Effects of Plasma-Chemical Composition on AISI 316L Surface Modification by Active Screen Nitrocarburizing Using Gaseous and Solid Carbon Precursors
Abstract
1. Introduction
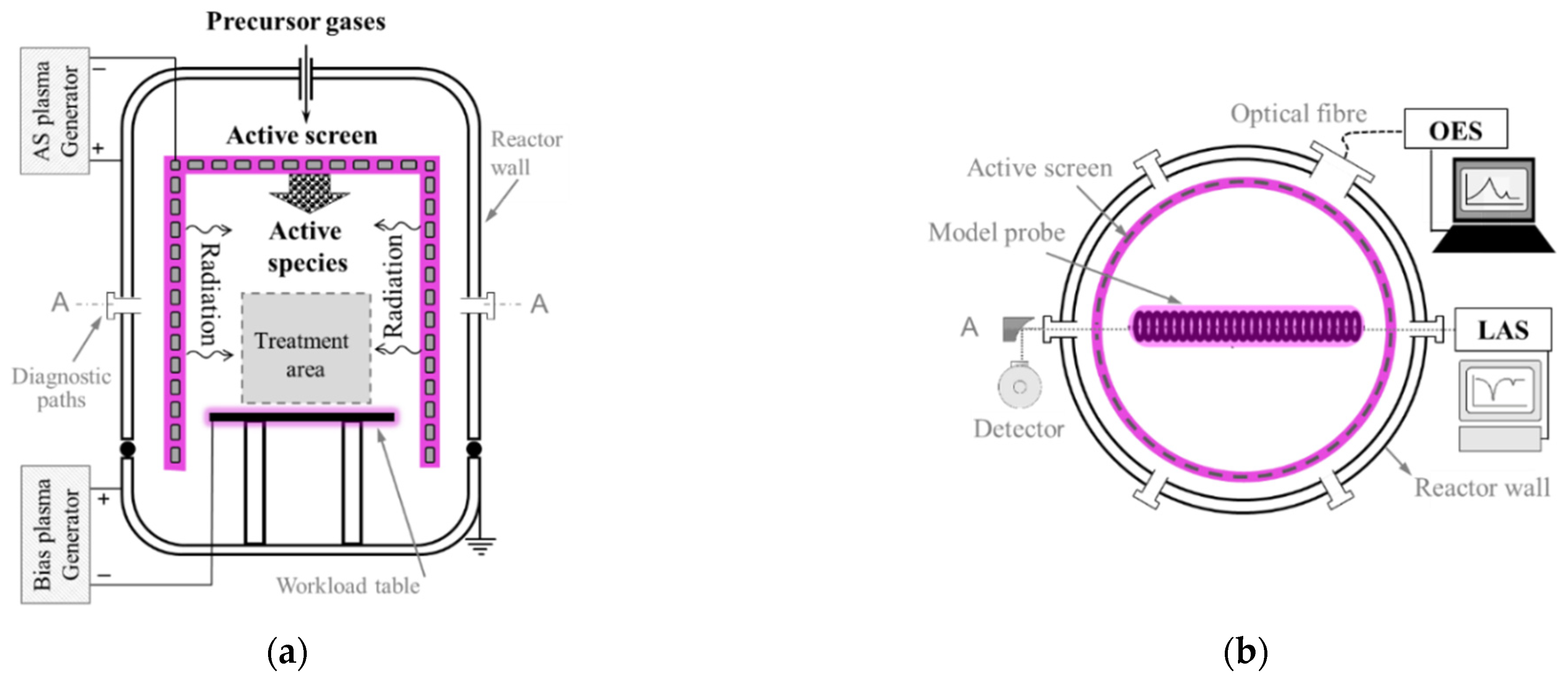
2. Materials and Methods
3. Results
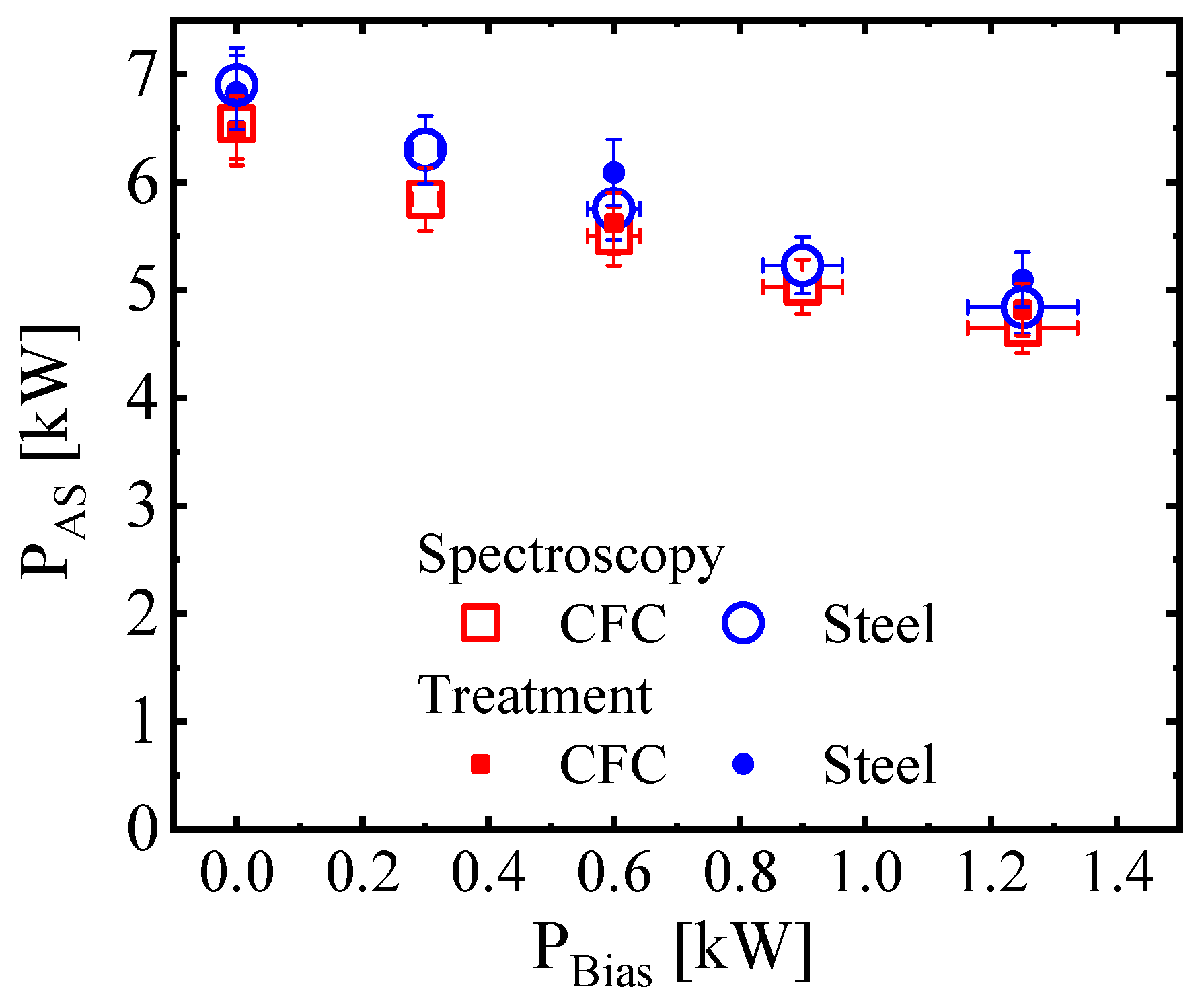
3.1. Interconnection of the AS and Bias Plasma Powers
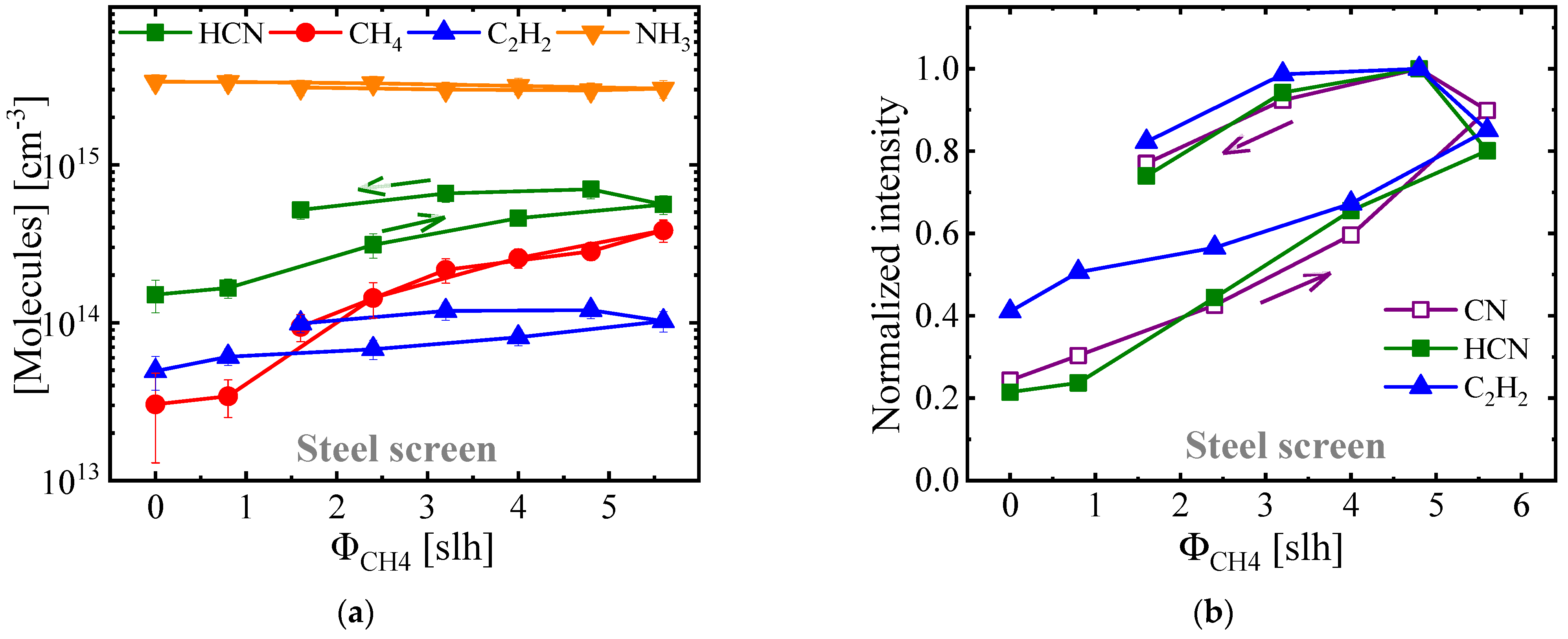
3.2. Plasma Diagnostics of ASPNC Treatment Using Steel AS
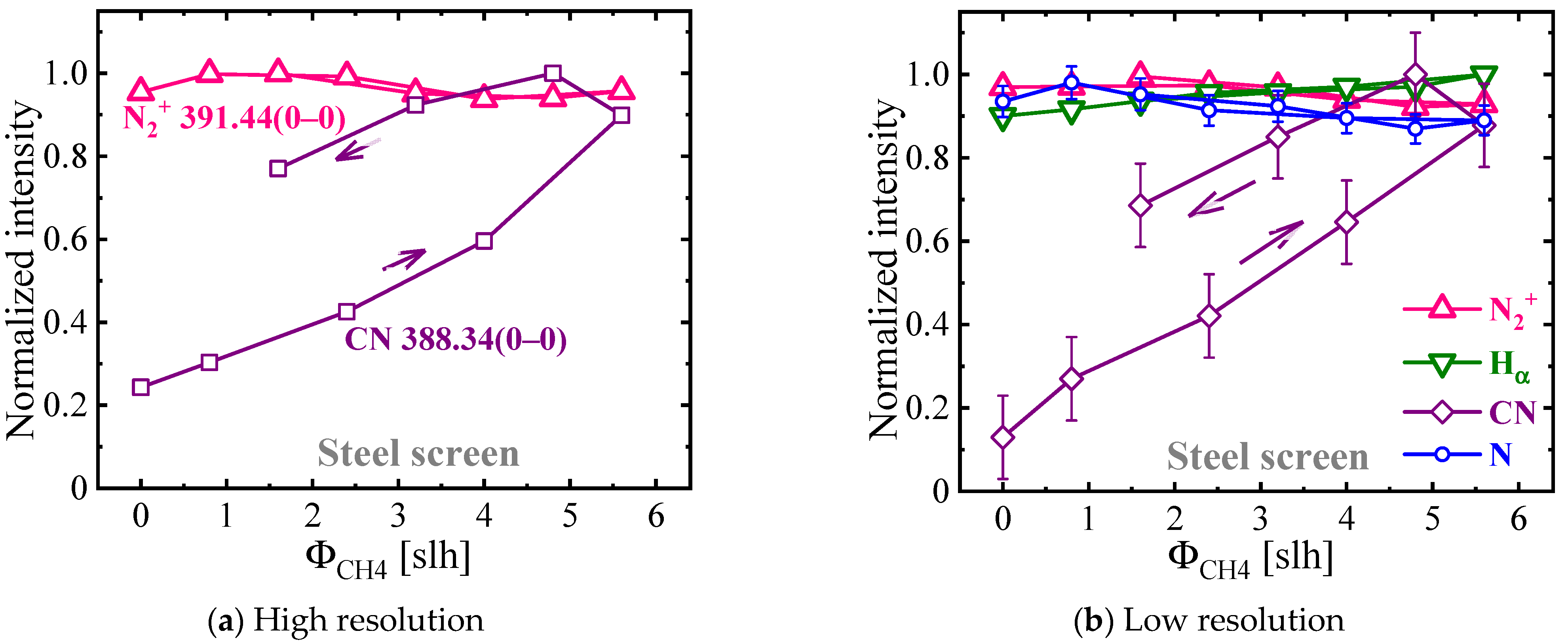
3.3. Comparison of Gaseous and Solid-State Carbon Precursors in ASPNC
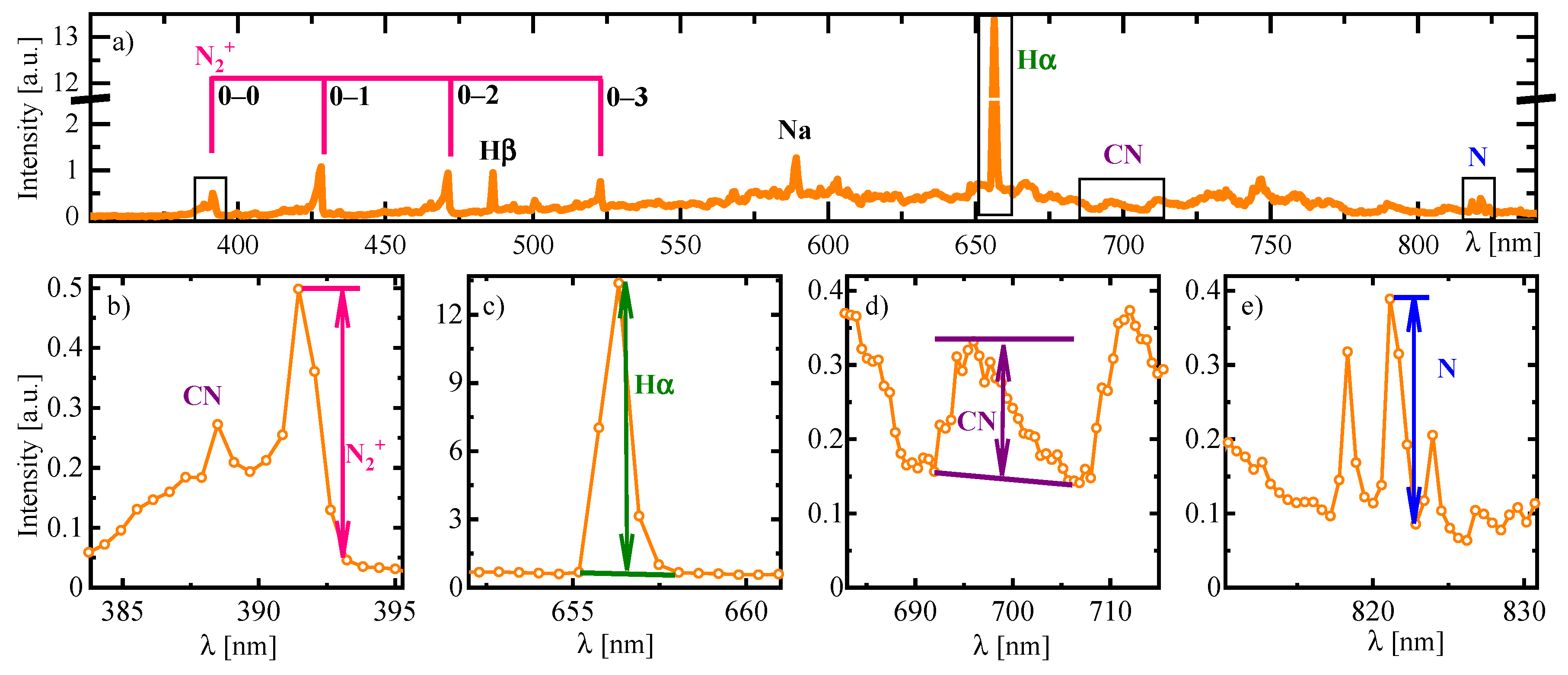
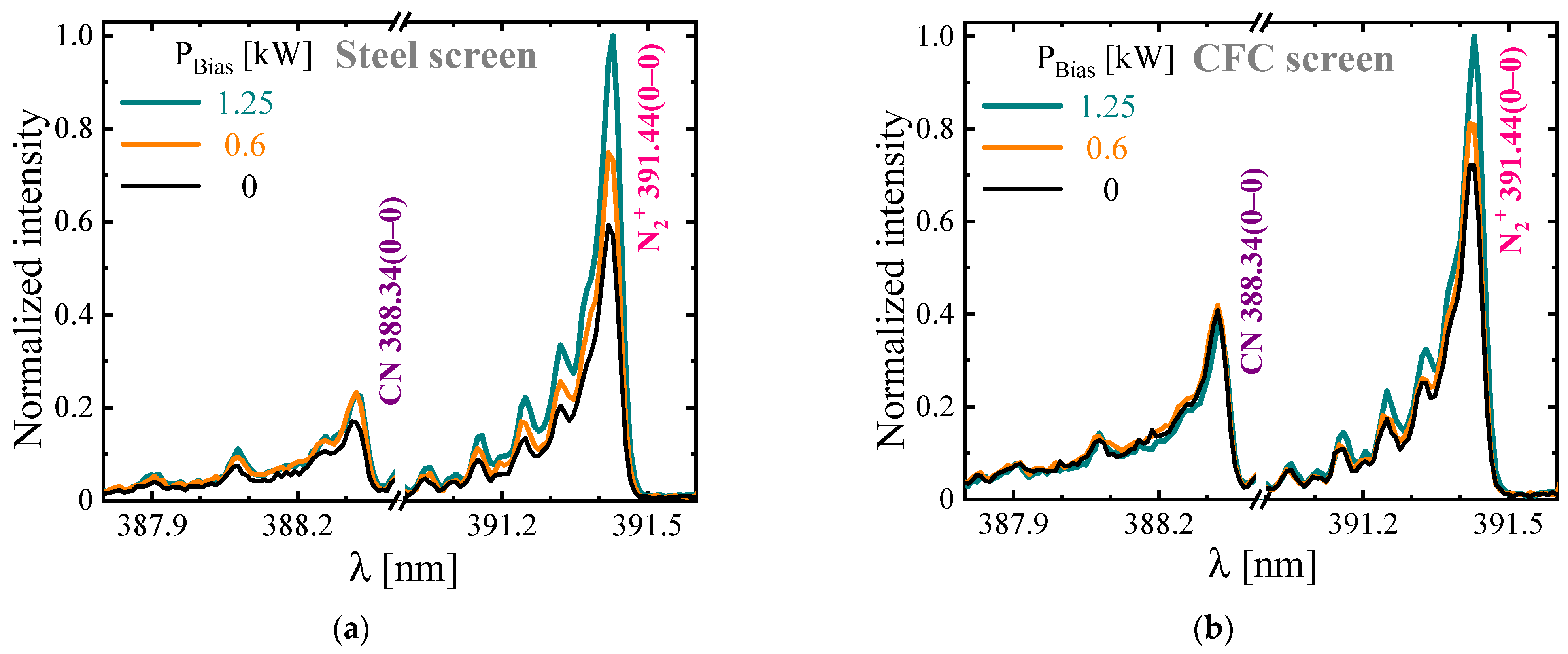
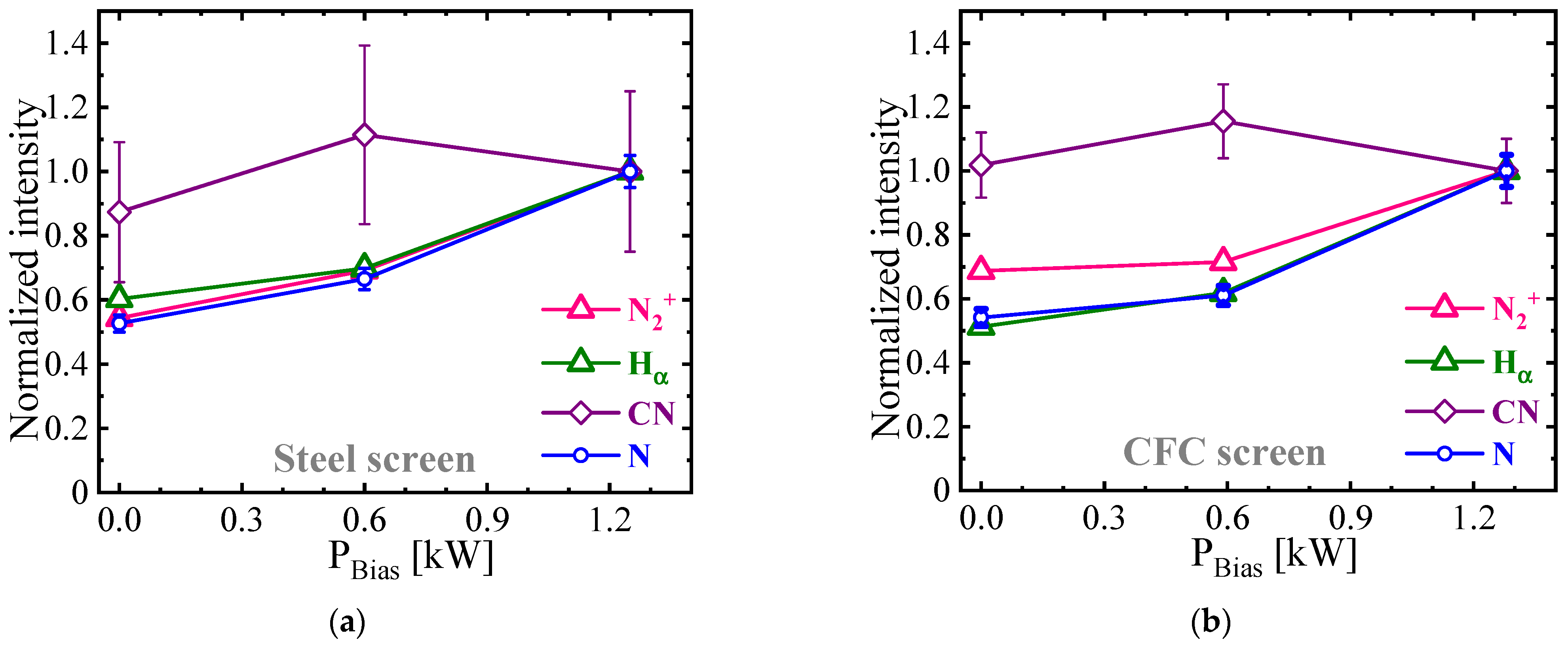
3.3.1. Plasma Diagnostics Using OES and LAS Techniques
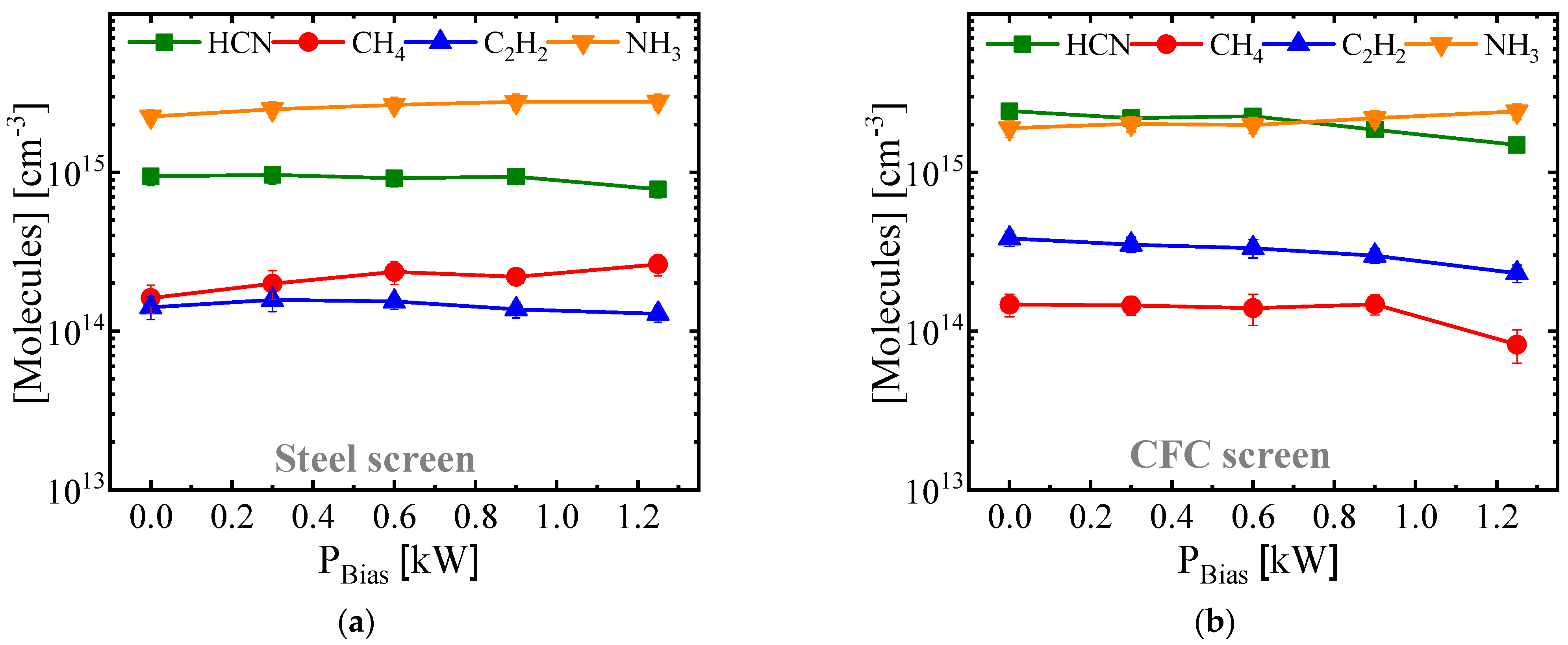
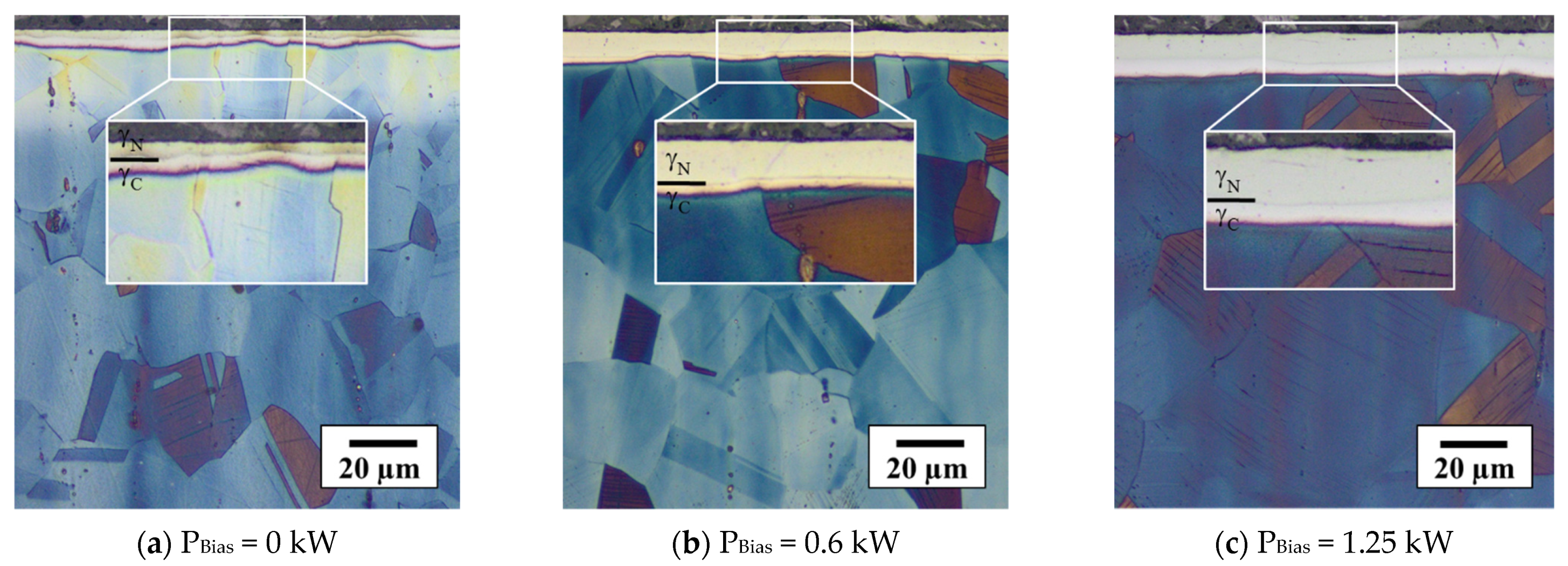
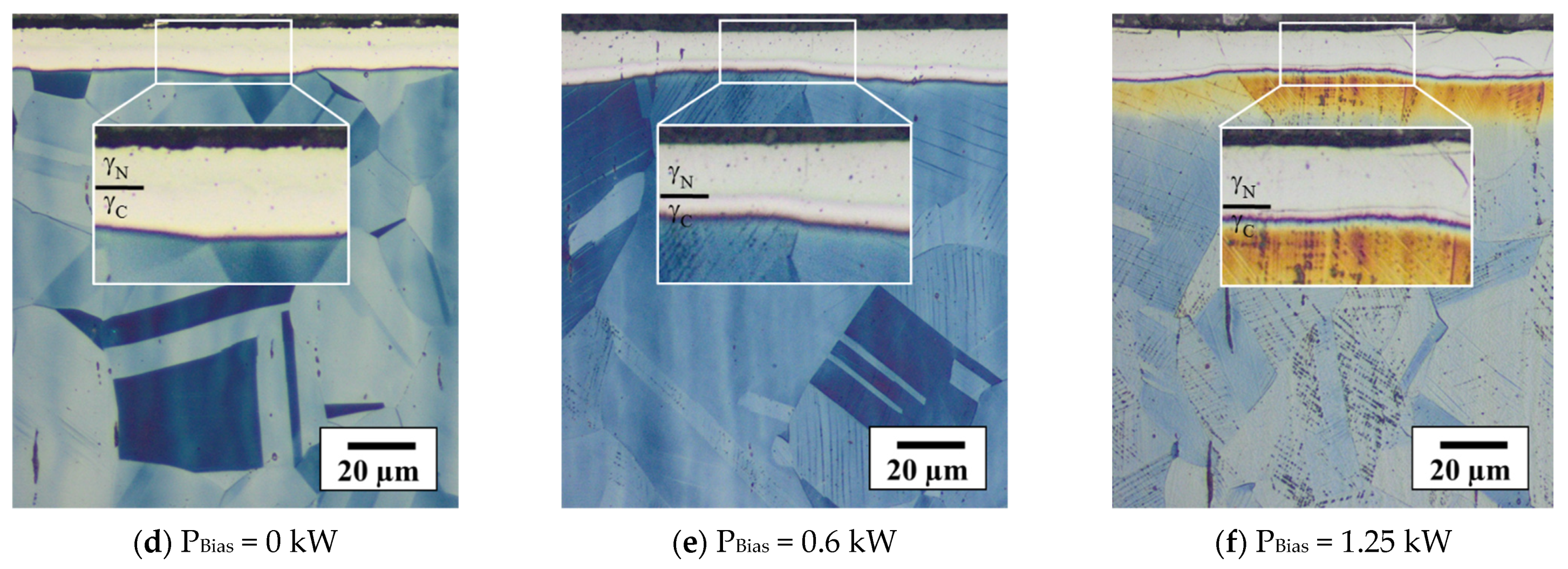
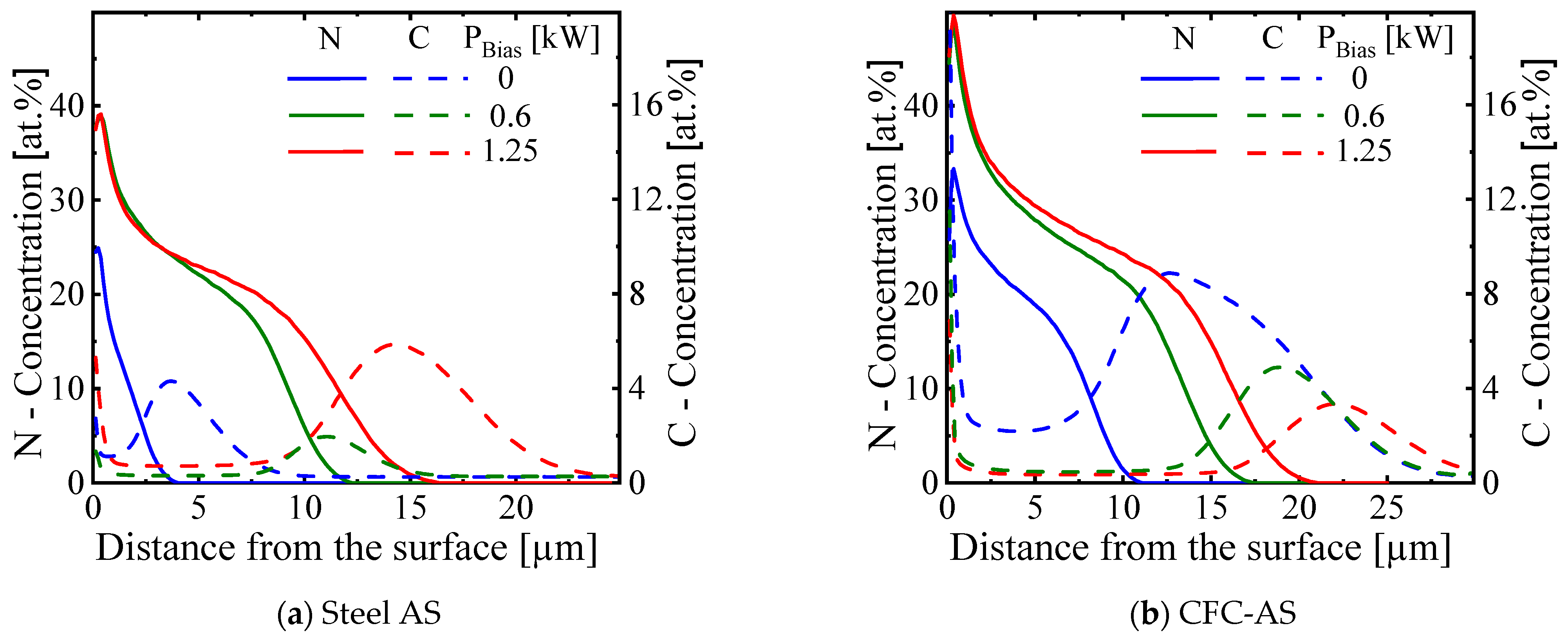
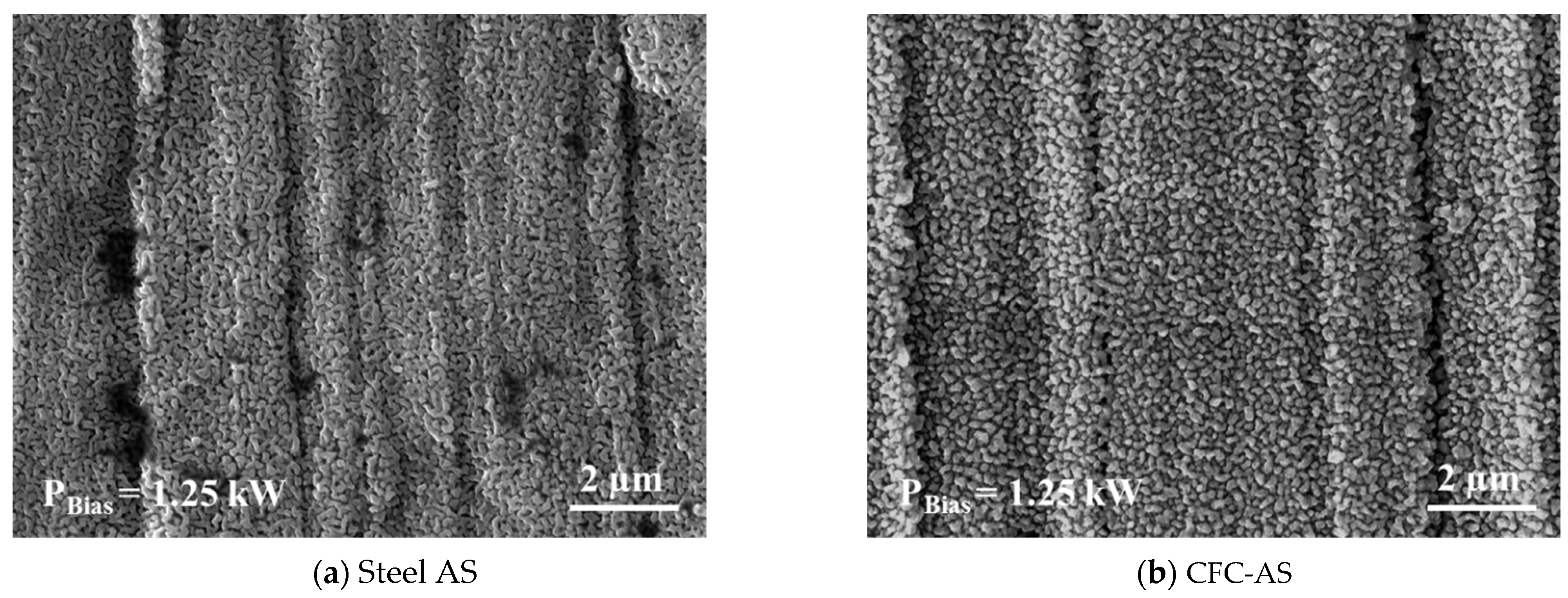
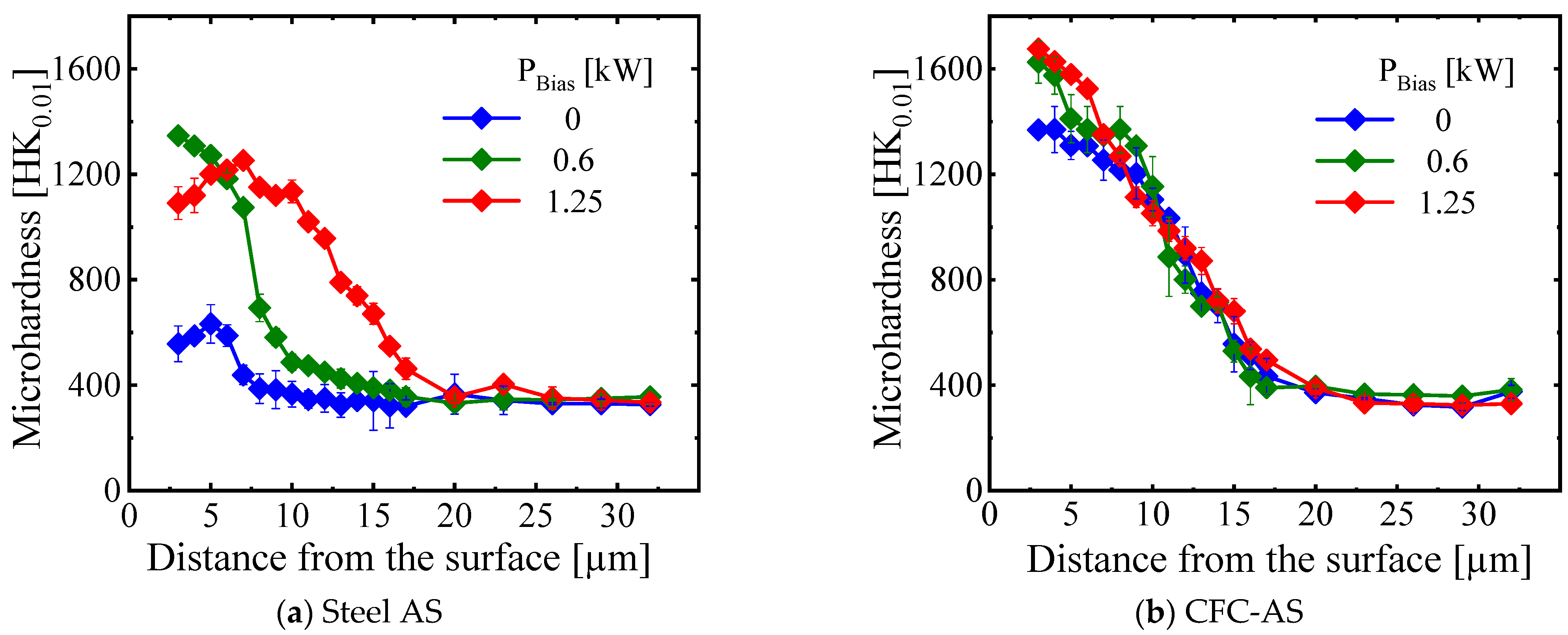
3.3.2. Characteristics of Expanded Austenite Layer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, T. Surface engineering of austenitic stainless steel. Surf. Eng. 2002, 18, 415–422. [Google Scholar] [CrossRef]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Dong, F.Y.; Zhang, P.; Pang, J.C.; Chen, D.M.; Yang, K.; Zhang, Z.F. Optimizing strength and ductility of austenitic stainless steels through equal-channel angular pressing and adding nitrogen element. Mater. Sci. Eng. A 2013, 587, 185–191. [Google Scholar] [CrossRef]
- Mendes, A.F.; Scheuer, C.J.; Joanidis, I.L.; Cardoso, R.P.; Mafra, M.; Klein, A.N.; Brunatto, S.F. Low-temperature plasma nitriding of sintered PIM 316L austenitic stainless steel. Mater. Res. 2014, 17, 100–109. [Google Scholar] [CrossRef][Green Version]
