Modification of Biocorrosion and Cellular Response of Magnesium Alloy WE43 by Multiaxial Deformation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Investigated
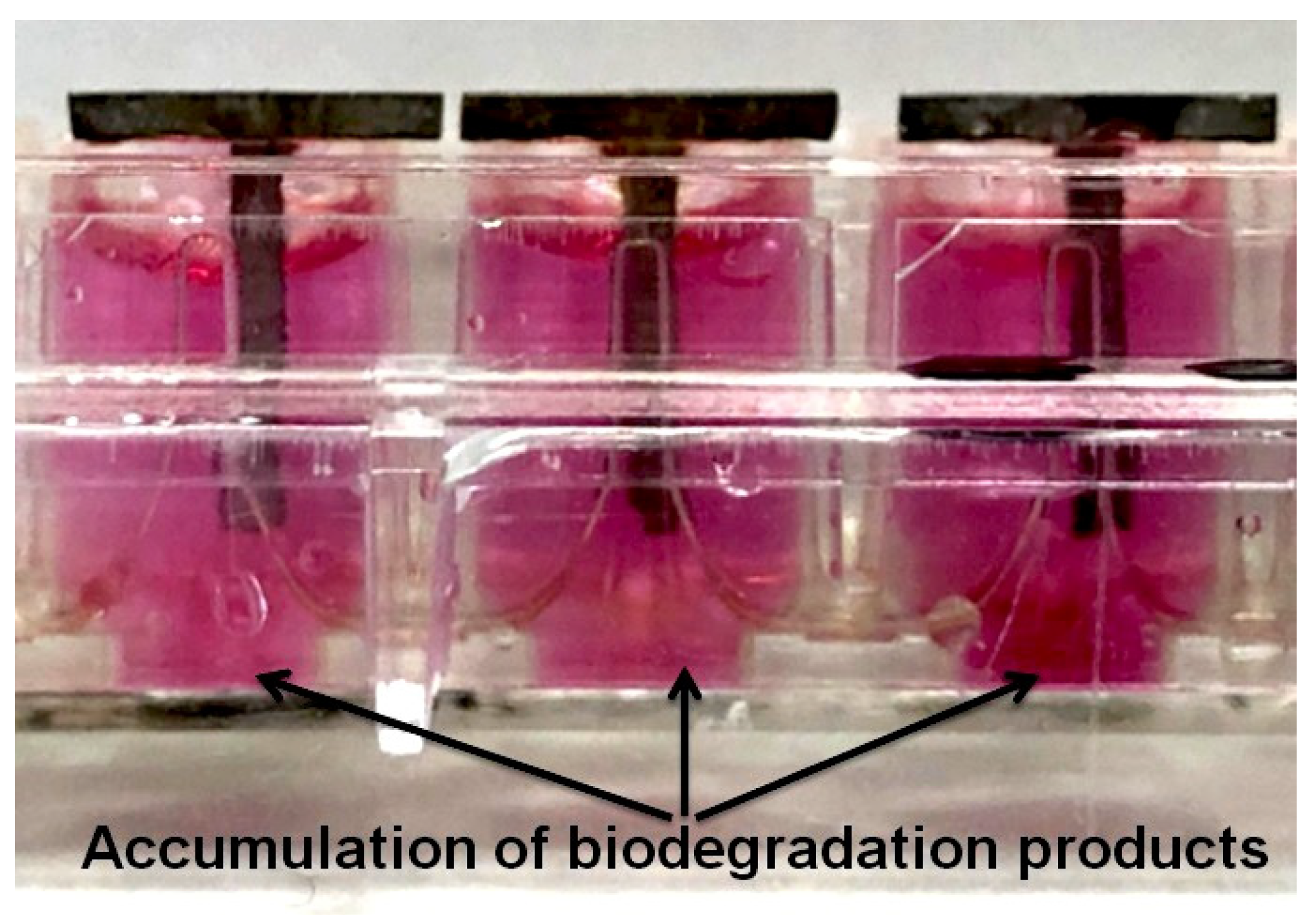
2.2. Accumulation of Biodegradation Products in a Bioactive Medium
2.3. pH Variation in the Immersion Medium
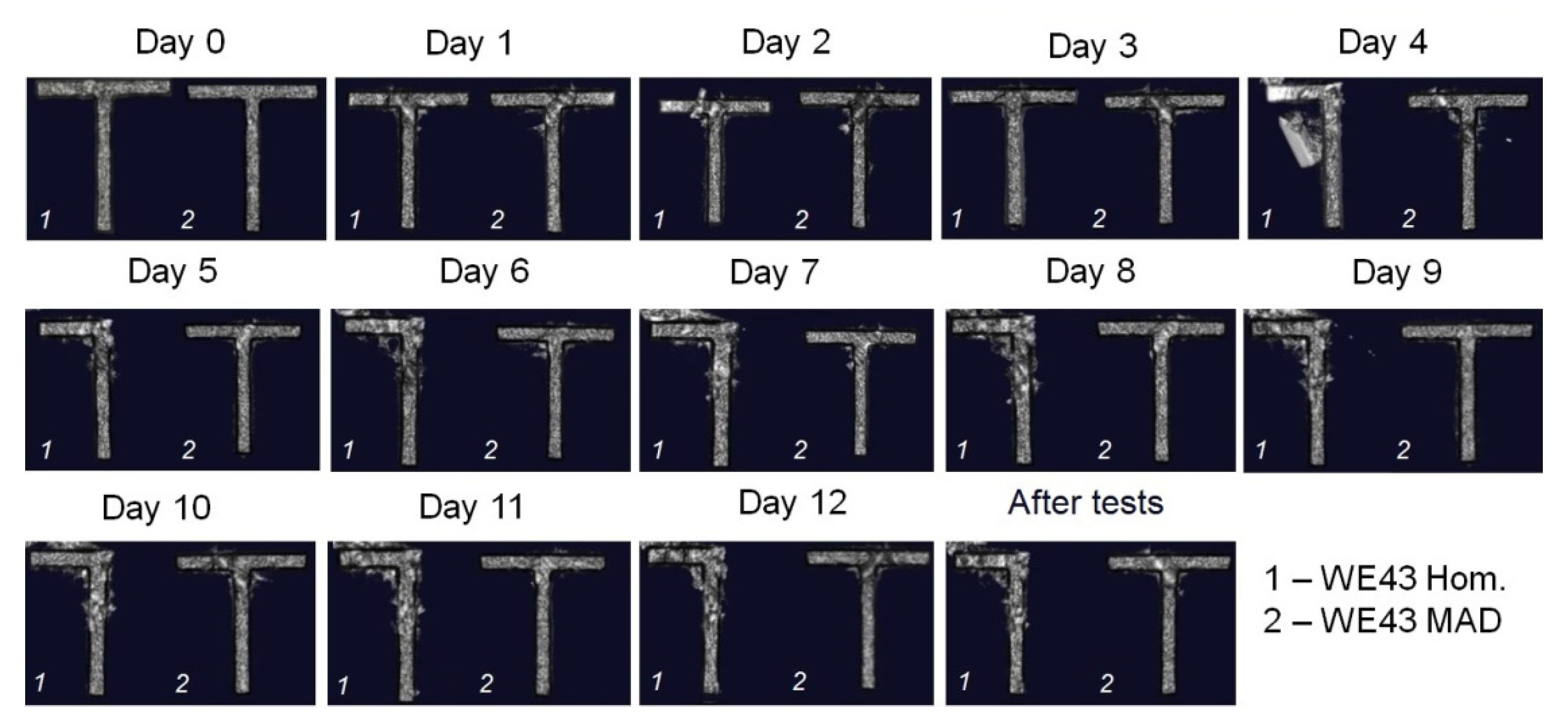
2.4. Volume CT Scan and Magnitude of Hounsfield Units
2.5. Mass Loss after Incubation in FBS and in the Complete Growth Medium
2.6. Cytotoxicity, Cell Viability and Proliferation
2.7. Statistical Analysis
3. Results
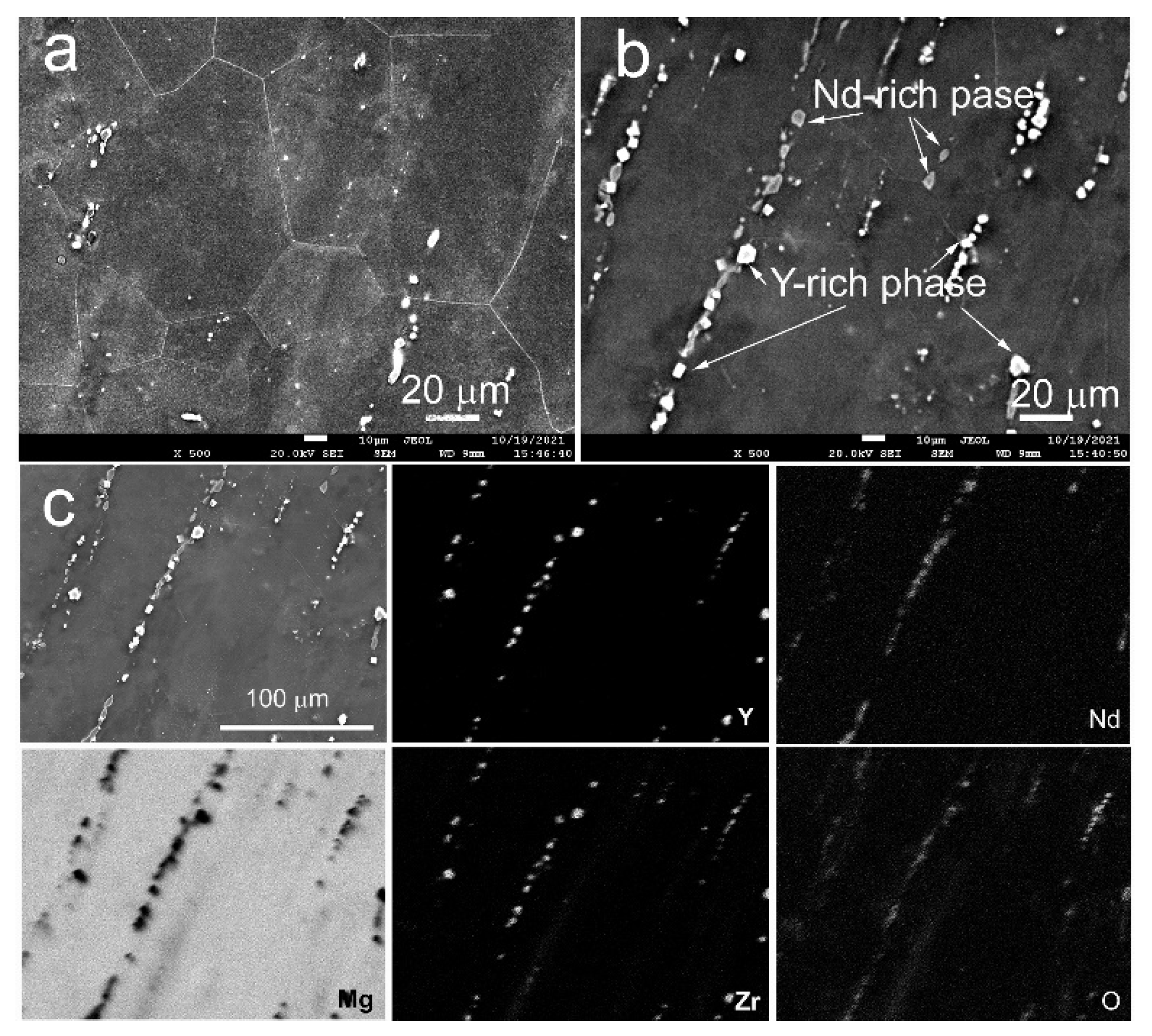
3.1. Study of the Microstructure
3.2. Accumulation of Biodegradation Products in a Bioactive Medium
3.3. Variation in pH of Incubation Medium
3.4. Volume CT Scan and Magnitude of Hounsfield Units in FBS In Vitro
3.5. Study of Alloy Degradation Products
3.6. Mass Loss after Incubation in FBS and DMEM
3.7. Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, J.; Siegel, N.; Reategui, A.; Faateh, M.; Manson, P.N.; Redett, R.J. Absorbable Fixation Devices for Pediatric Craniomaxillofacial Trauma: A Systematic Review of the Literature. Plast. Reconstr. Surg. 2019, 144, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J. Bioabsorbable polymers in cancer therapy: Latest developments. EPMA J. 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Sukegaw, S.; Furuki, Y.; Nariai, Y.; Sekin, J. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Gupta, A.; Grevious, M.; Reid, R.R. Use of resorbable implants for mandibular fixation: A systematic review. J. Craniofac. Surg. 2009, 20, 331–339. [Google Scholar] [CrossRef]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable Osteofixation Materials for Maxillofacial Bone Surgery: A Review on Polymers and Magnesium-Based Materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; de Visscher, J.G.A.M.; Hoppenreijs, T.J.M.; Bergsma, J.E.; van Minnen, B.; Stegenga, B.; Bos, R.R.M. