Abstract
To dispose of arsenic-containing tailings with low carbon and high efficiency, sodium sulphate (Na2SO4), sodium hydroxide (NaOH), calcium nitrate Ca(NO3)2 and calcium hydroxide Ca(OH)2 were independently added to metallurgical slag-based binder (MSB) solidification/stabilisation (S/S)-treated tailings (MSTs) to enhance the MST arsenic S/S performance. Results showed that only Ca(OH)2 could increase the unconfined compressive strength of MST from 16.3 to 20.49 MPa and decrease the leachate As concentration from 31 μg/L to below 10 μg/L. Na3AsO4·12H2O and NaAsO2 were used to prepare pure MSB paste for mechanism analysis. The results of microstructure analyses showed the high specific surface area and amorphous properties of calcium–sodium aluminosilicate hydrate facilitated the adsorption or solid-solution formation of As(V) and As(III). As(V) formed an inner-sphere complex in ettringite, whereas As(III) formed an outer-sphere complex, and the relatively larger size and charge of As(V) compared with SO42− restrict substitution inside channels without affecting the ettringite structure under high loading of As(V). The added Ca(OH)2 promoted the hydration reaction of MSBs and facilitated the formation of a Ca–As(V) precipitate with low solubility, from Ca4(OH)2(AsO4)2·4H2O (Ksp = 10−27.49) to Ca5(AsO4)3(OH) (Ksp = 10−40.12). This work is beneficial for the application of cement-free MSB in the S/S process.
1. Introduction
Arsenic (As) is a toxic and carcinogenic element, which can cause severe effects on human health, such as skin, lungs, kidney and liver cancers, even at a low concentration [1]. The mining, processing and smelting industry of nonferrous metals is the most widespread source of As emissions in China, accounting for 61.8% [2]. Mining wastewater, smelting slag, dust and tailings are piled up in tailings ponds, which have potential environmental risks and occupy a large percentage of soil [3]. Heavy metal pollution from a large number of tailings poses a major threat to the environment [4,5]. The treatment of heavy metal pollution has received increasing attention since the implementation of the Soil Pollution Prevention and Control Law of the People’s Republic of China in 2019 [6]. It is imperative to develop effective and economically viable technologies to reduce pollution from the nonferrous metal industry.
Solidification/stabilisation (S/S) technology has been used to dispose of metal(loid)-containing waste by forming a less soluble, mobile or toxic product through adsorption, co-precipitation and physical encapsulation [7]. Ordinary Portland cement (OPC) is a traditionally used binder in S/S processes, while the high carbon footprint of cement clinker manufacture is of concern [8,9]. To achieve an environment-friendly binder for the S/S of As-containing waste, many studies have been conducted to replace cement in part or whole using materials, such as red mud, blast furnace or metakaolin-assisted cement [1,10], cement-free clay-based binders [11], red mud-enhanced magnesium phosphate cement [12], pine sawdust biochar [13], coal fly ash [14], zero-valent iron or magnetic biochar [15]. However, there have been few reports on the use of cement-free metallurgical slag-based binders (MSB) [16,17], which consist of steel slag powder (SSP), ground-granulated blast furnace slag (GGBFS) and flue gas desulphurisation gypsum (FGDG). Steel slag is a by-product of a steel-making process, known as overburnt clinker because its mineral phase composition is similar to that of cement clinker [18]. In 2019, 149.45 million tonnes of it were produced in China; however, the usage rate is less than 40% [19]. A large accumulation of steel slag pressurises the environment [20]. GGBFS is a by-product of the steel-making industry and is used as a supplementary cementitious material. In the hydration process of MSB, the relatively high alkalinity of SSP facilitates the breakage of Si–O and Al–O bonds in the GGBFS vitreous structure to form SiO44− and AlO45− [21,22], which react with dissolved Ca2+ to form calcium silicoaluminate hydrate (C–A–S–H) gels [11,23]. The AlO45− dissolved in solution transforms from four coordinated aluminium ions to six coordinated aluminium ions [Al(OH)6]3− in alkaline environments, which reacted with the Ca2+ and SO42− dissolved from gypsum to form ettringite [24].
In this study, As-containing tailings were chosen as the S/S objects, as other hazardous elements’ leaching risk is very low after S/S treatment (Table S1). In order to dispose of As-containing tailings with low carbon and high efficiency, the MSB (60 wt% GGBFS, 30 wt% SSP, and 10 wt% FGDG) was chosen as the binder for the As-containing tailings, based on a previous study [25]. SO42− (Na2SO4), OH− (NaOH), Ca2+ (Ca(NO3)2 or Ca2+ and OH− (Ca(OH)2) was added to the MSB-S/S treated tailings (MST) to enhance the MST-As-S/S performance. Arsenic in tailing occurs in various states in the natural environment, and its main forms in regard to leaching and migration characteristics are soluble arsenite As(III) and arsenate As(V) compounds [26]. The As-S/S mechanism of MSB and the mechanism of efficiency improvement are both unclear; therefore, Na3AsO4·12H2O and NaAsO2 were used to study the S/S mechanisms through X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and scanning electron microscope-energy dispersive spectrometer (SEM-EDS) analysis. This study will improve the engineering application prospects of MSB and reusability of MST.
