Evaluation of Mass Transfer Coefficient during Scrap Melting
Abstract
1. Introduction
2. Experimental Determination of Mass Transfer Coefficient
2.1. Experimental Set-Up and Description
2.2. Calculation of Mass Transfer Coefficient
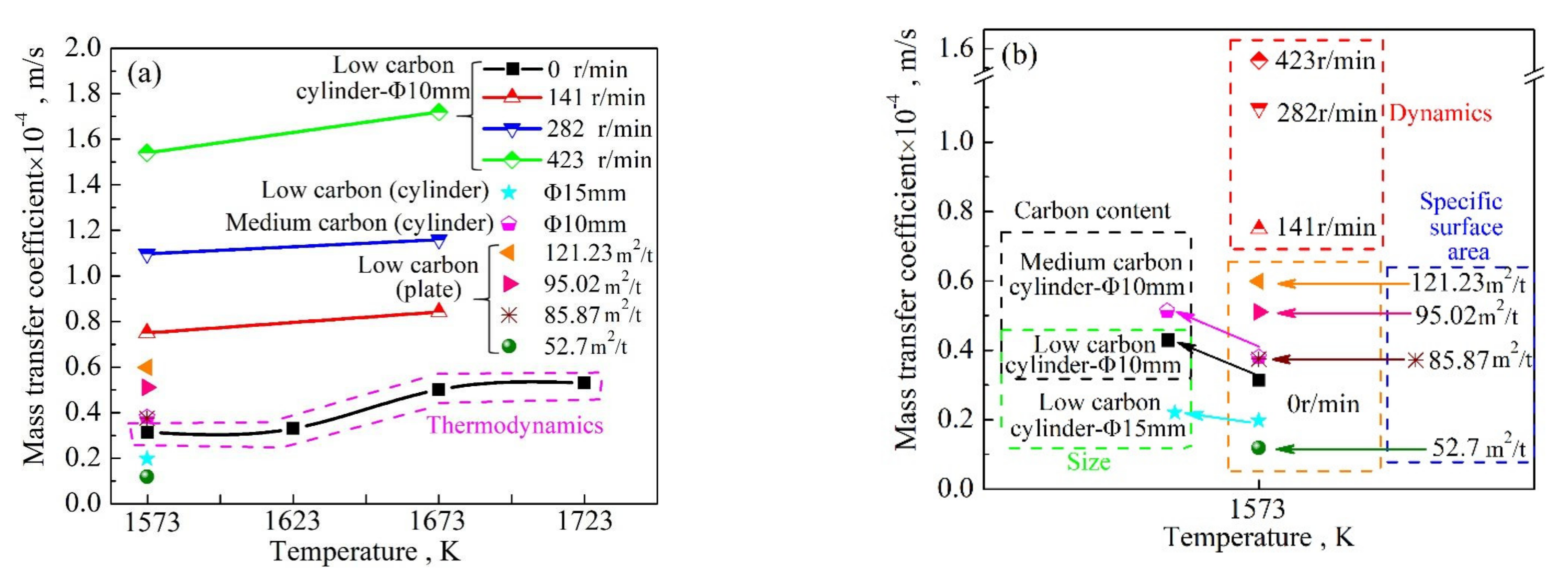
3. Qualitative Evaluation of Factors Affecting Mass Transfer Coefficient
3.1. Correlation Analysis of the Factors Affecting Mass Transfer Coefficient
3.2. Qualitative Evaluation of Mass Transfer Coefficient Using the Entropy Weight Method
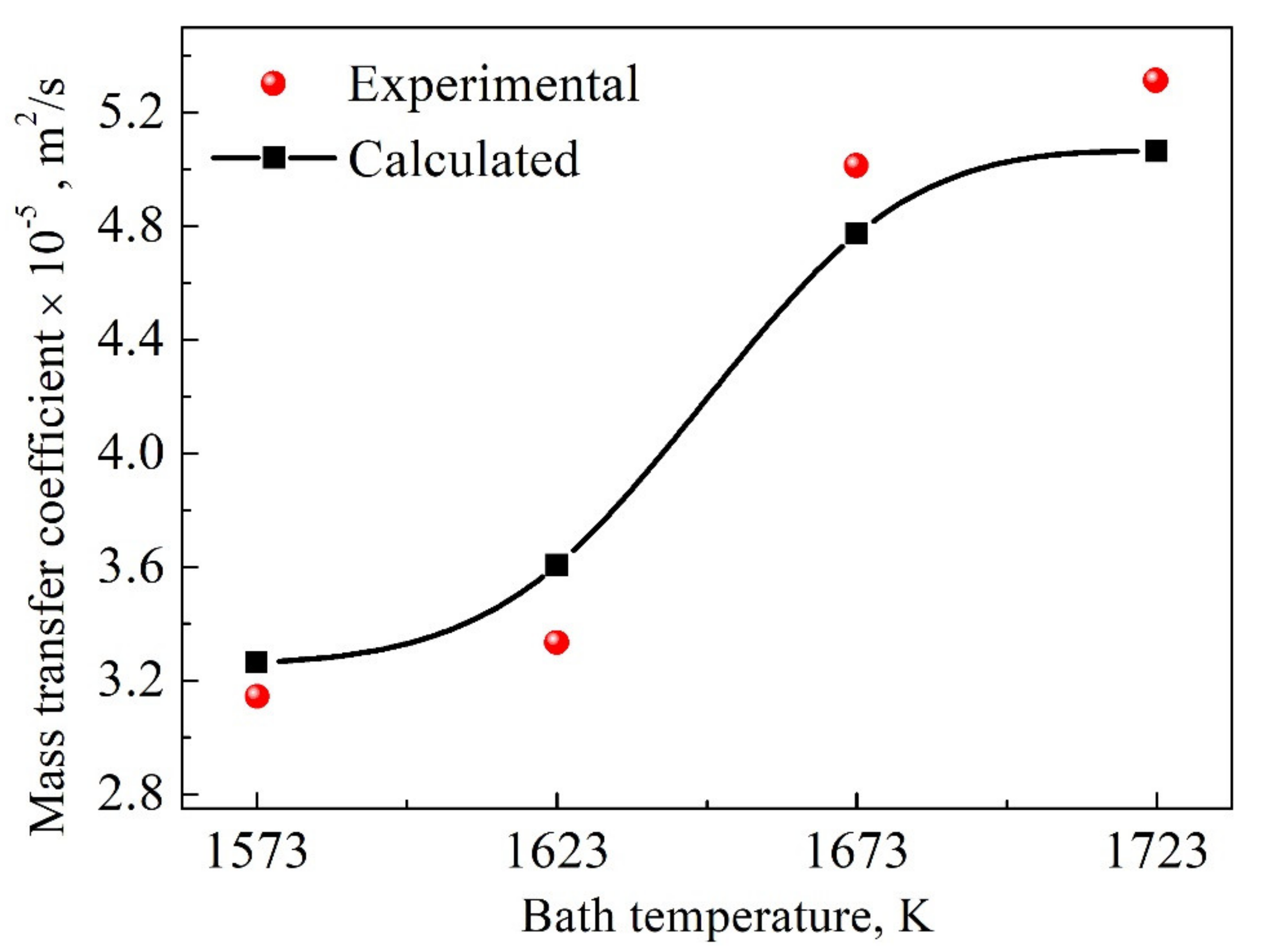
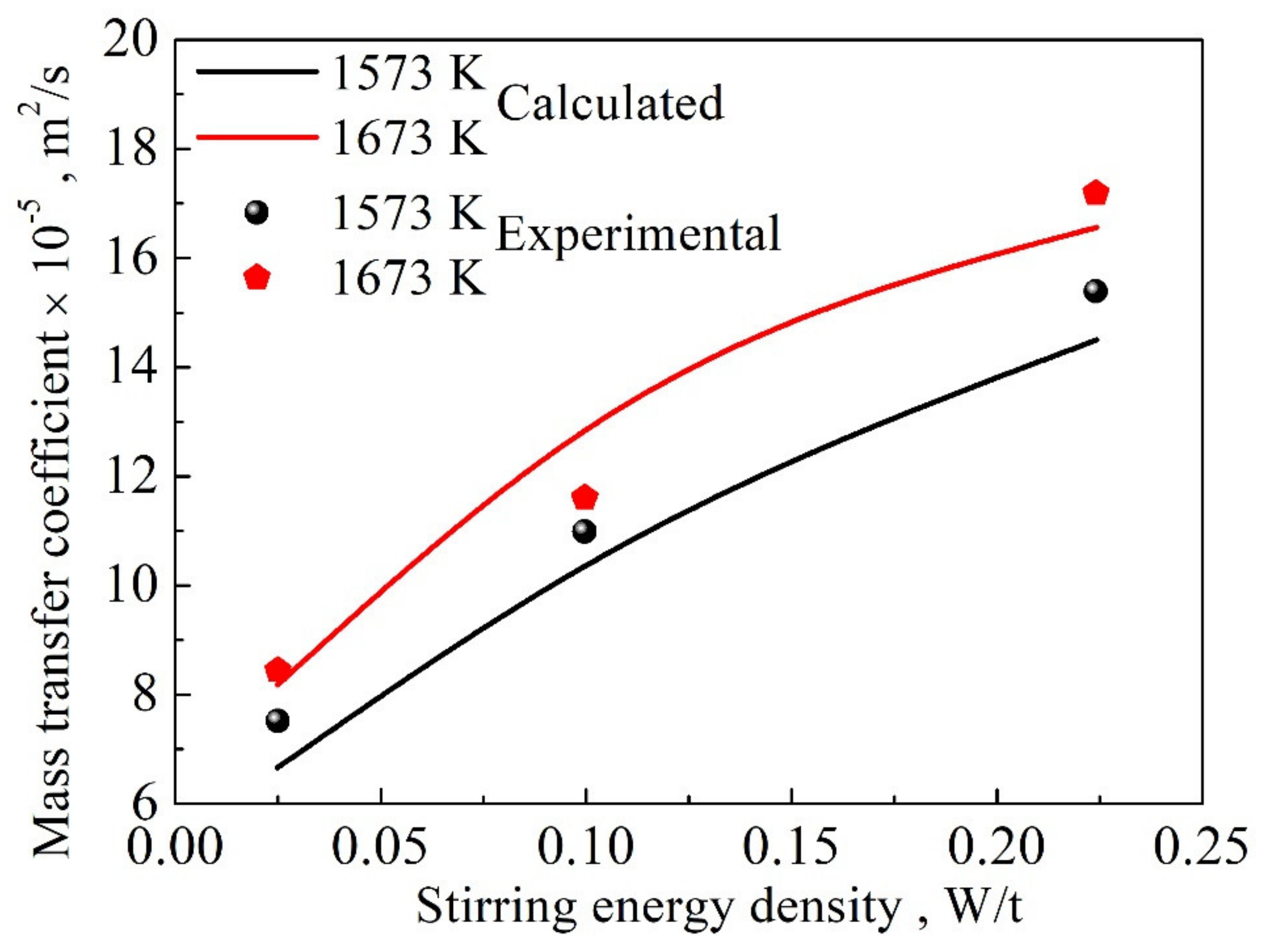
4. Quantitative Evaluation of Mass Transfer Coefficient Using Explicit Functions
4.1. Explicit Function under Natural Convection Conditions
4.2. Explicit Function under Forced Convection Conditions
4.3. Validation of Explicit Functions under Natural and Forced Convection Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brabie, L.C.; Kawakami, M. Kinetics of Steel Scrap Melting in Molten Fe-C Bath. High Temp. Mater. Process. 2000, 19, 241–256. [Google Scholar] [CrossRef]
- Yang, W.Y.; Zhang, X.G.; Yang, Y.T. The Study Overview of Utilizing Scrap in Convertor Steelmaking. In Proceedings of the 8th China Iron and Steel Annual Conference, Yantai, China, 25–28 October 2011. [Google Scholar]
- Yang, W.Y.; Jiang, X.F.; Li, L. Thermal Simulation Experiments on Scrap Melting. Iron Steel 2017, 52, 27–35. [Google Scholar]
- Kosaka, M.; Minowa, S. Dissolution of Steel Cylinder into Liquid Fe-C Alloy. (Stirring of Liquid Iron in H. F. Furnace-I). Tetsu-to-Hagane 1966, 52, 537–539, 1429–1432. [Google Scholar]