- De Las Heras, E.; Ybarra, G.; Lamas, D.; Cabo, A.; Dalibon, E.L.; Brühl, S.P. Plasma nitriding of 316L stainless steel in two different N2-H2 atmospheres—Influence on microstructure and corrosion resistance. Surf. Coat. Technol. 2017, 313, 47–54. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Bell, T. Low temperature plasma carburising of austenitic stainless steels for improved wear and corrosion resistance. Surf. Eng. 1999, 15, 49–54. [Google Scholar] [CrossRef]
- García Molleja, J.; Nosei, L.; Ferrón, J.; Bemporad, E.; Lesage, J.; Chicot, D.; Feugeas, J. Characterization of expanded austenite developed on AISI 316L stainless steel by plasma carburization. Surf. Coat. Technol. 2010, 204, 3750–3759. [Google Scholar] [CrossRef]
- Chen, F.-S.; Chang, C.-N. Effect of CH4 addition on plasma nitrocarburizing of austenitic stainless steel. Surf. Coat. Technol. 2003, 173, 9–18. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, C.X.; Dong, H.; Bell, T. Low temperature plasma nitrocarburising of AISI 316 austenitic stainless steel. Surf. Coat. Technol. 2005, 191, 195–200. [Google Scholar] [CrossRef]
- Czerwiec, T.; He, H.; Marcos, G.; Thiriet, T.; Weber, S.; Michel, H. Fundamental and Innovations in Plasma Assisted Diffusion of Nitrogen and Carbon in Austenitic Stainless Steels and Related Alloys. Plasma Process. Polym. 2009, 6, 401–409. [Google Scholar] [CrossRef]
- Sun, Y.; Haruman, E. Low Temperature Plasma Surface Alloying of Austenitic Stainless Steels. Solid State Phenom. 2006, 118, 85–90. [Google Scholar] [CrossRef]
- Manova, D.; Lotnyk, A.; Mändl, S.; Neumann, H.; Rauschenbach, B. CrN precipitation and elemental segregation during the decay of expanded austenite. Mater. Res. Express 2016, 3, 66502. [Google Scholar] [CrossRef]
