Effects of Process Control Agent Amount, Milling Time, and Annealing Heat Treatment on the Microstructure of AlCrCuFeNi High-Entropy Alloy Synthesized through Mechanical Alloying
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
3.1. Thermodynamics of the Alloy
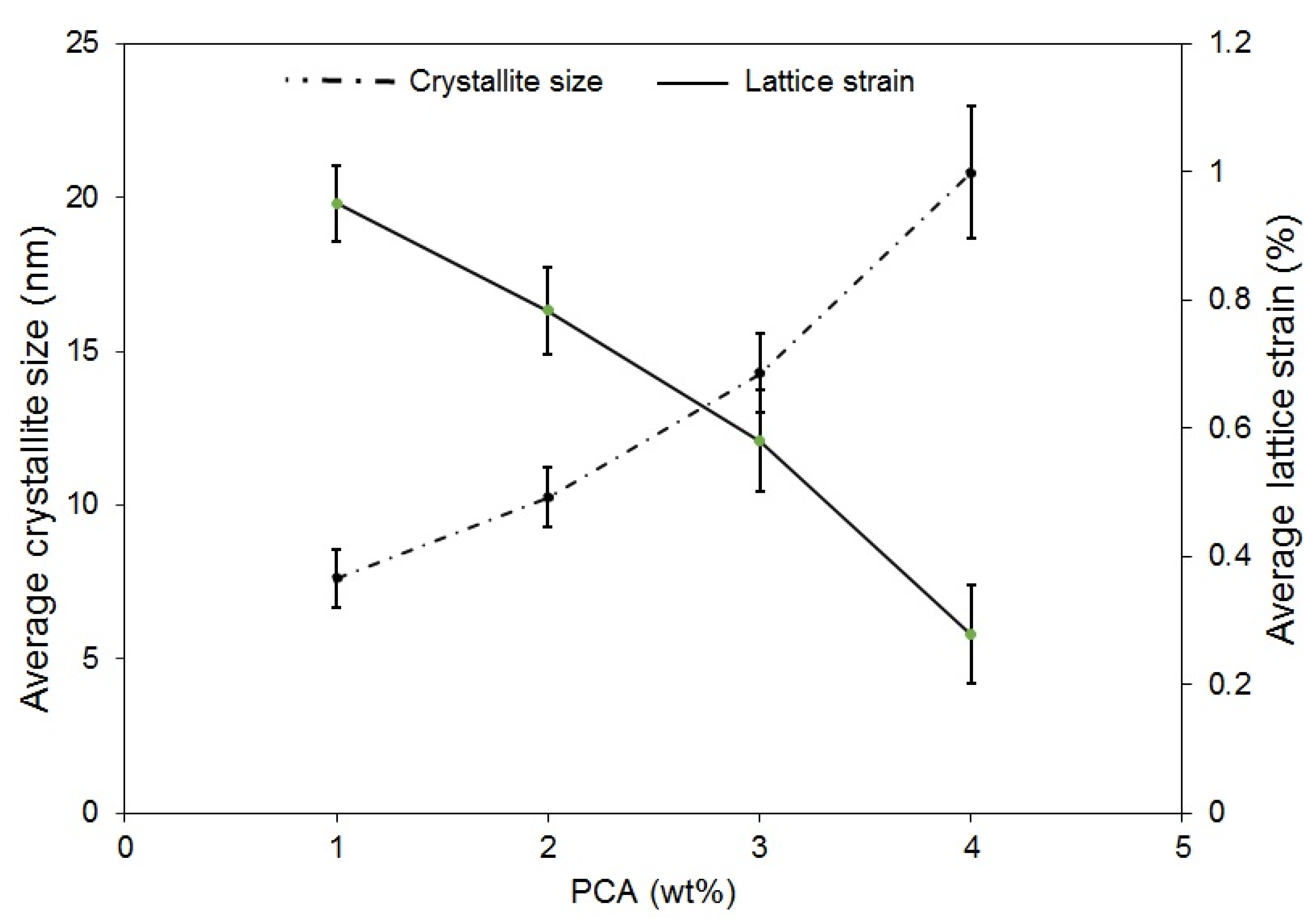
3.2. Effect of Process Control Agent Amount
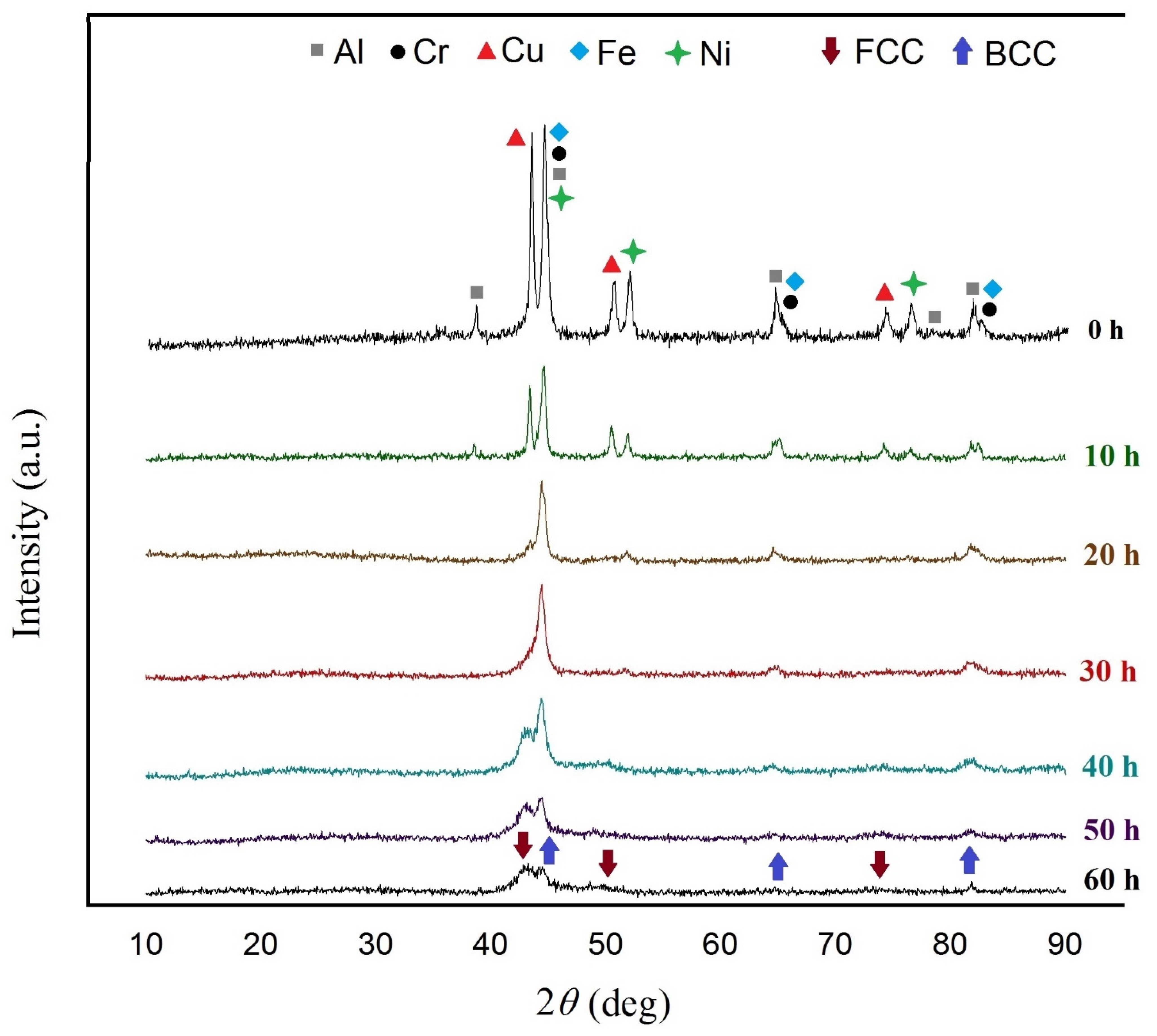
