The Efficient Removal of Calcium and Magnesium Ions from Industrial Manganese Sulfate Solution through the Integrated Application of Concentrated Sulfuric Acid and Ethanol
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Equipment
2.2. Simulation Software and Methods
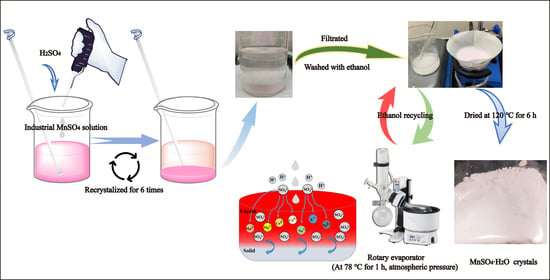
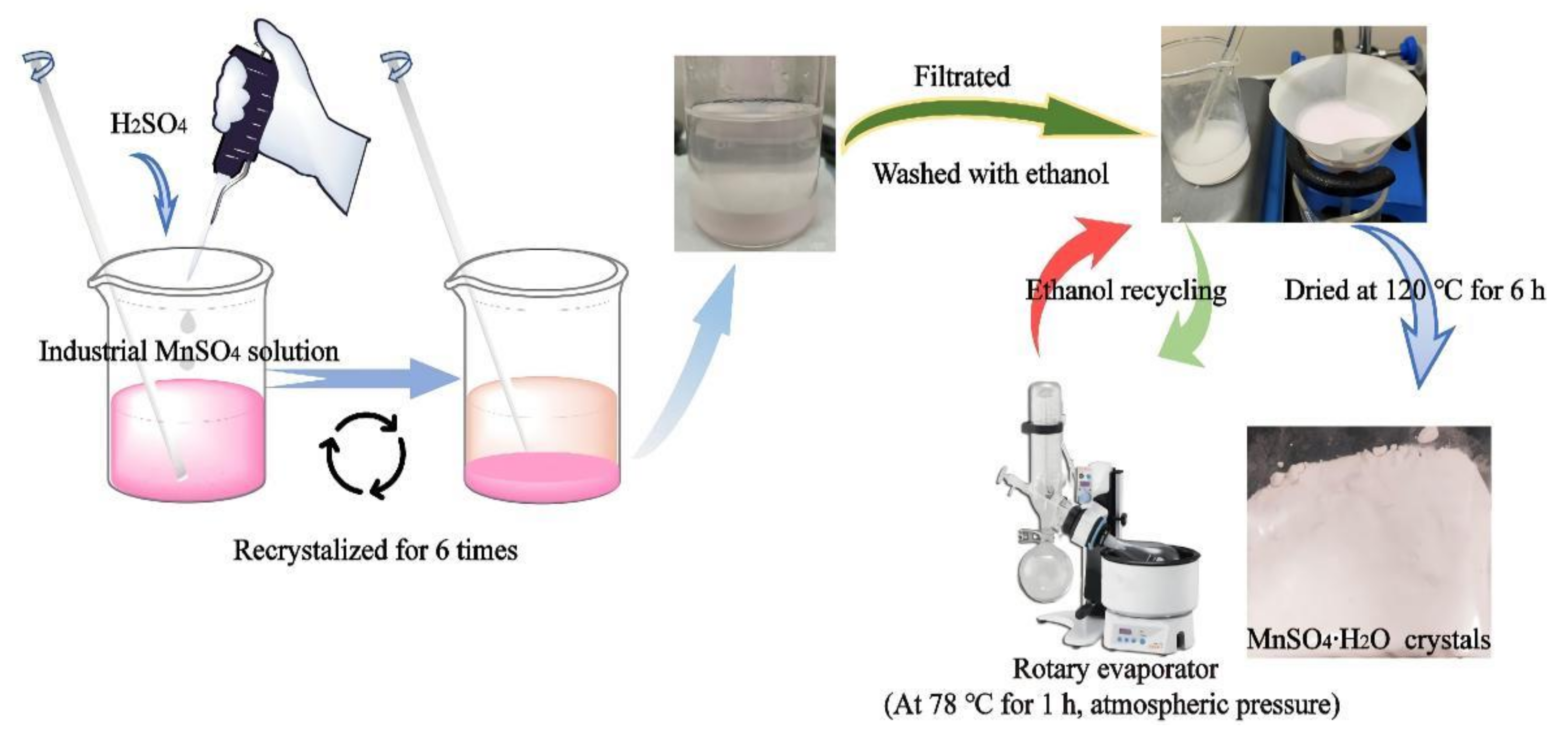
2.3. Preparation of High-Purity MnSO4·H2O