- Dimitrov, V.I.; D’Haen, J.; Knuyt, G.; Quaeyhaegens, C.; Stals, L.M. A method for determination of the effective diffusion coefficient and sputtering rate during plasma diffusion treatment. Surf. Coat. Technol. 1998, 99, 234–241. [Google Scholar] [CrossRef]
- Baranowska, J. Characteristic of the nitride layers on the stainless steel at low temperature. Surf. Coat. Technol. 2004, 180–181, 145–149. [Google Scholar] [CrossRef]
- Saker, A.; Leroy, C.; Michel, H.; Frantz, C. Properties of sputtered stainless steel-nitrogen coatings and structural analogy with low temperature plasma nitrided layers of austenitic steels. Mater. Sci. Eng. A 1991, 140, 702–708. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Hummelshøj, T.S.; Somers, M.A.J. Gaseous carburising of self-passivating Fe–Cr–Ni alloys in acetylene–hydrogen mixtures. Surf. Eng. 2011, 27, 602–608. [Google Scholar] [CrossRef]
- Song, Y.; Kim, J.-H.; Kim, K.-S.; Kim, S.; Song, P. Effect of C2H2/H2 Gas Mixture Ratio in Direct Low-Temperature Vacuum Carburization. Metals 2018, 8, 493. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Hummelshøj, T.S.; Somers, M.A.J. A Method of Activating an Article of Passive Ferrous or Non-Ferrous metal Prior to Carburizing, Nitriding and/or Nitrocarburizing. EP Patent 2278038 A1, 26 January 2011. [Google Scholar]
- Somers, M.A.J.; Christiansen, T.L. Carburizing in Hydrocarbon Gas. WO Patent 2006/136166 A1, 28 December 2006. [Google Scholar]
- Hoshino, K.; Miyashita, M.; Kawamura, T.; Totsuka, T.; Eiraku, H.; Yashiro, K.; Kurosawa, T. Method for Activating Surface of Metal Member. EP Patent 1707646 A1, 4 October 2006. [Google Scholar]