3.3. Synthesis of AlCrCuFeNi Alloy with 2 wt. % of Process Control Agent
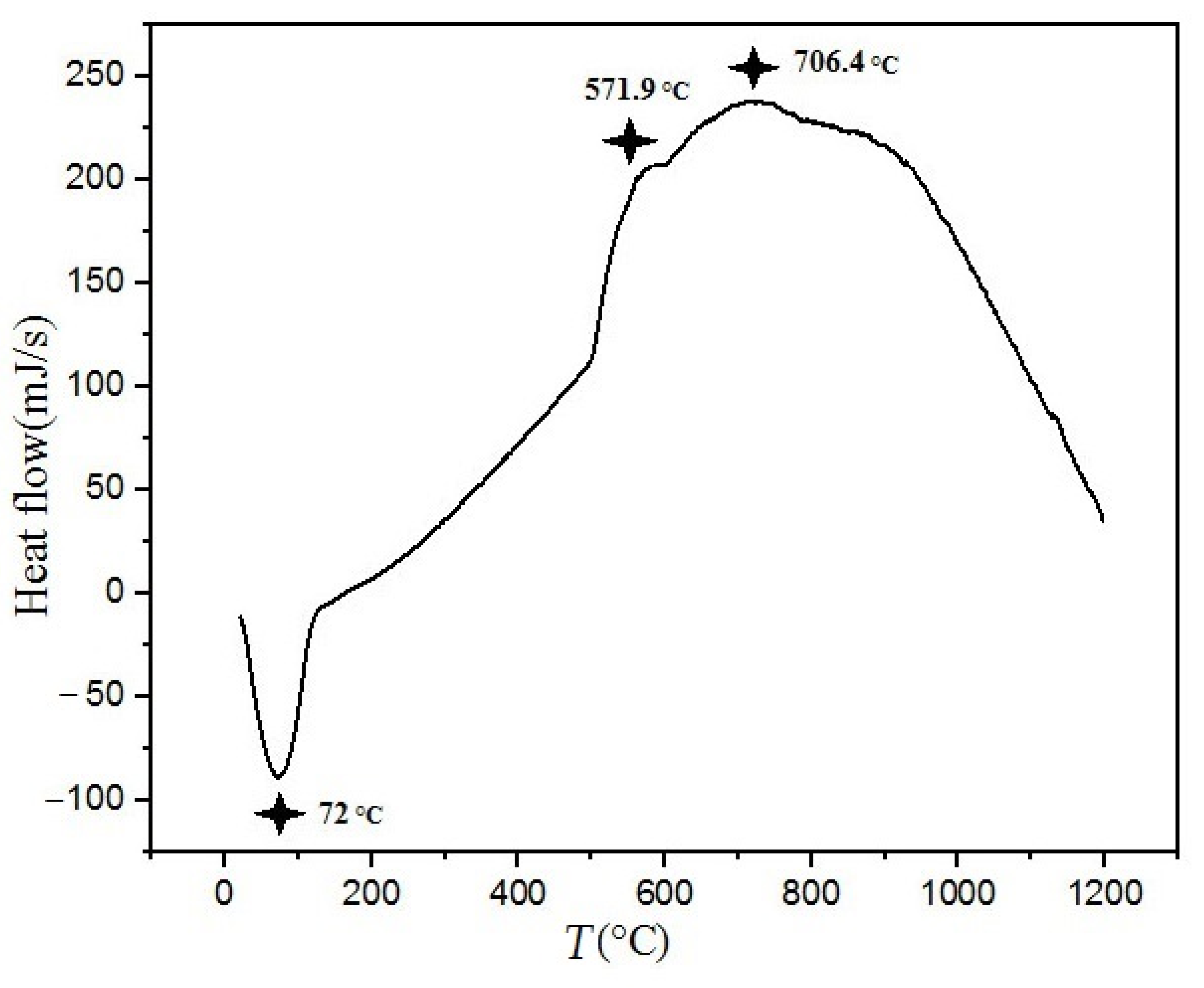
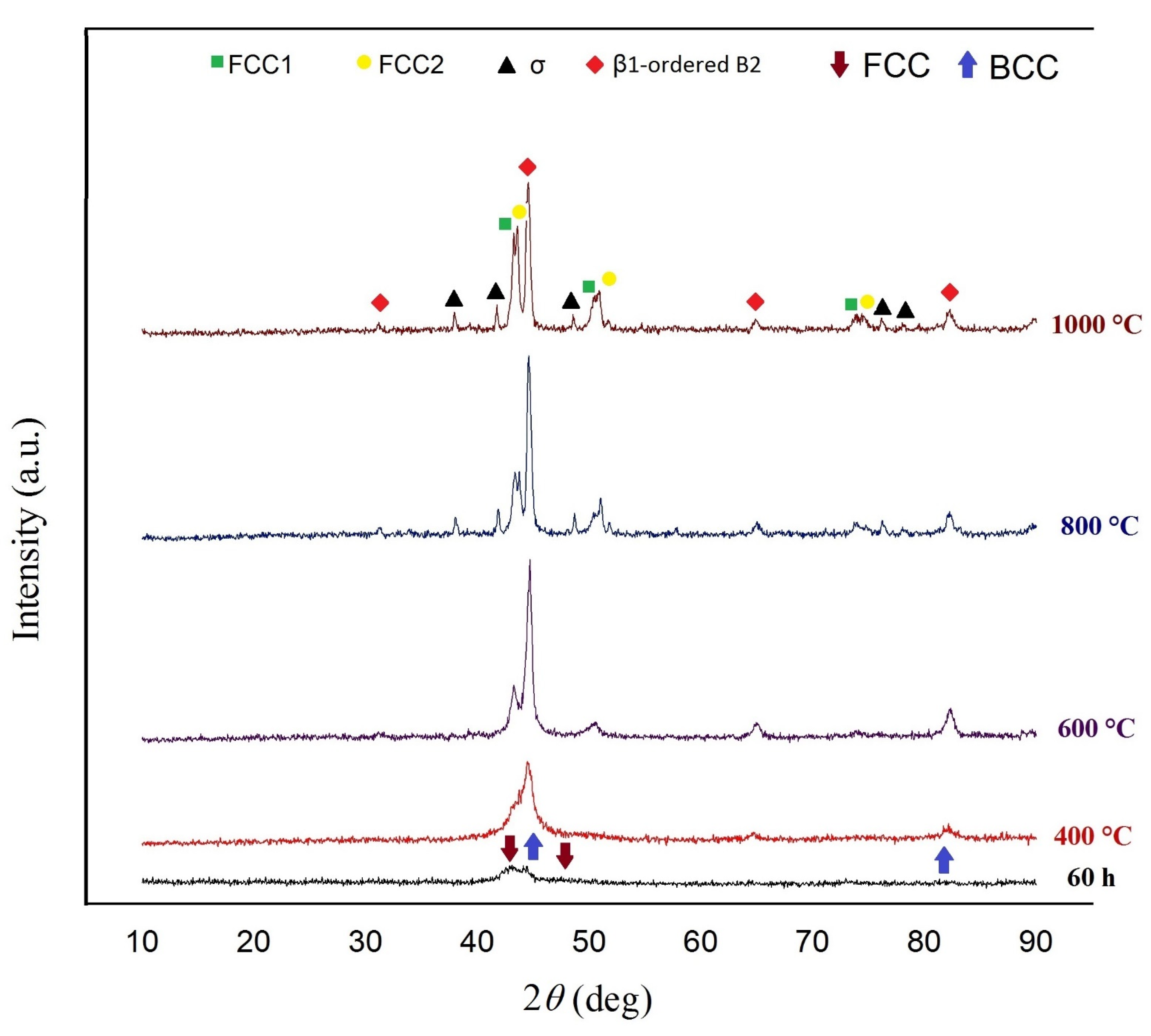
3.4. Thermal Behavior of the Alloy
4. Conclusions
- Thermodynamic measurements predict the formation of a dual-phase HEA in the AlCrCuFeNi alloying system, which was achieved after 60 h of milling including an FCC and a BCC solid solution phase.
