; Gao, B.; Li, L.; Liu, Y.; Ding, Z.G.; Liu, W.; Zhou, H.; Zhao, Y.H. Enhancing the strain hardening and ductility of Mg-Y alloy by introducing stacking faults. J. Magnes. Alloy. 2020, 8, 1221–1227. [Google Scholar] [CrossRef]
- Wei, K.; Hu, R.; Yin, D.D.; Xiao, L.R.; Pang, S.; Cao, Y.; Zhou, H.; Zhao, Y.H.; Zhu, Y.T. Grain size effect on tensile properties and slip systems of pure magnesium. Acta Mater. 2021, 206, 116604. [Google Scholar] [CrossRef]
- Lu, J.W.; Yin, D.D.; Huang, G.H.; Quan, G.F.; Zeng, Y.; Zhou, H.; Wang, Q.D. Plastic anisotropy and deformation behavior of extruded Mg-Y sheets at elevated temperatures. Mater. Sci. Eng. A 2017, 700, 598–608. [Google Scholar] [CrossRef]
- Yin, D.D.; Boehlert, C.J.; Long, L.J.; Huang, G.H.; Zhou, H.; Zheng, J.; Wang, Q.D. Tension-compression asymmetry and the underlying slip/twinning activity in extruded Mg–Y sheets. Int. J. Plast. 2021, 136, 102878. [Google Scholar] [CrossRef]
- Ma, L.N.; Xie, K.; Cai, J.; Hemker, K.J. Non-dissociated <c+a> dislocations in an AZ31 alloy revealed by transmission electron microscopy. Mater. Res. Lett. 2020, 81, 45–150. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Wang, Q.D.; Ye, B.; Li, X.C.; Liu, X.T.; Zhou, H. Microstructural refinement and homogenization of Mg-SiC nanocomposites by cyclic extrusion compression. Mate. Sci. Eng. A 2012, 556, 267–270. [Google Scholar] [CrossRef]
- Zhou, H.; Ning, H.Y.; Ma, X.L.; Yin, D.D.; Xiao, L.R.; Sha, X.C.; Yu, Y.D.; Wang, Q.D.; Li, Y.S. Microstructural evolution and mechanical properties of Mg-9.8Gd-2.7Y-0.4Zr alloy produced by repetitive upsetting. J. Mate. Sci. Technol. 2018, 34, 1067–1075. [Google Scholar] [CrossRef]
- Huang, G.H.; Yin, D.D.; Lu, J.W.; Zhou, H.; Zeng, Y.; Quan, G.F.; Wang, Q.D. Microstructure, texture and mechanical properties evolution of extruded fine-grained Mg-Y sheets during annealing. Mater. Sci. Eng. A 2018, 720, 24–35. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, B.; Liao, W.J.; Zhou, H.; Guo, W.; Wang, Q.D.; Jiang, H.Y.; Ding, W.J. Microstructure evolution and mechanical properties of AZ91D magnesium alloy processed by repetitive upsetting. Mater. Sci. Eng. A 2015, 641, 62–70. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Nie, J.F.; Wei, K.; Mao, Q.Z.; Lu, F.H.; Zhao, Y.H. Achieving ultra-strong Magnesium-lithium alloys by low-strain rotary swaging. Mater. Res. Lett. 2021, 9, 255–262. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Q.D.; Liu, M.P.; Peng, T.; Liu, X.T.; Zhou, H. Microstructure and mechanical performance of AZ31-1.7 wt.% Si alloy processed by cyclic channel die compression. Mater. Sci. Forum 2010, 667–669, 457–461. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Q.D.; Li, W.Z.; Zhou, H.; Zhang, L.; Liao, W.J. Enhanced microstructure homogeneity and mechanical properties of AZ91-SiC nanocomposites by cyclic closed-die forging. J. Compos. Mater. 2016, 51, 681–686. [Google Scholar] [CrossRef]
- Qiu, D.; Zhao, P.Y.; Trinkle, D.R.; Wang, Y.Z. Stress-dependent dislocation core structures leading to non-Schmid behavior. Mater. Res. Lett. 2021, 9, 134–140. [Google Scholar] [CrossRef]
- Xiao, L.R.; Cao, Y.; Li, S.; Zhou, H.; Ma, X.L.; Mao, L.; Sha, X.C.; Wang, Q.D.; Zhu, Y.T.; Han, X.D. The formation mechanism of a novel interfacial phase with high thermal stability in a Mg-Gd-Y-Ag-Zr alloy. Acta Mater. 2019, 162, 214–225. [Google Scholar] [CrossRef]
- Ni, R.; Ma, S.J.; Long, L.J.; Zheng, J.; Zhou, H.; Wang, Q.D.; Yin, D.D. Effects of precipitate on the slip activity and plastic heterogeneity of Mg-11Y-5Gd-2Zn-0.5Zr (wt. %) during room temperature compression. Mater. Sci. Eng. A 2021, 804, 140738. [Google Scholar] [CrossRef]