- Baranowska, J. Importance of surface activation for nitrided layer formation on austenitic stainless steel. Surf. Eng. 2010, 26, 293–298. [Google Scholar] [CrossRef]
- Spies, H.-J.; Eckstein, C.; Biermann, H.; Franke, A. Corrosion behaviour of stainless steels after low temperature thermochemical treatment. Mater. Werkst. 2010, 41, 133–141. [Google Scholar] [CrossRef]
- Sun, Y. Production of nitrogen and carbon S phases in austenitic stainless steels by hybrid plasma surface alloying. Surf. Eng. 2010, 26, 114–122. [Google Scholar] [CrossRef]
- Lin, K.; Li, X.; Sun, Y.; Luo, X.; Dong, H. Active screen plasma nitriding of 316 stainless steel for the application of bipolar plates in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2014, 39, 21470–21479. [Google Scholar] [CrossRef]
- Georges, J. Nitriding Process and Nitriding Furnace Therefor. U.S. Patent 5,989,363, 23 November 1999. [Google Scholar]
- Li, C.X.; Georges, J.; Li, X.Y. Active screen plasma nitriding of austenitic stainless steel. Surf. Eng. 2002, 18, 453–457. [Google Scholar] [CrossRef]
- Hubbard, P.; Dowey, S.J.; Doyle, E.D.; McCulloch, D.G. Influence of bias and in situ cleaning on through cage (TC) or active screen plasma nitrided (ASPN) steels. Surf. Eng. 2006, 22, 243–247. [Google Scholar] [CrossRef]
- Hubbard, P.; Dowey, S.J.; Partridge, J.G.; Doyle, E.D.; McCulloch, D.G. Investigation of nitrogen mass transfer within an industrial plasma nitriding system II: Application of a biased screen. Surf. Coat. Technol. 2010, 204, 1151–1157. [Google Scholar] [CrossRef]
- Olzon-Dionysio, M.; Campos, M.; Kapp, M.; de Souza, S.; de Souza, S.D. Influences of plasma nitriding edge effect on properties of 316L stainless steel. Surf. Coat. Technol. 2010, 204, 3623–3628. [Google Scholar] [CrossRef]