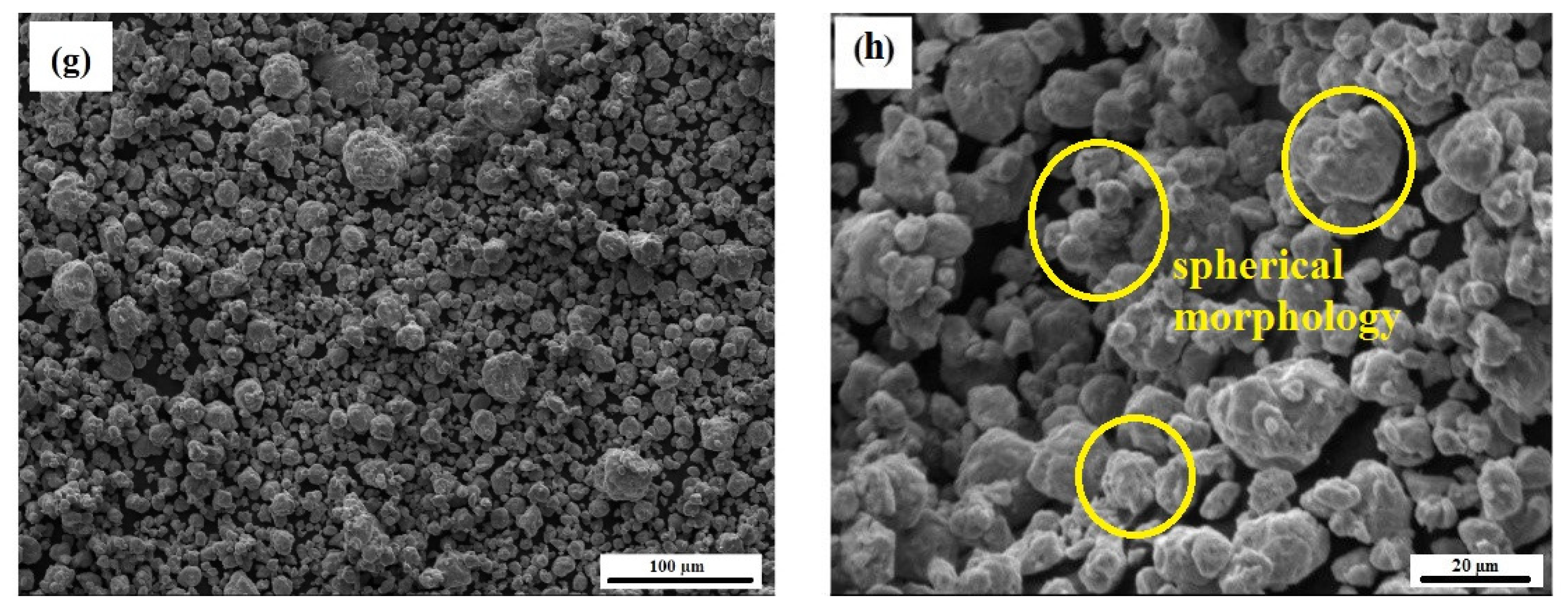
- It was seen that increasing the amount of PCA can postpone the alloying process during MA and lead to a decrease in lattice strain and an increase in crystallite size. The morphology of particles also changed from a spherical shape to a plate-like shape with increase in PCA amount.
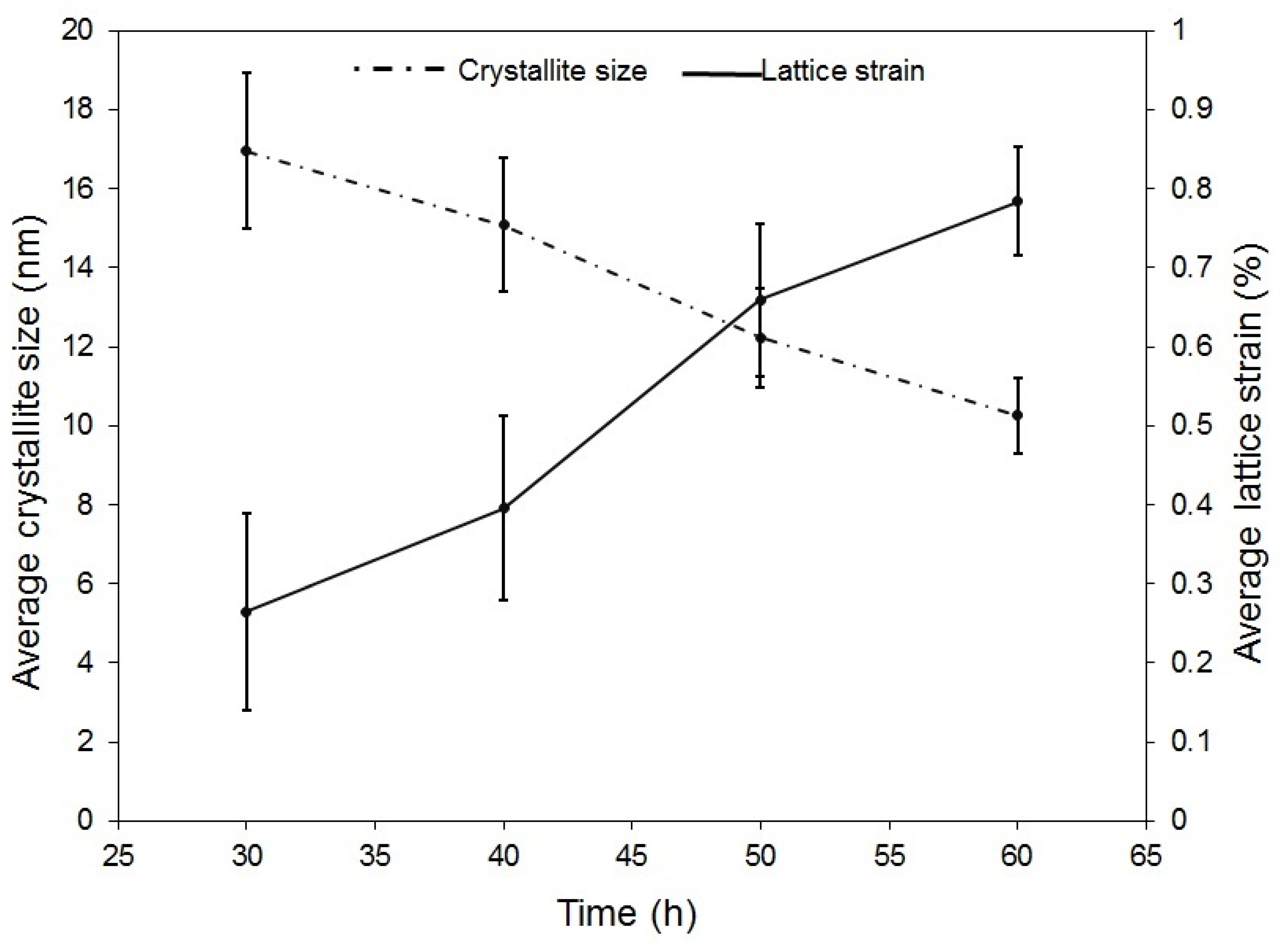
- Investigation of milling time revealed that with increase in milling time, lattice strain declines and crystallite size increases, and a 60-h-milling time was needed for achieving the final dual-phase HEA.
- Thermal analysis revealed phase stability up to 400 °C. At 600 °C, a sigma phase (σ), and a B2-ordered solid solution formed in the alloy. In addition, with the increasing of the heat treatment temperature to 800 °C the FCC phase decomposed into two new FCC phases.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tsai, M.-H.; Yeh, J.-W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Shabani, A.; Toroghinejad, M.R. Investigation of microstructure, texture, and mechanical properties of FeCrCuMnNi multiphase high entropy alloy during recrystallization. Mater. Charact. 2019, 154, 253–263. [Google Scholar] [CrossRef]
- Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F. Mechanical alloying synthesis and spark plasma sintering consolidation of CoCrFeNiAl high-entropy alloy. J. Alloy Compd. 2014, 589, 61–66. [Google Scholar] [CrossRef]
- Shabani, A.; Toroghinejad, M.R. Evaluation of microstructure and texture formation during annealing of cold-rolled FeCrCuMnNi multiphase high-entropy alloy. Trans. Nonferrous Met. Soc. China 2020, 30, 449–462. [Google Scholar] [CrossRef]
- Shabani, A.; Toroghinejad, M.R.; Shafyei, A.; Logé, R.E. Microstructure and mechanical properties of a multiphase FeCrCuMnNi high-entropy alloy. J. Mater. Eng. Perform. 2019, 28, 2388–2398. [Google Scholar] [CrossRef]
- Praveen, S.; Murty, B.; Kottada, R.S. Alloying behavior in multi-component AlCoCrCuFe and NiCoCrCuFe high entropy alloys. Mater. Sci. Eng. A 2012, 534, 83–89. [Google Scholar] [CrossRef]
- Kumar, A.; Arora, A.; Chandrakar, R.; Rao, K.R.; Chopkar, M. Nano-crystalline high entropy alloys prepared by mechanical alloying. Mater. Today Proc. 2020, 27, 1310–1314. [Google Scholar] [CrossRef]