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: A multicenter randomized controlled trial. PLoS ONE 2017, 12, e0177152. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; van Bakelen, N.B.; Dijkstra, P.U.; Vissink, A.; Bos, R.R.M.; van Minnen, B. Biodegradable versus titanium osteosynthesis in maxillofacial traumatology: A systematic review with meta-analysis and trial sequential analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, N.; Betts, L.; Zhu, D. Bio-Adaption between Magnesium Alloy Stent and the Blood Vessel: A Review. J. Mater. Sci. Technol. 2016, 32, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.Y.; Kwok, J.S.; Nguyen, C.T.; Fox, K. Evaluating magnesium alloy WE43 for bioresorbable coronary stent applications. MRS Adv. 2021, 6, 54–60. [Google Scholar] [CrossRef]
- Oshibe, N.; Marukawa, E.; Yoda, T.; Harada, H. Degradation and interaction with bone of magnesium alloy WE43 implants: A long-term follow-up in vivo rat tibia study. J. Biomater. Appl. 2019, 33, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Dobatkin, S.; Martynenko, N.; Anisimova, N.; Kiselevskiy, M.; Prosvirnin, D.; Terentiev, V.; Yurchenko, N.; Salishchev, G.; Estrin, Y. Mechanical Properties, Biodegradation, and Biocompatibility of Ultrafine Grained Magnesium Alloy WE43. Materials 2019, 12, 3627. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, N.; Lukyanova, E.; Anisimova, N.; Kiselevskiy, M.; Serebryany, V.; Yurchenko, N.; Raab, G.; Birbilis, N.; Salishchev, G.; Dobatkin, S.; et al. Improving the property profile of a bioresorbable Mg-Y-Nd-Zr alloy by deformation treatments. Materialia 2020, 13, 100841. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, C.O.; Torres-García, S.E.; Olvera-Sandoval, C.; Ramírez-Castillo, F.Y.; Muro, A.L.; Avelar-Gonzalez, F.J.; Guerrero-Barrera, A.L. Cell culture: History, development and prospects. Int. J. Curr. Res. Aca. Rev. 2014, 2, 188–200. [Google Scholar]