- Isobe, K.; Maede, H.; Ozawa, K.; Umezawa, K.; Saito, C. Analysis of the Scrap Melting Rate in High Carbon Molten Iron. Tetsu-to-Hagane 1990, 76, 2033–2040. [Google Scholar] [CrossRef][Green Version]
- Wei, G.; Zhu, R.; Tang, T.; Dong, K. Study on the Melting Characteristics of Steel Scrap in Molten Steel. Ironmak. Steelmak. 2019, 46, 609–617. [Google Scholar] [CrossRef]
- Li, R.H. The Basis of Mass Transfer; Beijing University Press: Beijing, China, 1987; pp. 195–251. [Google Scholar]
- Don, W.P. Chemical Engineers’ Handbook, 7th ed; Science Press: Beijing, China, 2001; p. 5. [Google Scholar]
- Wright, J.K. Steel Dissolution in Quiescent and Gas Stirred Fe/C Melts. Metall. Mater. Trans. B 1989, 20, 363–374. [Google Scholar] [CrossRef]
- Kim, Y.U.; Pehlke, R. Mass Transfer During Dissolution of a Solid into Liquid in the Iron—Carbon System. Metall. Mater. Trans. A 1974, 5, 2527–2532. [Google Scholar] [CrossRef]
- Kosaka, M.; Minowa, S. Dissolution of Steel Cylinder into Liquid Fe-C Alloy. Tetsu-to-Hagane 1967, 53, 983–997. [Google Scholar] [CrossRef]
- Kosaka, M.; Minowa, S. Mass-Transfer from Graphite Cylinder into Liquid Fe-C Alloy. Tetsu-to-Hagane 2010, 53, 1467–1477. [Google Scholar] [CrossRef]
- Shukla, A.K.; Deo, B.; Robertson, D.G.C. Scrap Dissolution in Molten Iron Containing Carbon for the Case of Coupled Heat and Mass Transfer Control. Metall. Mater. Trans. B 2013, 44, 1407–1427. [Google Scholar] [CrossRef]
- Szekely, J.; Chuang, Y.K.; Hlinka, J.W. The Melting and Dissolution of Low-Carbon Steels in Iron—Carbon Melts. Metall. Mater. Trans. B 1972, 3, 2825–2833. [Google Scholar] [CrossRef]