2. Materials and Methods
2.1. As-Containing Tailings and Metallurgical Slag-Based Binders
As-containing tailings were collected from a closed lead-zinc tailings pond in Hechi Nandan, Guangxi (China). The main components of the tailings were quartz, calcite and fluorite, of which the D50 and D90 particle sizes were 42 and 188 μm, respectively (Figure S1). The total concentration of As in the tailings was 2098 mg/kg, as determined using an Agilent 7500a ICP-MS (Agilent Technologies, Santa Clara, CA, USA) after aqua regia digestion (1/3 concentrated HNO3 and HCl (v/v)) based on Chinese standard method HJ 803-2016. The leaching concentration of As in the tailings after horizontal oscillation-leaching tests was 0.57 mg/L, based on the Chinese standard method HJ 557-2010 (Table 1). The MSB consisted of GGBFS, SSP and FGDG. The chemical components of raw materials are shown in Table 1, and the XRD spectra of the binders are shown in Figure S2. The analytical reagents, Na2SO4, NaOH, Ca(NO3)2 and Ca(OH)2, were used as added additive for the binders, respectively. A polycarboxylic acid water-reducer (WR) was used to achieve an acceptable flowability of mortar during S/S treatment. The analytical reagents, Na3AsO4·12H2O and NaAsO2, were used to prepare the pure MSB paste to study the S/S mechanisms of As(III) and As(V).

Table 1.
Raw material chemical components, blaine fineness, component concentrations and leaching concentration of As.
2.2. Cement-Free S/S Treatment
For the preparation of MST mortar samples, the different constituents of binders were mixed with tailings at specific ratios (Table 2). MSB with 60 wt% GGBFS, 30 wt% SSP and 10 wt% FGDG was chosen due to its high compressive strength, according to the findings of previous studies [25]. Four additives (Na2SO4, NaOH, Ca(NO3)2 and Ca(OH)2) were incorporated into the MSB mass individually at 5 wt%, 10 wt% and 15 wt%. The binders-to-tailings (B/T), water-to-solids (W/S) and WR-to-solids (WR/S) mass ratios were maintained at 0.25, 0.2 and 0.008, respectively, in all MST samples. After the tailings and binders were mixed for 1 min using a standard cement mortar mixer, distilled water used to dissolve the WR was added, and the blended mortar was mixed for another 5 min, before being cast into steel moulds (40 mm × 40 mm × 160 mm) to prepare the MST samples. To prepare MSB paste samples, 5 wt% concentrations of the analytical reagent, Na3AsO4·12H2O or NaAsO2, was added (Table 2), which were then mixed with water until the mass ratio of water-to-binders (W/B) reached 0.35. The paste samples were stirred for 5 min and then cast into steel moulds (30 mm × 30 mm × 50 mm). All mortar and paste samples were cured in a moist cabinet at (40 ± 2) °C and (90 ± 1)% relative humidity (similar to the underground filling environment in Guangxi [27]) for 3 day, and then the samples were demoulded and placed under the same curing conditions until the appropriate degree of sample ageing was achieved.

Table 2.
Mixture formulations of MST and MSB samples (wt%).
2.3. S/S Performance and Spectroscopic/Microscopic Analysis
The S/S performance was evaluated in terms of the unconfined compressive strength (UCS) and As leachability of the MST samples. The UCS of the samples was assessed on the basis of the Chinese standard GB/T 17671-1999 method of testing cements. The determination of UCS on three replicates at each curing time, and the average value with standard deviations of less than 5% was reported. The leachability of MST was assessed using the Chinese standard method HJ 557-2010, wherein the ratio of leachant (deionised water) volume to solid specimen weight (crushed to size < 3mm) was maintained at 10:1 (L/kg). Leachate samples were oscillated horizontally at 110 ± 10 times/min for 8 h and then left to stand at room temperature for 16 h. The pH values were measured, and the leachate was filtered through a 0.45-μm polypropylene microfiltration membrane. Afterwards, the filtered leachate samples were acidified to below pH 2 using concentrated HNO3 and then stored at 4 °C in the dark before As detection using a PerkinElmer Optima 8300 ICP-OES (Perkin Elmer, Cumberland, NJ, USA) or an Agilent 7500a ICP-MS (Agilent Technologies, Santa Clara, CA, USA), with all samples analysed in triplicate to ensure accuracy. Mean results for triplicate sample analysis (with standard deviations of less than 5%) and error bars, are presented in this study. The As curing rate was evaluated using Equation (1):
where Cs/s is the As leaching concentration of MST, and μg/L; CT is the As leaching concentration of untreated tailings.