- Johnson, I.; Liu, H. A study on factors affecting the degradation of magnesium and a magnesium-yttrium alloy for biomedical applications. PLoS ONE 2013, 8, e65603. [Google Scholar] [CrossRef] [PubMed]
- Janning, C.; Willbold, E.; Vogt, C.; Nellesen, J.; Meyer-Lindenberg, A.; Windhagen, H.; Thorey, F.; Witte, F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010, 6, 1861–1868. [Google Scholar] [CrossRef]
- Cooper, S.L.; Peppas, N.A. Biomaterials, Interfacial Phenomena and Applications.; American Chemical Society: Washington, DC, USA, 1982. [Google Scholar]
- Liu, C.-L.; Zhang, Y.; Zhang, C.-Y.; Wang, W.; Huang, W.-J.; Chu, P.K. Synergistic effect of chloride ion and albumin on the corrosion of pure magnesium. Front. Mater. Sci. 2014, 8, 244–255. [Google Scholar] [CrossRef]
- Hedberg, Y.; Wang, X.; Hedberg, J.; Lundin, M.; Blomberg, E.; Odnevall Wallinder, I. Surface-protein interactions on different stainless steel grades: Effects of protein adsorption, surface changes and metal release. J. Mater. Sci. Mater. Med. 2013, 24, 1015–1033. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.V.; Muñoz, A.I. Electrochemical characterisation of biomedical alloys for surgical implants in simulated body fluids. Corros. Sci. 2008, 50, 1954–1961. [Google Scholar] [CrossRef]
- Bamford, C.; Cooper, S.L.; Tsurutta, T. The Vroman Effect; ASC Publications: Washington, DC, USA, 1992. [Google Scholar]
- Heimke, G. Osseo-Integrated Implants; CRC Press: Boca Raton, FL, USA, 1990; Volume 2. [Google Scholar]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Parfenov, E.V.; Kulyasova, O.B.; Mukaeva, V.R.; Mingo, B.; Farrakhov, R.G.; Cherneikina, Y.V.; Yerokhin, A.; Zheng, Y.F.; Valiev, R.Z. Influence of ultra-fine grain structure on corrosion behaviour of biodegradable Mg-1Ca alloy. Corros. Sci. 2020, 163, 108303. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, A.; Jiang, J.; Xu, H.; Song, D.; Lu, F.; Nishida, Y. Enhanced biodegradation behavior of ultrafine-grained ZE41A magnesium alloy in Hank’s solution. Prog. Nat. Sci. Mater. 2013, 23, 420–424. [Google Scholar] [CrossRef][Green Version]