- Gao, M. Fundamentals of Scrap Melting in the Steelmaking Process. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2021. [Google Scholar]
- Sun, H.; Liu, Y.; Lin, C.; Muh-Jung, L.U. Experimental Observation of Spherical Scrap Melting in Hot Metal. In Proceedings of the 6th International Congress on the Science & Technology of Steelmaking, Beijing, China, 12–14 May 2015; pp. 136–139. [Google Scholar]
- Pehlke, R.D.; Goodell, P.D.; Dunla, R.W. Kinetics of Steel Dissolution in Molten Pig Iron. Trans. Metall. Soc. AlME 1964, 233, 1420–1427. [Google Scholar]
- Liu, Y.S.; Gao, J.P. Water Model Study on the Feature of Scrap Melting in the Oxygen Top-Bottom Combined Blowing Process. J. Iron Steel Res. 1990, 2, 1–7. [Google Scholar]
- Kruskopf, A. A Model for Scrap Melting in Steel Converter. Metall. Mater. Trans. B 2015, 46, 1195–1206. [Google Scholar] [CrossRef]
- Yang, G.; Deng, S.; Xu, A.J.; Dai, X.Q. Analysis and Calculation of Steel Scrap Melting in Multifunctional Hot Metal Ladle. J. Cent. South Univ. (Sci. Tech.) 2019, 5, 1021–1027. [Google Scholar]
- Wu, M.H. Simulation Study on the Stirring of the Converter Bath by the Bottom Blowing. Hebei Metall. 1987, 4, 3–17. [Google Scholar]
- Zuo, D.W. Research and Practice on Reducing Tapping Temperature of BOF. Hunan Metall. 2003, 2, 32–35. [Google Scholar]
- Wanibe, Y.; Shoji, T.; Fujisawa, T.; Sakao, H. Temperature-Dependency of Interdiffusion in Molten Fe-C Alloy. Iron Steel Inst. Jpn. Trans. 1982, 22, 560–565. [Google Scholar] [CrossRef][Green Version]