Curing Rate = (1 − CS/S/CT) × 100%
The hydration of pulverised and sieved paste samples was terminated using alcohol drenching at the specific testing age, with samples then dried at 50 °C under a vacuum for 24 h for further characterisation. Mineralogy was detected using a high-resolution powered Rigaku D/max-RB XRD with CuKα radiation, a voltage of 40 kV, current of 200 mA and 2θ scanning, ranging between 10° and 70°. The morphology and elemental mapping of the samples were analysed using SEM-EDS (JSM-6701F). Structural and chemical bond analyses were performed using GX Perkin-Elmer FTIR (Perkin Elmer, Cumberland, NJ, USA) between wavelengths of 400–4000 cm−1 and at a resolution of 0.125 cm−1.
3. Results
3.1. The S/S Performance of MST Samples
The UCS results for MST samples (Figure 1a) demonstrate that MST combined with various additives performed differently. The 3, 7 and 28 day UCS results for T were 11, 13.8 and 16.3 MPa, respectively. An increase in concentrations of Na2SO4, NaOH and Ca(NO3)2 reduced the USC of MST at every curing time, especially for the addition of NaOH, which inhibits hydration reactions and reduced the USC of MST (the lowest 28 day USC (T-H3 3.7 MPa) was only 22.7% of T). The addition of Ca(OH)2 enhanced mechanical properties (the highest 28 d UCS (T-C3 20.49 MPa) was 125.7% of T). The USC of all samples could meet the filling requirements (5 MPa, the black line in Figure 1a), except for T-H3.
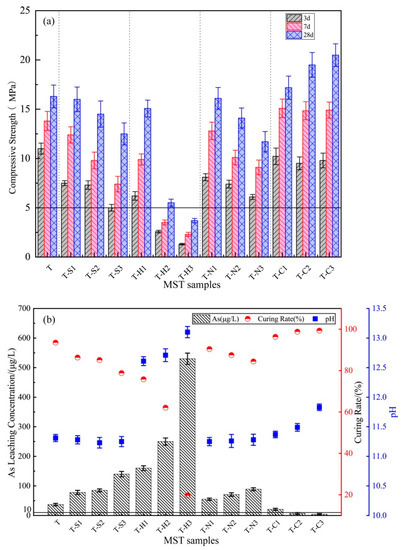
Figure 1.
(a) UCS of MST samples at 3, 7 and 28 day; (b) As leachability of 28 day MST samples.
Figure 1b shows leachate As concentrations and the pH values of 28 day MST samples after the horizontal oscillation-leaching experiments. The leachate As concentration of T samples was 31 μg/L, which exceeded the prescribed As concentration limit value in the Chinese standard GB 5749-2006 for drinking water quality (10 μg/L, the black line in Figure 1b), indicating a relevant risk of environmental pollution. The addition of Na2SO4, NaOH or Ca(NO3)2 enhanced leachate As concentration, particularly that of T-H3, which reached 530 μg/L. The addition of Ca(OH)2 reduced the leachate As concentration, particularly that of T-C2 and T-C3, which was less than 10 μg/L.
3.2. Spectroscopic/Microscopic Analysis
3.2.1. XRD Pattern Analysis
The XRD patterns of MSB paste hydrated for 3 and 28 day are presented in Figure 2a,b), respectively. The main mineral phases of the B sample, as shown in Figure 2a, are C-S-H gel, ettringite (Ca6Al2(SO4)3(OH)12·26H2O) and un-hydrated gypsum (CaSO4·2H2O), C2F (2CaO·Fe2O3), RO phase ((MgO)0.239(FeO)0.761), akermanite (Ca2MgSi2O7) and C2S (2CaO·SiO2) (Figure S2). With the addition of As(III) (sample B(III)), the peaks of ettringite decreased. However, when As(V) was added (B(V)), the peaks of ettringite disappeared due to the formation of a new phase, (Ca4Al2SO10·16H2O), and the peaks of Ca–As co-precipitation Ca4(OH)2(AsO4)2·4H2O appeared.
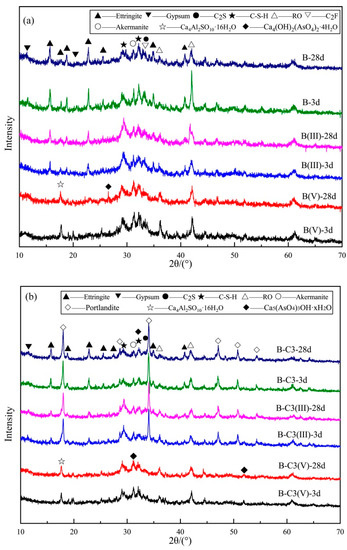
Figure 2.
The XRD patterns of (a) B, B(III) and B(V) paste; (b) B-C3, B-C3(III) and B-C3(V) paste.