- Shivam, V.; Shadangi, Y.; Basu, J.; Mukhopadhyay, N. Evolution of phases, hardness and magnetic properties of AlCoCrFeNi high entropy alloy processed by mechanical alloying. J. Alloy Compd. 2020, 832, 154826. [Google Scholar] [CrossRef]
- Qiao, Y.; Tang, Y.; Li, S.; Ye, Y.; Liu, X.; Bai, S. Preparation of TiZrNbTa refractory high-entropy alloy powder by mechanical alloying with liquid process control agents. Intermetallics 2020, 126, 106900. [Google Scholar] [CrossRef]
- Singh, N.; Shadangi, Y.; Shivam, V.; Mukhopadhyay, N.K. MgAlSiCrFeNi low-density high entropy alloy processed by mechanical alloying and spark plasma sintering: Effect on phase evolution and thermal stability. J. Alloy Compd. 2021, 875, 159923. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Kamaraj, M.; Murty, B. Synthesis and characterization of nanocrystalline AlFeTiCrZnCu high entropy solid solution by mechanical alloying. J. Alloy Compd. 2008, 460, 253–257. [Google Scholar] [CrossRef]
- Yurkova, A.I.; Cherniavsky, V.; Bolbut, V.; Krüger, M.; Bogomol, I. Structure formation and mechanical properties of the high-entropy AlCuNiFeCr alloy prepared by mechanical alloying and spark plasma sintering. J. Alloy Compd. 2019, 786, 139–148. [Google Scholar] [CrossRef]
- Thangaraju, S.; Bouzy, E.; Hazotte, A. Phase stability of a mechanically alloyed CoCrCuFeNi high entropy alloy. Adv. Eng. Mater. 2017, 19, 1700095. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, M.-R.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Chang, S.-Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Canakci, A.; Varol, T.; Nazik, C. Effects of amount of methanol on characteristics of mechanically alloyed Al–Al2O3 composite powders. Mater. Technol. 2012, 27, 320–327. [Google Scholar] [CrossRef]
- Long, B.; Zuhailawati, H.; Umemoto, M.; Todaka, Y.; Othman, R. Effect of ethanol on the formation and properties of a Cu–NbC composite. J. Alloy Compd. 2010, 503, 228–232. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Maurice, D.; Courtney, T. Modeling of mechanical alloying: Part I. deformation, coalescence, bdand fragmentation mechanisms. Metall. Mater. Trans. A 1994, 25, 147–158. [Google Scholar] [CrossRef]
- Shaw, L.; Villegas, J.; Luo, H.; Zawrah, M.; Miracle, D. Effects of process-control agents on mechanical alloying of nanostructured aluminum alloys. Metall. Mater. Trans. A 2003, 34, 159–170. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.; Wen, C. Effect of process control agent on the porous structure and mechanical properties of a biomedical Ti–Sn–Nb alloy produced by powder metallurgy. Acta Biomater. 2010, 6, 1630–1639. [Google Scholar] [CrossRef]