- Jiang, J.; Zhang, F.; Ma, A.; Song, D.; Chen, J.; Liu, H.; Qiang, M. Biodegradable Behaviors of Ultrafine-Grained ZE41A Magnesium Alloy in DMEM Solution. Metals 2016, 6, 3. [Google Scholar] [CrossRef]
- Hosaka, T.; Yoshihara, S.; Amanina, I.; MacDonald, B.J. Influence of Grain Refinement and Residual Stress on Corrosion Behavior of AZ31 Magnesium Alloy Processed by ECAP in RPMI-1640 Medium. Procedia Eng. 2017, 184, 432–441. [Google Scholar] [CrossRef]
- Kavyani, M.; Ebrahimi, G.R.; Ezatpour, H.R.; Jahazi, M. Microstructure refinement, mechanical and biocorrosion properties of Mg–Zn–Ca–Mn alloy improved by a new severe plastic deformation process. J. Magnes. Alloys 2021. [Google Scholar] [CrossRef]
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magnes. Alloys 2021. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Martynenko, N.S.; Anisimova, N.Y.; Temralieva, D.R.; Kiselevskiy, M.V.; Morozov, M.M.; Yusupov, V.S.; Dobatkin, S.V.; Estrin, Y.Z. Effect of rotary swaging and subsequent aging on the implant-relevant properties of magnesium alloy WE43. J. Phys. Conf. Ser. 2020, 1688, 012006. [Google Scholar] [CrossRef]
- Barinov, S.M.; Komlev, V.S. Calcium Phosphate Bone Cements. Inorg. Mater. 2011, 47, 1470–1485. [Google Scholar] [CrossRef]
- Bohner, M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cells Mater. 2010, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.A.; Krohicheva, P.A.; Fomin, A.S.; Khairutdinova, D.R.; Antonova, O.S.; Baikin, A.S.; Smirnov, V.V.; Fomina, A.A.; Leonov, A.V.; Mikheev, I.V.; et al. In situ magnesium calcium phosphate cements formation: From one pot powders precursors synthesis to in vitro investigations. Bioact. Mater. 2020, 5, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Mestres, G.; Ginebra, M.-P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Kreczy, D.; Brückner, T.; Gbureck, U.