- Ahangarani, S.; Sabour, A.R.; Mahboubi, F. Surface modification of 30CrNiMo8 low-alloy steel by active screen setup and conventional plasma nitriding methods. Appl. Surf. Sci. 2007, 254, 1427–1435. [Google Scholar] [CrossRef]
- Burlacov, I.; Hamann, S.; Spies, H.-J.; Röpcke, J.; Biermann, H. In-line Process Control in the Active Screen Plasma Nitrocarburizing Using a Combined Approach Based on Infrared Laser Absorption Spectroscopy and Bias Power Management. HTM J. Heat Treat. Mater. 2016, 71, 141–147. [Google Scholar] [CrossRef]
- Burlacov, I.; Hamann, S.; Spies, H.-J.; Röpcke, J.; Biermann, H. On the influence of carbon contamination of reactor parts in active screen plasma nitrocarburizing processes. J. Appl. Phys. 2018, 123, 233302. [Google Scholar] [CrossRef]
- Haruman, E.; Bell, T.; Sun, Y. Compound Layer Characteristics Resulting from Plasma Nitrocarburising in Atmospheres Containing Carbon Dioxide Gas Additions. Surf. Eng. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Burlacov, I.; Hamann, S.; Spies, H.-J.; Dalke, A.; Röpcke, J.; Biermann, H. A Novel Approach of Plasma Nitrocarburizing Using a Solid Carbon Active Screen—a Proof of Concept. HTM J. Heat Treat. Mater. 2017, 72, 254–259. [Google Scholar] [CrossRef]
- Dalke, A.; Burlacov, I.; Spies, H.-J.; Biermann, H. Use of a solid carbon precursor for DC plasma nitrocarburizing of AISI 4140 steel. Vacuum 2018, 149, 146–149. [Google Scholar] [CrossRef]
- Burlacov, I.; Spies, H.-J.; Biermann, H. Vorrichtung zur Vorrichtung zur Plasmagestützten Erzeugung von Hochreaktiven Prozessgasen auf Basis ungesättigter H-C-N-Verbindungen, die zur Anreicherung der Randschicht von metallischen Bauteilen mit erhöhtem Stickstoff- und/oder Kohlenstoffanteil beitragen. DE Patent 10 2013 006 589 A1, 19 April 2018. [Google Scholar]