- Duan, Y.; Pang, H.; Wen, X.; Zhang, X.; Wang, T. Microwave absorption performance of FeCoNiAlCr0.9 alloy powders by adjusting the amount of process control agent. J. Mater. Sci. Technol. 2021, 77, 209–216. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Y. Influence of process control agent on interdiffusion between Al and Mg during mechanical alloying. J. Alloy Compd. 1999, 290, 279–283. [Google Scholar] [CrossRef]
- Shabani, A.; Toroghinejad, M.R.; Shafyei, A.; Logé, R.E. Evaluation of the mechanical properties of the heat treated FeCrCuMnNi high entropy alloy. Mater. Chem. Phys. 2019, 221, 68–77. [Google Scholar] [CrossRef]
- Shabani, A.; Toroghinejad, M.R.; Aminaei, M. Effect of prior cold deformation on recrystallization behavior of a multi-phase FeCrCuMnNi high entropy alloy. Mater. Chem. Phys. 2021, 272, 124991. [Google Scholar] [CrossRef]
- Koundinya, N.; Babu, C.S.; Sivaprasad, K.; Susila, P.; Babu, N.K.; Baburao, J. Phase evolution and thermal analysis of nanocrystalline AlCrCuFeNiZn high entropy alloy produced by mechanical alloying. J. Mater. Eng. Perform. 2013, 22, 3077–3084. [Google Scholar] [CrossRef]
- Guo, S. Phase selection rules for cast high entropy alloys: An overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Tan, X.-R.; Zhang, G.-P.; Zhi, Q.; Liu, Z.-X. Effects of milling on the microstructure and hardness of Al2NbTi3V2Zr high-entropy alloy. Mater. Des. 2016, 109, 27–36. [Google Scholar] [CrossRef]
- Murali, M.; Kumaresh Babu, S.; Majhi, J.; Vallimanalan, A.; Mahendran, R. Processing and characterisation of nano crystalline AlCoCrCuFeTix high-entropy alloy. Powder Metall. 2018, 61, 139–148. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Z.; Zhang, J.; Shi, J.; Wang, W.; Wang, H.; Wang, Y.; Zhang, Q. Nanocrystalline CoCrFeNiCuAl high-entropy solid solution synthesized by mechanical alloying. J. Alloy Compd. 2009, 485, L31–L34. [Google Scholar] [CrossRef]
- Shabani, A.; Toroghinejad, M.R.; Shafyei, A.; Cavaliere, P. Effect of cold-rolling on microstructure, texture and mechanical properties of an equiatomic FeCrCuMnNi high entropy alloy. Materialia 2018, 1, 175–184. [Google Scholar] [CrossRef]