- Li, Z.H.; Sasaki, T.T.; Shiroyama, T.; Miura, A.; Uchida, K.; Hono, K. Simultaneous achievement of high thermal conductivity, high strength and formability in Mg-Zn-Ca-Zr sheet alloy. Mater. Res. Lett. 2020, 8, 335–340. [Google Scholar] [CrossRef]
- Wu, G.H.; Jafari Nodooshan, H.R.; Zeng, X.Q.; Liu, W.C.; Li, D.J.; Ding, W.J. Microstructure and High Temperature Tensile Properties of Mg-10Gd-5Y-0.5Zr Alloy after Thermo-Mechanical Processing. Metals 2018, 8, 980. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.Y.; Han, X.Z.; Zhou, Z.Y.; Wang, Y.L.; Li, X.S.; Wu, J.P. Effects of Microstructure and Texture Evolution on Strength Improvement of an Extruded Mg-10Gd-2Y-0.5Zn-0.3Zr Alloy. Metals 2018, 8, 1087. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.H.; Liu, S.J.; Wu, R.Z.; Hou, L.; Zhang, M.L. Recent developments in high-strength Mg-RE-based alloys: Focusing on Mg-Gd and Mg-Y systems. J. Magnes. Alloy. 2018, 6, 277–291. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, W.Z.; Jian, W.W.; Cheng, G.M.; Ma, X.L.; Guo, W.; Mathaudhu, N.S.; Wang, Q.D.; Zhu, Y.T. A new metastable precipitate phase in Mg-Gd-Y-Zr alloy. Philos. Mag. 2014, 94, 2403–2409. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Mishara, R.S.; Brennan, R.C.; Cho, K. Achieving extraordinary structural efficiency in a wrought magnesium rare earth alloy. Mater. Res. Lett. 2020, 8, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.R.; Chen, X.F.; Ning, H.Y.; Jiang, P.; Liu, Y.; Chen, B.; Yin, D.D.; Zhou, H.; Zhu, Y.T. Unexpected high-temperature brittleness of a Mg-Gd-Y-Ag alloy. J. Magnes. Alloy. 2021, in press. [Google Scholar] [CrossRef]
- Jafari Nodooshan, H.R.; Wu, G.H.; Liu, W.C.; Wei, G.L.; Li, Y.L.; Zhang, S. Effect of Gd content on high temperature mechanical properties of Mg-Gd-Y-Zr alloy. Mater. Sci. Eng. A 2016, 651, 840–847. [Google Scholar] [CrossRef]
- Su, N.; Wu, Y.J.; Deng, Q.; Chang, Z.; Wu, Q.; Xue, Y.; Yang, K.; Chen, Q.; Peng, L. Synergic effects of Gd and Y contents on the age-hardening response and elevated-temperature mechanical properties of extruded Mg-Gd(-Y)-Zn-Mn alloys. Mater. Sci. Eng. A 2021, 810, 141019. [Google Scholar] [CrossRef]
- Mo, N.; McCarroll, I.; Tan, Q.; Ceguerra, A.; Liu, Y.; Cairney, J.; Dieringa, H.; Huang, Y.; Jiang, B.; Pan, F.; et al. Understanding solid solution strengthening at elevated temperatures in a creep-resistant Mg-Gd-Ca alloy. Acta Mater. 2019, 181, 185–199. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.M.; Mahmudi, R. Effects of Gd, Y, and La Rare-Earth Elements on the Microstructural Stability and Elevated-Temperature Mechanical Properties of AZ81 Magnesium Alloy. Metall. Mater. Trans. A 2019, 50, 5957–5968. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Wang, C.; Ju, J.; Sun, J.P.; Wu, Y.N.; Jiang, J.H.; Ma, A.B. Comparative Study of Two Aging Treatments on Microstructure and Mechanical Properties of an Ultra-Fine Grained Mg-10Y-6Gd-1.5Zn-0.5Zr Alloy. Metals 2018, 8, 658. [Google Scholar] [CrossRef] [Green Version]
- Kandalam, S.; Agrawal, P.; Avadhani, G.S.; Kumar, S.; Suwas, S. Precipitation response of the magnesium alloy WE43 in strained and unstrained conditions. J. Alloy. Compd. 2015, 623, 317–323. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.F.; Duan, Y.B.; Wang, K.J.; Wang, Y.T.; Zhang, W.J.; Hu, J. Precipitation processes during the peak-aged and over-aged stages in an Mg-Gd-Y-Zr alloy. J. Alloy. Compd. 2019, 788, 541–548. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, M.Y.; Wu, K.; Wang, E.D.; Fan, G.H.; Xu, S.W.; Kamado, S.; Liu, X.D.; Wang, G.J.; Lv, X.Y. Effect of ageing treatment on the precipitation behaviour of Mg-Gd-Y-Zn-Zr alloy. J. Alloy. Compd. 2013, 550, 50–56. [Google Scholar] [CrossRef]