- Schlüter, M.; Hopf, C.; Jacob, W. Chemical sputtering of carbon by combined exposure to nitrogen ions and atomic hydrogen. New J. Phys. 2008, 10, 53037. [Google Scholar] [CrossRef]
- Jacob, W.; Hopf, C.; Schlüter, M. Chemical sputtering of carbon by nitrogen ions. Appl. Phys. Lett. 2005, 86, 204103. [Google Scholar] [CrossRef]
- Puth, A.; Hamann, S.; Kusýn, L.; Burlacov, I.; Dalke, A.; Spies, H.-J.; Biermann, H.; Röpcke, J. Spectroscopic investigations of plasma nitrocarburizing processes using an active screen made of carbon in a model reactor. Plasma Sources Sci. Technol. 2018, 27, 75017. [Google Scholar] [CrossRef]
- Hamann, S.; Burlacov, I.; Spies, H.-J.; Biermann, H.; Röpcke, J. Spectroscopic investigations of plasma nitriding processes: A comparative study using steel and carbon as active screen materials. J. Appl. Phys. 2017, 121, 153301. [Google Scholar] [CrossRef]
- Hamann, S.; Börner, K.; Burlacov, I.; Spies, H.-J.; Röpcke, J. Spectroscopic diagnostics of active screen plasma nitriding processes: On the interplay of active screen and model probe plasmas. J. Phys. D Appl. Phys. 2015, 48, 345204. [Google Scholar] [CrossRef]
- Cleugh, D. Plasma species analysis for insitu assessment of surface treatments. Surf. Eng. 2002, 18, 133–139. [Google Scholar] [CrossRef]
- Dalke, A.; Burlacov, I.; Hamann, S.; Puth, A.; Spies, H.-J.; Röpcke, J.; Biermann, H. Plasma Nitrocarburizing of AISI 316L Austenitic Stainless Steel Applying a Carbon Active Screen: Status and Perspectives. HTM J. Heat Treat. Mater. 2018, 73, 246–257. [Google Scholar] [CrossRef]
- Dalke, A.; Burlacov, I.; Hamann, S.; Puth, A.; Böcker, J.; Spies, H.-J.; Röpcke, J.; Biermann, H. Solid carbon active screen plasma nitrocarburizing of AISI 316L stainless steel: Influence of N2-H2 gas composition on structure and properties of expanded austenite. Surf. Coat. Technol. 2019, 357, 1060–1068. [Google Scholar] [CrossRef]