- Florian Penz, M.; Johannes, S. Diffusive steel scrap melting in carbon-saturated hot metal—Phenomenological investigation at the solid-liquid interface. Materials 2019, 12, 1358. [Google Scholar] [CrossRef]
- Florian Penz, M.; Johannes, S. A Review of Steel Scrap Melting in Molten Iron-Carbon Melts. Steel Res. Int. 2019, 90, 1900124. [Google Scholar] [CrossRef]
- Florian Penz, M.; Johannes, S. Dissolution of Scrap in Hot Metal under Linz Donawitz (LD) Steelmaking Conditions. Metals 2018, 8, 1078. [Google Scholar] [CrossRef]


| C | Si | P | Mn | S | |
|---|---|---|---|---|---|
| Q235 low-carbon steel rod | 0.168 | 0.110 | 0.041 | 0.391 | 0.0227 |
| Q235 low-carbon steel plate | 0.182 | 0.17~0.37 | ≤0.030 | 0.35–0.65 | 0.0011 |
| 45# steel rod | 0.491 | 0.27 | 0.035 | 0.65 | 0.035 |
| QT500-7 Ductile iron rod | 3.69 | 1.653 | 0.0077 | 0.0825 | 0.0125 |
| Iron/carbon bath | 4.61 | 0.42 | 0.17 | 0.33 | 0.04 |
| Serial Number | Bath Temperature | Stirring Energy Density | Specific Surface Area | Carbon Content | Mass Transfer Coefficient |
|---|---|---|---|---|---|
| - | K | W/t | m2/t | wt% | ×10−4 m/s |
| 1 | 1573 | 0 | 56.03 | 0.168 | 0.314 |
| 2 | 1623 | 0 | 56.03 | 0.168 | 0.333 |
| 3 | 1673 | 0 | 56.03 | 0.168 | 0.501 |
| 4 | 1723 | 0 | 56.03 | 0.168 | 0.531 |
| 5 | 1573 | 0.0249 | 56.03 | 0.168 | 0.750 |
| 6 | 1573 | 0.0997 | 56.03 | 0.168 | 1.097 |
| 7 | 1573 | 0.2243 | 56.03 | 0.168 | 1.540 |
| 8 | 1673 | 0.0249 | 56.03 | 0.168 | 0.842 |
| 9 | 1673 | 0.0997 | 56.03 | 0.168 | 1.159 |
| 10 | 1673 | 0.2243 | 56.03 | 0.168 | 1.720 |
| 11 | 1573 | 0 | 56.03 | 0.491 | 0.379 |
| 12 | 1573 | 0 | 52.7 | 0.180 | 0.131 |