- Sha, X.C.; Xiao, L.R.; Chen, X.F.; Cheng, G.M.; Yu, Y.D.; Yin, D.D.; Zhou, H. Atomic structure of γ″ phase in Mg-Gd-Y-Ag alloy induced by Ag addition. Philos. Mag. 2019, 99, 1957–1969. [Google Scholar] [CrossRef]
- Li, R.G.; Nie, J.F.; Huang, G.J.; Xin, Y.C.; Liu, Q. Development of high-strength magnesium alloys via combined processes of extrusion, rolling and ageing. Scripta Mater. 2011, 64, 950–953. [Google Scholar] [CrossRef]
- He, S.M.; Zeng, X.Q.; Peng, L.M.; Gao, X.; Nie, J.F.; Ding, W.J. Precipitation in a Mg-10Gd-3Y-0.4Zr (wt.%) alloy during isothermal ageing at 250 °C. J. Alloy. Compd. 2006, 421, 309–313. [Google Scholar] [CrossRef]
- Nie, J.F. Precipitation and Hardening in Magnesium Alloys. Metall. Mater. Trans. A. 2021, 43, 3891–3939. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.F. Effects of precipitate shape and orientation on dispersion strengthening in magnesium alloys. Scripta Mater. 2003, 48, 1009–1015. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, T.W.; Fu, L.; Tang, B.Y.; Peng, L.M.; Ding, W.J. Ab-initio study of the effect of rare-earth elements on the stacking faults of Mg solid solutions. Intermetallics 2012, 29, 21–26. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Qian, Q.; Jiang, Y.; Wang, Y.R.; Zhu, Y.M.; Nie, J.F. Incoherent tilt grain boundaries stabilized by stacking faults and solute-cluster segregation: A case-study of an Mg-Gd alloy. Mater. Res. Lett. 2020, 8, 268–274. [Google Scholar] [CrossRef]
- Sun, W.W.; Zhu, Y.M.; Marceau, R.; Wang, L.Y.; Zhang, Q.; Gao, X.; Hutchinson, C. Precipitation strengthening of aluminum alloys by room-temperature cyclic plasticity. Science 2019, 363, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Antion, C.; Donnadieu, P.; Perrard, F.; Deschamps, A.; Tassin, C.; Pisch, A. Hardening precipitation in a Mg-4Y-3RE alloy. Acta Mater. 2003, 51, 5335–5348. [Google Scholar] [CrossRef]
- Zheng, J.X.; Li, Z.; Tan, L.D.; Xu, X.S.; Luo, R.C.; Chen, B. Precipitation in Mg-Gd-Y-Zr Alloy: Atomic-scale insights into structures and transformations. Mater. Charact. 2016, 117, 76–83. [Google Scholar] [CrossRef]
| Aging Time | Size Along Short Axis (dt, nm) | Size Along Long Axis (lt, nm) | Size Along <0001> Direction (nm) |
|---|---|---|---|
| 10 h | 5.3 | 5.81 | 12.83 |
| 30 h | 11.46 | 7.58 | 28.18 |
| 50 h | 13.72 | 8.1 | 31.83 |
| 70 h | 16.97 | 9.1 | 45.69 |
| 200 h | 16.64 | 10.68 | 48.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Song, Y.; Li, N.; Sha, X.; Xu, M.; Chen, B.; Gao, B.; Xiao, L.; Zhou, H. Mechanical Properties and Microstructure Evolution of Mg-Gd Alloy during Aging Treatment. Metals 2022, 12, 39. https://doi.org/10.3390/met12010039
Liu Y, Song Y, Li N, Sha X, Xu M, Chen B, Gao B, Xiao L, Zhou H. Mechanical Properties and Microstructure Evolution of Mg-Gd Alloy during Aging Treatment. Metals. 2022; 12(1):39. https://doi.org/10.3390/met12010039
Chicago/Turabian StyleLiu, Yi, Yang Song, Na Li, Xuechao Sha, Mengning Xu, Bin Chen, Bo Gao, Lirong Xiao, and Hao Zhou. 2022. "Mechanical Properties and Microstructure Evolution of Mg-Gd Alloy during Aging Treatment" Metals 12, no. 1: 39. https://doi.org/10.3390/met12010039
APA StyleLiu, Y., Song, Y., Li, N., Sha, X., Xu, M., Chen, B., Gao, B., Xiao, L., & Zhou, H. (2022). Mechanical Properties and Microstructure Evolution of Mg-Gd Alloy during Aging Treatment. Metals, 12(1), 39. https://doi.org/10.3390/met12010039