- Maniee, A.; Mahboubi, F.; Soleimani, R. The study of tribological and corrosion behavior of plasma nitrided 34CrNiMo6 steel under hot and cold wall conditions. Mater. Des. 2014, 60, 599–604. [Google Scholar] [CrossRef]
- Pye, D. Practical Nitriding and Ferritic Nitrocarburizing; ASM International: Materials Park, OH, USA, 2003. [Google Scholar]
- Jafarpour, S.M.; Puth, A.; Dalke, A.; Böcker, J.; Pipa, A.V.; Röpcke, J.; van Helden, J.H.; Biermann, H. Solid carbon active screen plasma nitrocarburizing of AISI 316L stainless steel in cold wall reactor: Influence of plasma conditions. J. Mater. Res. Technol. 2020, 9, 9195–9205. [Google Scholar] [CrossRef]
- Pintassilgo, C.D.; Jaoul, C.; Loureiro, J.; Belmonte, T.; Czerwiec, T. Kinetic modelling of a N2 flowing microwave discharge with CH 4 addition in the post-discharge for nitrocarburizing treatments. J. Phys. D Appl. Phys. 2007, 40, 3620–3632. [Google Scholar] [CrossRef]
- Pintassilgo, C.D.; Loureiro, J.; Cernogora, G.; Touzeau, M. Methane decomposition and active nitrogen in a N2-CH4glow discharge at low pressures. J. Phys. D Appl. Phys. 1999, 8, 463–478. [Google Scholar] [CrossRef]
- Heydarzadeh Sohi, M.; Ebrahimi, M.; Honarbakhsh Raouf, A.; Mahboubi, F. Effect of plasma nitrocarburizing temperature on the wear behavior of AISI 4140 steel. Surf. Coat. Technol. 2010, 205, S84–S89. [Google Scholar] [CrossRef]