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; Fuchs, A. Development and Bone Regeneration Capacity of Premixed Magnesium Phosphate Cement Pastes. Materials 2019, 12, 2119. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Lazoryak, B.I.; Davidova, G.A.; Murzakhanov, F.F.; Gabbasov, B.F.; Petrakova, N.V.; Fosca, M.; Barinov, S.M.; Vadalà, G.; Uskoković, V.; et al. Antibacterial and cell-friendly copper-substituted tricalcium phosphate ceramics for biomedical implant applications. Mater. Sci. Eng. C 2021, 129, 112410. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anisimova, N.; Martynenko, N.; Novruzov, K.; Rybalchenko, O.; Kiselevskiy, M.; Rybalchenko, G.; Straumal, B.; Salishchev, G.; Mansharipova, A.; Kabiyeva, A.; et al. Modification of Biocorrosion and Cellular Response of Magnesium Alloy WE43 by Multiaxial Deformation. Metals 2022, 12, 105. https://doi.org/10.3390/met12010105
Anisimova N, Martynenko N, Novruzov K, Rybalchenko O, Kiselevskiy M, Rybalchenko G, Straumal B, Salishchev G, Mansharipova A, Kabiyeva A, et al. Modification of Biocorrosion and Cellular Response of Magnesium Alloy WE43 by Multiaxial Deformation. Metals. 2022; 12(1):105. https://doi.org/10.3390/met12010105
Chicago/Turabian StyleAnisimova, Natalia, Natalia Martynenko, Keryam Novruzov, Olga Rybalchenko, Mikhail Kiselevskiy, Georgy Rybalchenko, Boris Straumal, Gennady Salishchev, Almagul Mansharipova, Aigul Kabiyeva, and et al. 2022. "Modification of Biocorrosion and Cellular Response of Magnesium Alloy WE43 by Multiaxial Deformation" Metals 12, no. 1: 105. https://doi.org/10.3390/met12010105
APA StyleAnisimova, N., Martynenko, N., Novruzov, K., Rybalchenko, O., Kiselevskiy, M., Rybalchenko, G., Straumal, B., Salishchev, G., Mansharipova, A., Kabiyeva, A., Gabdullin, M., Dobatkin, S., & Estrin, Y. (2022). Modification of Biocorrosion and Cellular Response of Magnesium Alloy WE43 by Multiaxial Deformation. Metals, 12(1), 105. https://doi.org/10.3390/met12010105