3.2.2. FTIR Spectra Analysis
Figure 3a displays the FTIR spectra of the B, B(III) and B(V) samples. The spectra of the six samples are similar, presenting analogous adsorption bands. The pure B paste spectra (Figure 3a ① and ②) exhibit a small band at 3639 cm−1 associated with the O–H stretching vibration; bands at 3424 and 1646 cm−1 are related to the O–H stretching and bending modes of molecular interlayer water, respectively, and bands near 1450 (1478, 1428) cm−1 and at 876 cm−1 are related to the anti-symmetric stretching (v3) and out-of-plane bending (v2) modes of CO32− ions, respectively. The bands at 973 cm−1 and near 450 (462, 422) cm−1 are associated with anti-symmetric Si–O(Al) stretching vibrations (v3) and in-plane Si–O bending vibrations (v2) in the SiO4 tetrahedra of the C–(A)–S–H gel. The lack of a sharp band at 973 cm−1 indicates the wide distribution of SiQn(mAl) units. Moreover, the 3 day and 28 day infra-red spectra of B samples display large bands at 1114 cm−1 corresponding to the S–O4 stretching mode (v3), with a shoulder at 537 cm−1, potentially due to out-of-plane Si–O bending vibrations (v4).
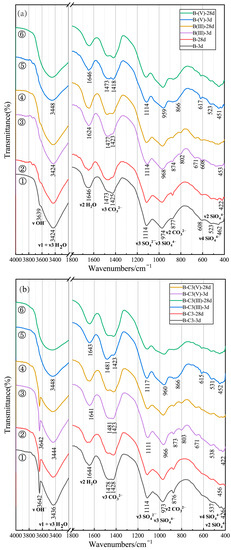
Figure 3.
FTIR spectra of (a) ① and ② B sample, ③ and ④ B(III) sample and ⑤ and ⑥ B(V) sample; (b) ① and ② B-C3 sample, ③ and ④ B-C3(III) sample and ⑤ and ⑥ B-C3(V) sample.
Nevertheless, some differences were observed in the infrared spectra of B(III) and B(V) samples. The weak band at 3639 cm−1 in the B sample (Figure 3a ① and ②) disappeared in the B(V) (Figure 3a ⑤ and ⑥) samples, indicating that OH− reacts with the added As(V), which is consistent with the result of XRD analysis (Figure 2a). The anti-symmetric Si–O(Al) stretching vibrations (v3) of the C–(A)–S–H gel (973 cm−1 for B) decreased to 968 cm−1 for B(III) and 964 cm−1 for B(V), indicating that the added As(III) and As(V) were combined in the microstructure network and changed the degree of polymerisation of the C–(A)–S–H gel. In addition, the O–H stretching band (3424 cm−1 for B) increased to 3448 cm−1 and became broader for B(V) due to the absence of ettringite, leading to the change of interchannel molecular water, which combined with the result of XRD pattern (Figure 2a). Moreover, some new bands were observed, with the appearance of broadband at 802 and 671 cm−1 for B(III), and at 866 cm−1 for B(V) (Figure 3a ③–⑥), which was due to the As–O stretching vibration of the As(III) and As(V) species [28,29].
Figure 3b display the FTIR spectra of the B-C3, B-C3(III) and B-C3(V) samples, respectively. These spectra are all similar to those presented in Figure 3a, except that the spectra of the B-C3 and B-C3(III) samples exhibited sharp adsorption bands at 3642 cm−1 due to the addition of Ca(OH)2. However, the sharp adsorption bands at 3642 cm−1 did not appear in B-C3(V), illustrating that Ca(OH)2 reacted with As(V). Bands around 1110 cm−1, corresponding to the S–O4 stretching mode (v3) in Figure 3b ①–⑥, were offset at 1114 cm−1 in B-C3 sample, 1111 cm−1 in B-C3(III) and 1117 cm−1 in B-C3(V), indicating that, after As(III) and As(V) were added, the hydration products associated with SO42− were affected, as shown by the XRD pattern (Figure 2b).
3.2.3. SEM-EDS Analysis
Figure 4 show SEM-EDS images for B, B(V) and B(III) at hydration ages of 3 and 28 day. Dense structures with amorphous gels and needle-like mineral phases are visible in the cement-free B paste in Figure 4a,b, which are calcium-sodium aluminosilicate hydrate (C–(N)–A–S–H) gels and ettringite, combined with the component analysis results in Table S2 (b-1, b-2 and b-3). After As(V) was added, the needle-like ettringite structure was undetected (Figure 4c,d), and a small amount of As(V) was found in the short columnar crystals of sodium sulphate, combined with the component analysis results (Table S2 (d-1)). Moreover, according to the SEM-EDS mapping images (Figure S3) of the red box in Figure 4d, the distribution of As(V) was almost similar to that of Ca, Si and Al, and the distribution of Na was similar to that of S. Therefore, the majority of the added As(V) entered the C–A–S–H gels through isomorphism substitution or was adsorbed and encapsulated by the gels, whereas the tiny minority of that entered the sodium sulphate to form sodium arsenic alunite. After As(III) was added, combined with the component analysis results (Table S2 (e-1,f-3)), different amounts of As(III) were detected in the C–A–S–H, ettringite, Na2SO4 and Na2Ca(SO4)2 (Figure 4e,f).