- Kittel, C.; McEuen, P.; McEuen, P. Introduction to Solid State Physics; Wiley: New York, NY, USA, 1996; Volume 8. [Google Scholar]
- Sadeghi, B.; Cavaliere, P. Progress of Flake Powder Metallurgy Research. Metals 2021, 11, 931. [Google Scholar] [CrossRef]
- Deng, H.; Xie, Z.; Wang, M.; Chen, Y.; Liu, R.; Yang, J.; Zhang, T.; Wang, X.; Fang, Q.; Liu, C. A nanocrystalline AlCoCuNi medium-entropy alloy with high thermal stability via entropy and boundary engineering. Mater. Sci. Eng. A 2020, 774, 138925. [Google Scholar] [CrossRef]
- Qin, G.; Chen, R.; Mao, H.; Yan, Y.; Li, X.; Schönecker, S.; Vitos, L.; Li, X. Experimental and theoretical investigations on the phase stability and mechanical properties of Cr7Mn25Co9Ni23Cu36 high-entropy alloy. Acta Mater. 2021, 208, 116763. [Google Scholar] [CrossRef]





| Item | Mixing Enthalpy (ΔHmix, kJ/mol) | Mixing Entropy (ΔSmix, J/k.mol) | Radius Difference | Valence Electron Concentration (VEC) |
|---|---|---|---|---|
| Criterions | −10 < ΔH < +5 | ≥13.38 | δ < 6.6% | 6.87 ≤ VEC < 8 for FCC + BCC |
| Calculated for AlCrCuFeNi | −4 | 13.38 | 5.62% | 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdani, N.; Toroghinejad, M.R.; Shabani, A.; Cavaliere, P. Effects of Process Control Agent Amount, Milling Time, and Annealing Heat Treatment on the Microstructure of AlCrCuFeNi High-Entropy Alloy Synthesized through Mechanical Alloying. Metals 2021, 11, 1493. https://doi.org/10.3390/met11091493
Yazdani N, Toroghinejad MR, Shabani A, Cavaliere P. Effects of Process Control Agent Amount, Milling Time, and Annealing Heat Treatment on the Microstructure of AlCrCuFeNi High-Entropy Alloy Synthesized through Mechanical Alloying. Metals. 2021; 11(9):1493. https://doi.org/10.3390/met11091493
Chicago/Turabian StyleYazdani, Negar, Mohammad Reza Toroghinejad, Ali Shabani, and Pasquale Cavaliere. 2021. "Effects of Process Control Agent Amount, Milling Time, and Annealing Heat Treatment on the Microstructure of AlCrCuFeNi High-Entropy Alloy Synthesized through Mechanical Alloying" Metals 11, no. 9: 1493. https://doi.org/10.3390/met11091493
APA StyleYazdani, N., Toroghinejad, M. R., Shabani, A., & Cavaliere, P. (2021). Effects of Process Control Agent Amount, Milling Time, and Annealing Heat Treatment on the Microstructure of AlCrCuFeNi High-Entropy Alloy Synthesized through Mechanical Alloying. Metals, 11(9), 1493. https://doi.org/10.3390/met11091493