- Naeem, M.; Iqbal, J.; Zakaullah, M.; Shafiq, M.; Mujahid, Z.I.; Díaz-Guillén, J.C.; Lopez-Badillo, C.M.; Sousa, R.R.M.; Khan, M.A. Enhanced wear and corrosion resistance of AISI-304 steel by duplex cathodic cage plasma treatment. Surf. Coat. Technol. 2019, 375, 34–45. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.Y.; Bell, T. X-ray diffraction characterisation of low temperature plasma nitrided austenitic stainless steels. J. Mater. Sci. 1999, 34, 4793–4802. [Google Scholar] [CrossRef]
- Slycke, J.; Ericsson, T. A study of reactions occurring during the carbonitriding process part II. J. Heat Treat. 1981, 2, 97–112. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafarpour, S.M.; Pipa, A.V.; Puth, A.; Dalke, A.; Röpcke, J.; van Helden, J.-P.H.; Biermann, H. Effects of Plasma-Chemical Composition on AISI 316L Surface Modification by Active Screen Nitrocarburizing Using Gaseous and Solid Carbon Precursors. Metals 2021, 11, 1411. https://doi.org/10.3390/met11091411
Jafarpour SM, Pipa AV, Puth A, Dalke A, Röpcke J, van Helden J-PH, Biermann H. Effects of Plasma-Chemical Composition on AISI 316L Surface Modification by Active Screen Nitrocarburizing Using Gaseous and Solid Carbon Precursors. Metals. 2021; 11(9):1411. https://doi.org/10.3390/met11091411
Chicago/Turabian StyleJafarpour, Saeed M., Andrei V. Pipa, Alexander Puth, Anke Dalke, Jürgen Röpcke, Jean-Pierre H. van Helden, and Horst Biermann. 2021. "Effects of Plasma-Chemical Composition on AISI 316L Surface Modification by Active Screen Nitrocarburizing Using Gaseous and Solid Carbon Precursors" Metals 11, no. 9: 1411. https://doi.org/10.3390/met11091411
APA StyleJafarpour, S. M., Pipa, A. V., Puth, A., Dalke, A., Röpcke, J., van Helden, J.-P. H., & Biermann, H. (2021). Effects of Plasma-Chemical Composition on AISI 316L Surface Modification by Active Screen Nitrocarburizing Using Gaseous and Solid Carbon Precursors. Metals, 11(9), 1411. https://doi.org/10.3390/met11091411