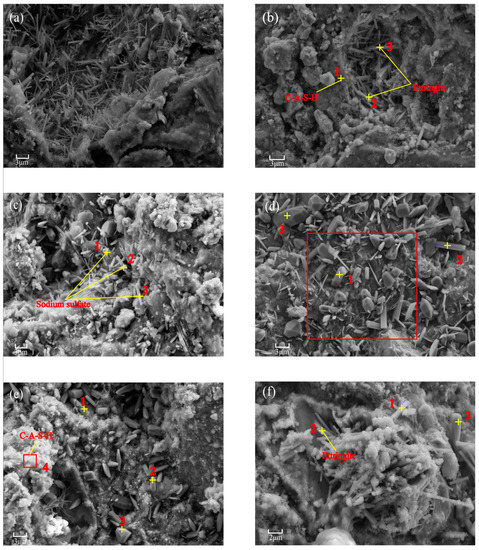
Figure 4.
SEM-EDS result for (a) B-3d; (b) B-28 d; (c) B(V)-3 d; (d) B(V)-28 d; (e) B(III)-3 d; (f) B(III)-28 d.
Figure 5 show SEM-EDS images of B-C3, B-C3(V) and B-C3(III) at hydration ages of 3 and 28 day. In addition, amorphous C–A–S–H gels interwoven with needle-like ettringite structures were detected in the B-C3 paste. The images of B-C3(V) and B-C3(III) are similar to those for B(V) and B(III), except for the appearance of calcium hydroxide flakes in B-C3(V)-3d (Figure 5c) and calcium monosulfoaluminate hydrate (AFm) flakes in B-C3(III)-3d (Figure 5e), combined with the component analysis results in Table S3, which may be due to their content being lower than the detection limit of the XRD technique; both kinds of flakes disappeared as the hydration reaction proceeded.
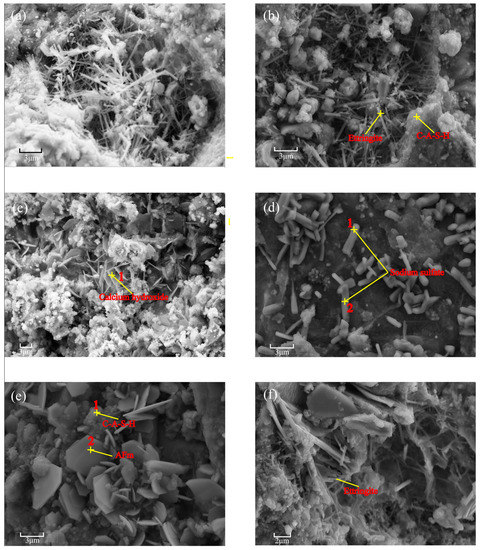
Figure 5.
SEM-EDS result for (a) B-C3-3 d; (b) B-C3-28 d; (c) B-C3(V)-3 d; (d) B-C3(V)-28 d; (e) B-C3(III)-3 d; (f) B-C3(III)-28 d.
4. Discussion
This study demonstrates the different As S/S performance of MST samples after four additives (Na2SO4, NaOH, Ca(NO3)2 and Ca(OH)2) were added, as shown in Table S4. The MSB is selective for additives and only Ca(OH)2 exerted a positive effect on the As S/S performance. The addition of Na2SO4 had little effect on the pH values, UCS and As curing rate, probably because gypsum in the MSB system (10 wt% FGDG in B) was sufficient to provide sulphate for the hydration reaction. The addition of NaOH resulted in a pH value above 13, which inhibited the dissolution of Ca2+ and hindered the hydration reaction, leading to a sharp decrease in UCS [30]. The amphoteric nature of arsenic in an alkaline environment and repulsion by OH− during the adsorption process of the hydration products led to the sharp decrease of the As curing rate [31,32]. Neither NaOH nor Ca(NO3)2 improved the As S/S performance, confirming that the individual addition of OH− without Ca2+ would not improve As S/S efficiency and had a negative effect [33,34].
In addition, this study demonstrates that both the As(V) and As(III) S/S mechanism of MSB, and the effect of Ca(OH)2 addition on As(V) and As(III) S/S are different. The addition of Ca(OH)2 to MSB raised the pH of the reaction mixture (Figure 1), facilitated GGBFS dissolution and provided a source of Ca2+, thus improving the As(V) and As(III) S/S related to the hydration products. Adsorption or solid solution formation of As(V) and As(III) occurred due to the high specific surface area and amorphous properties of C–A–S–H, based on the shift in anti-symmetric Si–O(Al) stretching vibrations (v3) in the discussion above (Figure 3) and SEM-EDS results (Figure 4 and Figure 5).