| 13 | 1573 | 0 | 95.02 | 0.180 | 0.452 |
| 14 | 1573 | 0 | 121.2 | 0.180 | 0.569 |
| 15 | 1573 | 0 | 85.87 | 0.180 | 0.375 |
| Bath Temperature | Stirring Energy Density | Scrap Specific Surface Area | Scrap Carbon Content | |
|---|---|---|---|---|
| Mass transfer coefficient | 0.533 | 0.960 | 0.362 | 0.016 |
| Influencing Factors | Factors | Serial Number | Value | Rank | |
|---|---|---|---|---|---|
| Bath temperature | Bath temperature | r1 | 0.0453 | 0.0453 | 2 |
| r2 | 0.0359 | ||||
| r3 | 0.0541 | ||||
| r4 | 0.0573 | ||||
| Stirring of the molten pool | Rotating speed | r5 | 0.1279 | 0.1279 | 1 |
| r6 | 0.1184 | ||||
| r7 | 0.1663 | ||||
| r8 | 0.0909 | ||||
| r9 | 0.1251 | ||||
| r10 | 0.1857 | ||||
| Scrap type | Carbon content | r11 | 0.0411 | 0.0411 | 3 |
| Scrap specific surface area | r12 | 0.0141 | |||
| r13 | 0.0488 | ||||
| r14 | 0.0614 | ||||
| r15 | 0.0405 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Gao, J.-T.; Zhang, Y.-L. Evaluation of Mass Transfer Coefficient during Scrap Melting. Metals 2021, 11, 1368. https://doi.org/10.3390/met11091368
Gao M, Gao J-T, Zhang Y-L. Evaluation of Mass Transfer Coefficient during Scrap Melting. Metals. 2021; 11(9):1368. https://doi.org/10.3390/met11091368
Chicago/Turabian StyleGao, Ming, Jin-Tao Gao, and Yan-Ling Zhang. 2021. "Evaluation of Mass Transfer Coefficient during Scrap Melting" Metals 11, no. 9: 1368. https://doi.org/10.3390/met11091368
APA StyleGao, M., Gao, J.-T., & Zhang, Y.-L. (2021). Evaluation of Mass Transfer Coefficient during Scrap Melting. Metals, 11(9), 1368. https://doi.org/10.3390/met11091368