Anion substitution in ettringite can occur via reaction with surface sites (ligand exchange), replacing Al/Ca coordinated surface OH− or substituting for sulphate inside channels (isomorphic substitution) [35]. This occurs as the ettringite surfaces are negatively charged under alkaline pH range conditions and due to the column and channel-like structure of ettringite, which is composed of columns of Ca6[Al(OH)6]2·24H2O6+ and channels of [(SO4)3·2H2O] 6− [36]. The extent of channel substitution of ettringite may be inversely proportional to the difference in size and electronegativity of the oxyanion compared with SO42− (0.29 Å), resulting in As(V) (0.47 Å) being likelier to be channel-substituted with sulphur than As(III) (0.69 Å) [37]. Thus, As(V) tended to form an inner-sphere complex in ettringite, whereas As(III) could form an outer-sphere complex [38], and the relatively larger size and charge of As(V) compared with SO42− restricts the substitution inside channels without affecting the ettringite structure under high loading of As(V), which is consistent with the XRD, IR and SEM-EDS analysis results.
Previous studies [39,40,41] have verified the formation of Ca–As precipitates under alkaline conditions. In the present study, the distinct Ca–As(III) mineral phase characteristic peak was not found in the XRD patterns, maybe because of the content being below the detection limit of XRD analysis or mainly existing as an amorphous or more complex structure. When Ca(OH)2 was added, Ca–As(V) changed from Ca4(OH)2(AsO4)2·4H2O (Ksp = 10−27.49) to Ca5(AsO4)3(OH) (Ksp = 10−40.12) (Figure 2), which restricted As leaching [42].
Based on the findings of this study, alkaline solid waste with high calcium ion equilibrium concentrations and high alkalinity can be used to replace Ca(OH)2 added to MSB for the S/S of high As-containing solid wastes for low carbon and environmental protection.
5. Conclusions
In this study, the effects of four additives (Na2SO4, NaOH, Ca(NO3)2 or Ca(OH)2) on the As S/S performance of MST to enhance the As S/S efficiency were investigated. As-containing analytical reagents were used to prepare pure MSB paste to study the S/S mechanisms of As(III) and As(V) and the mechanism of efficiency improvement using Ca(OH)2, based on XRD, IR and SEM-EDS analysis. Based on the results obtained from this study, the following conclusions can be drawn:
- An MSB is selective for the additives, and only Ca(OH)2 exerts a positive effect on the S/S performance of MST.
- As(V) can form an inner-sphere complex in ettringite, whereas As(III) can form an outer-sphere complex, and the relatively larger size and charge of As(V) compared with SO42− restrict its substitution inside channels without affecting the ettringite structure under high loading of As(V).
- The added Ca(OH)2 facilitates the encapsulation and adsorption of As(V) and As(III) in MSB, and changes the Ca–As(V) from Ca4(OH)2(AsO4)2·4H2O (Ksp = 10−27.49) to Ca5(AsO4)3(OH) (Ksp = 10−40.12).
- Cement-free MSB with Ca(OH)2 added is feasible to dispose of high As-containing solid wastes with high efficiency and a low carbon footprint.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/met11091389/s1, Figure S1: (a) XRD pattern and (b) grain size results of tailings, Figure S2: The XRD pattern of raw materials: (a) ground granulated blast furnace slag (GGBFS), C2S: dicalcium silicate; (b) steel slag powder (SSP), C3A: tricalcium aluminate, RO phase: (MgO)0.239(FeO)0.761, C2S: dicalcium silicate, C2F: dicalcium ferrite; (c) flue gas desulfurisation gypsum (FGDG), Figure S3: The SEM-EDS electron image and mapping images of Figure 4 (d), Table S1: Leached concentration of raw materials and T in Table 2, Table S2: Component analysis result of point in the Figure 4, Table S3: Component analysis result of point in the Figure 5, Table S4: Assessment summary of the S/S performance effects of Na2SO4, NaOH, Ca(NO3)2 and Ca(OH)2 at different doses (wt%).
Author Contributions
Formal analysis, W.G.; funding acquisition, P.F. and H.Y.; investigation, W.G. and Y.Z.; methodology, W.G.; project administration, S.Z.; resources, S.Z.; supervision, S.Z.; validation, W.G., Z.L., S.Z. and W.N.; writing—original draft, W.G.; writing—review and editing, Z.L., S.Z. and W.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China, grant number 2020YFC1807804 and the National Key Research and Development Program of China, grant number 2019YFC1803503.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Special thanks are addressed to Xuming Ma and Qihui Yan for their support with the interpretation of XRD results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Chen, L.; Tsang, D.C.; Zhou, Y.; Rinklebe, J.; Song, H.; Kwon, E.E.; Baek, K.; Ok, Y.S. Mechanistic insights into red mud, blast furnace slag, or metakaolin-assisted stabilization/solidification of arsenic-contaminated sediment. Environ. Int. 2019, 133, 105247. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Annual Report on Environmental Statistics. 2017. Available online: http://www.mee.gov.cn/hjzl/sthjzk/sthjtjnb/ (accessed on 8 August 2021).
- Cheng, X.; Qi, W.; Huang, Q.; Zhao, X.; Fang, R.; Xu, J. Typical Geo-Hazards and Countermeasures of Mines in Yunnan Province, Southwest China. IOP Conf. Ser. Earth Environ. Sci. 2016, 44, 022008. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Wang, S.; Li, R.; Wang, J.; Zhang, Z. Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol. Environ. Saf. 2017, 141, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and health risk assessment of heavy metals in China’s lead–zinc mine tailings: A meta–analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Status and countermeasures on soil heavy metals pollution control in nonferrous metals industry in China. Nonferrous Met. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Shi, C.; Spence, R. Designing of Cement-Based Formula for Solidification/Stabilization of Hazardous, Radioactive, and Mixed Wastes. Crit. Rev. Environ. Sci. Technol. 2004, 34, 391–417. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental impacts and decarbonization strategies in the cement and concrete industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-S.; Chen, L.; Zhan, B.; Wang, L.; Poon, C.S.; Tsang, D.C. Sustainable stabilization/solidification of arsenic-containing soil by blast slag and cement blends. Chemosphere 2021, 271, 129868. [Google Scholar] [CrossRef]
- Wang, L.; Cho, D.-W.; Tsang, D.C.; Cao, X.; Hou, D.; Shen, Z.; Alessi, D.; Ok, Y.S.; Poon, C.S. Green remediation of As and Pb contaminated soil using cement-free clay-based stabilization/solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Guo, B.; Tsang, D.C.; Huang, L.; Ok, Y.S.; Mechtcherine, V. Red mud-enhanced magnesium phosphate cement for remediation of Pb and as contaminated soil. J. Hazard. Mater. 2020, 400, 123317. [Google Scholar] [CrossRef]
- Beiyuan, J.; Awad, Y.M.; Beckers, F.; Tsang, D.; Ok, Y.S.; Rinklebe, J. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 2017, 178, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Tsang, D.C.W.; Yip, A.; Olds, W.E.; Weber, P. Arsenic and copper stabilisation in a contaminated soil by coal fly ash and green waste compost. Environ. Sci. Pollut. Res. 2014, 21, 10194–10204. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wang, F.; Wang, L.; Liu, J.; Hashimoto, Y.; Hosomi, M. Arsenic immobilization and removal in contaminated soil using zero-valent iron or magnetic biochar amendment followed by dry magnetic separation. Sci. Total Environ. 2021, 768, 144521. [Google Scholar] [CrossRef]
- Criado, M.; Ke, X.; Provis, J.; Bernal, S.A. Alternative inorganic binders based on alkali-activated metallurgical slags. In Sustainable and Nonconventional Construction Materials using Inorganic Bonded Fiber Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 185–220. [Google Scholar] [CrossRef]
- Zhou, M.; Cheng, X.; Chen, X. Studies on the Volumetric Stability and Mechanical Properties of Cement-Fly-Ash-Stabilized Steel Slag. Materials 2021, 14, 495. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhang, B.; Yang, Y.; Liu, M.; Tang, G.; Liu, X. Research on the status of comprehensive utilization of steel slag. Ind. Miner. Process. 2021, 4, 31–35. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.-C.; Shi, C.; Pan, S.-Y. Characteristics of steel slags and their use in cement and concrete—A review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Wang, Q.; Liu, Z.; Qian, W.; Jin, C.; Chen, L.; Li, L. Study on Cementitious Properties and Hydration Characteristics of Steel Slag. Pol. J. Environ. Stud. 2018, 27, 357–364. [Google Scholar] [CrossRef]
- Li, Y.; Ni, W.; Gao, W.; Zhang, Y.; Yan, Q.; Zhang, S. Corrosion evaluation of steel slag based on a leaching solution test. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 41, 790–801. [Google Scholar] [CrossRef]
- Wang, L.; Geddes, D.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C. The role of zinc in metakaolin-based geopolymers. Cem. Concr. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Nguyen, H.; Carvelli, V.; Kunther, W.; Illikainen, M.; Kinnunen, P. Phase evolution and mechanical performance of an ettringite-based binder during hydrothermal aging. Cem. Concr. Res. 2021, 143, 106403. [Google Scholar] [CrossRef]
- Gao, W.; Ni, W.; Zhang, Y.; Li, Y.; Shi, T.; Li, Z. Investigation into the semi-dynamic leaching characteristics of arsenic and antimony from solidified/stabilized tailings using metallurgical slag-based binders. J. Hazard. Mater. 2019, 381, 120992. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Wan, Y.; Mi, Z.; Wang, Q.; Wang, Q.; Li, H. The fate of arsenic in rice plants (Oryza sativa L.): Influence of different forms of selenium. Chemosphere 2020, 264, 128417. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Ni, W.; Yan, Q.; Gao, W.; Li, Y. Immobilisation of high-arsenic-containing tailings by using metallurgical slag-cementing materials. Chemosphere 2019, 223, 117–123. [Google Scholar] [CrossRef]
- NanrRu, Y.; WenrHai, Y. The Handbook of Inorganic Matalloid Material Atlas; Wuhan University of Technology Press: Wuhan, China, 2000. [Google Scholar]
- Zhang, G.-S.; Qu, J.-H.; Liu, H.-J.; Liu, R.-P.; Li, G.-T. Removal Mechanism of As(III) by a Novel Fe−Mn Binary Oxide Adsorbent: Oxidation and Sorption. Environ. Sci. Technol. 2007, 41, 4613–4619. [Google Scholar] [CrossRef]
- Guan, X.; Chen, J.; Gao, Y.; Gao, J. Mechanical properties and microstructure of coal gangue mortar blocks excited by NaOH Alkali. J. Xi An Univ. Sci. Technol. 2020, 40, 658–664. [Google Scholar]
- Yazdani, M.R.; Tuutijärvi, T.; Bhatnagar, A.; Vahala, R. Adsorptive removal of arsenic (V) from aqueous phase by feldspars: Kinetics, mechanism, and thermodynamic aspects of adsorption. J. Mol. Liq. 2016, 214, 149–156. [Google Scholar] [CrossRef]
- Phearom, S.; Shahid, M.K.; Choi, Y.-G. Optimization of Arsenic Adsorption by Mill Scale–Derived Magnetite Particles Using Response Surface Methodology. J. Hazard. Toxic Radioact. Waste 2021, 25, 04021022. [Google Scholar] [CrossRef]
- Yi, Y.; Shi, J.; Tian, Q.; Guo, X.-Y. Arsenic removal from high-arsenic dust by NaOH-Na 2 S alkaline leaching. Chin. J. Nonferrous Met. 2015, 25, 806–814. [Google Scholar]
- Zhang, W.; Ma, B.; Wang, C. Thermodynamics analysis on formation of calcium arsenate slag. Nonferrous Met. 2019, 9, 61–66. [Google Scholar] [CrossRef]
- Avalos, N.M.; Varga, T.; Mergelsberg, S.T.; Silverstein, J.A.; Saslow, S.A. Behavior of iodate substituted ettringite during aqueous leaching. Appl. Geochem. 2020, 125, 104863. [Google Scholar] [CrossRef]
- Saslow, S.A.; Kerisit, S.N.; Varga, T.; Mergelsberg, S.T.; Corkhill, C.L.; Snyder, M.M.V.; Avalos, N.M.; Yorkshire, A.S.; Bailey, D.J.; Crum, J. Immobilizing Pertechnetate in Ettringite via Sulfate Substitution. Environ. Sci. Technol. 2020, 54, 13610–13618. [Google Scholar] [CrossRef]
- Jiménez, A.; Prieto, M. Thermal Stability of Ettringite Exposed to Atmosphere: Implications for the Uptake of Harmful Ions by Cement. Environ. Sci. Technol. 2015, 49, 7957–7964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.; Sasaki, K.; Hirajima, T. Selenite and selenate uptaken in ettringite: Immobilization mechanisms, coordination chemistry, and insights from structure. Cem. Concr. Res. 2017, 100, 166–175. [Google Scholar] [CrossRef]
- Li, Y.-C.; Min, X.-B.; Chai, L.-Y.; Shi, M.-Q.; Tang, C.-J.; Wang, Q.-W.; Liang, Y.-J.; Lei, J.; Liyang, W.-J. Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. J. Environ. Manag. 2016, 181, 756–761. [Google Scholar] [CrossRef]
- Lei, J.; Peng, B.; Min, X.; Liang, Y.; You, Y.; Chai, L. Modeling and optimization of lime-based stabilization in high alkaline arsenic-bearing sludges with a central composite design. J. Environ. Sci. Health Part. A 2017, 52, 1–10. [Google Scholar] [CrossRef]
- Lei, J.; Peng, B.; Liang, Y.-J.; Min, X.-B.; Chai, L.-Y.; Ke, Y.; You, Y. Effects of anions on calcium arsenate crystalline structure and arsenic stability. Hydrometallurgy 2018, 177, 123–131. [Google Scholar] [CrossRef]
- Cornelis, G.; Johnson, C.A.; Van Gerven, T.; Vandecasteele, C. Leaching mechanisms of oxyanionic metalloid and metal species in alkaline solid wastes: A review. Appl. Geochem. 2008, 23, 955–976. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).