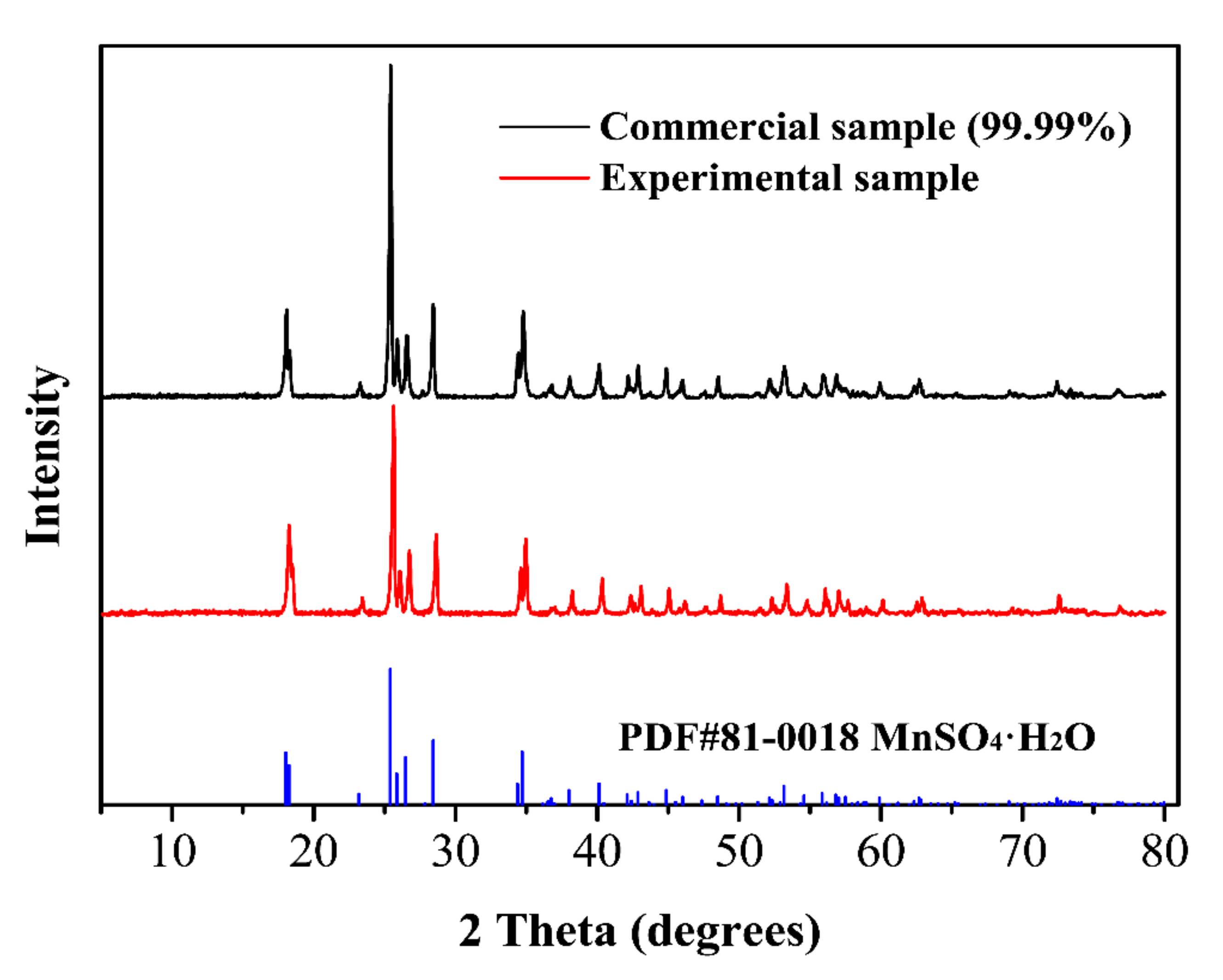
2.4. Characterization
3. Results and Discussions
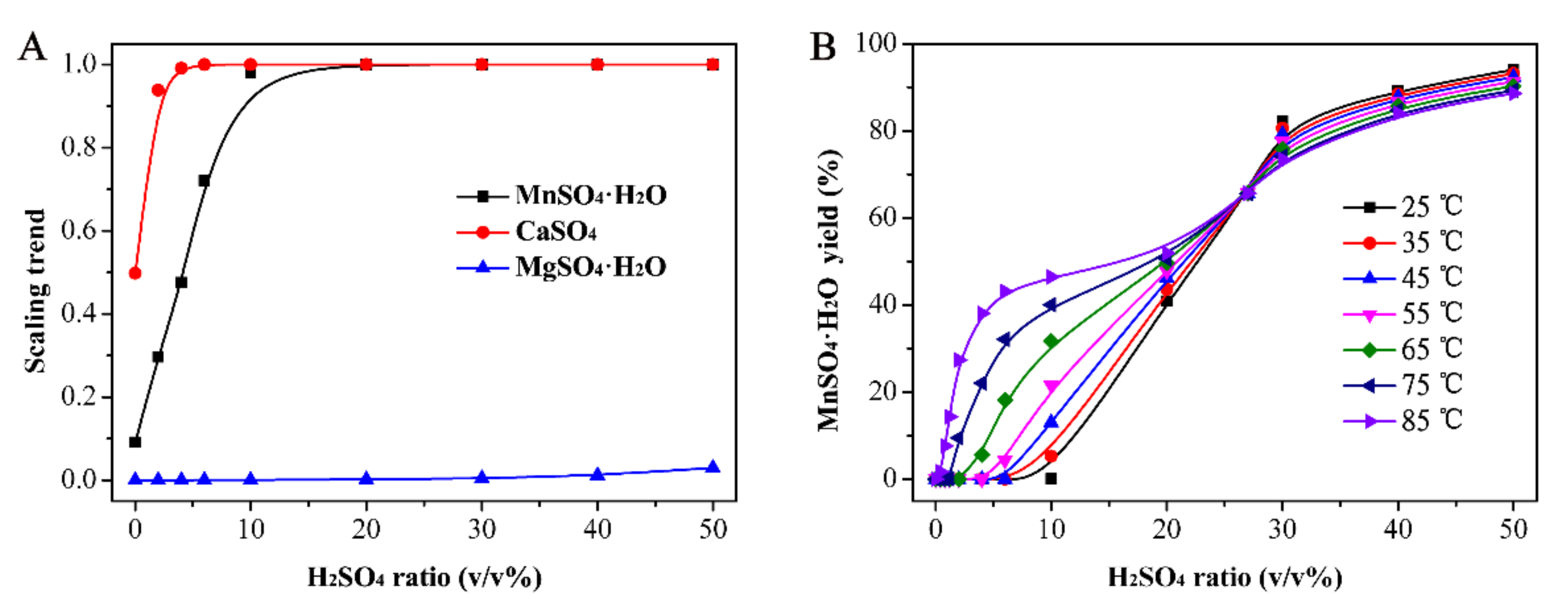
3.1. Simulation Results of OLI Stream Analyzer Software
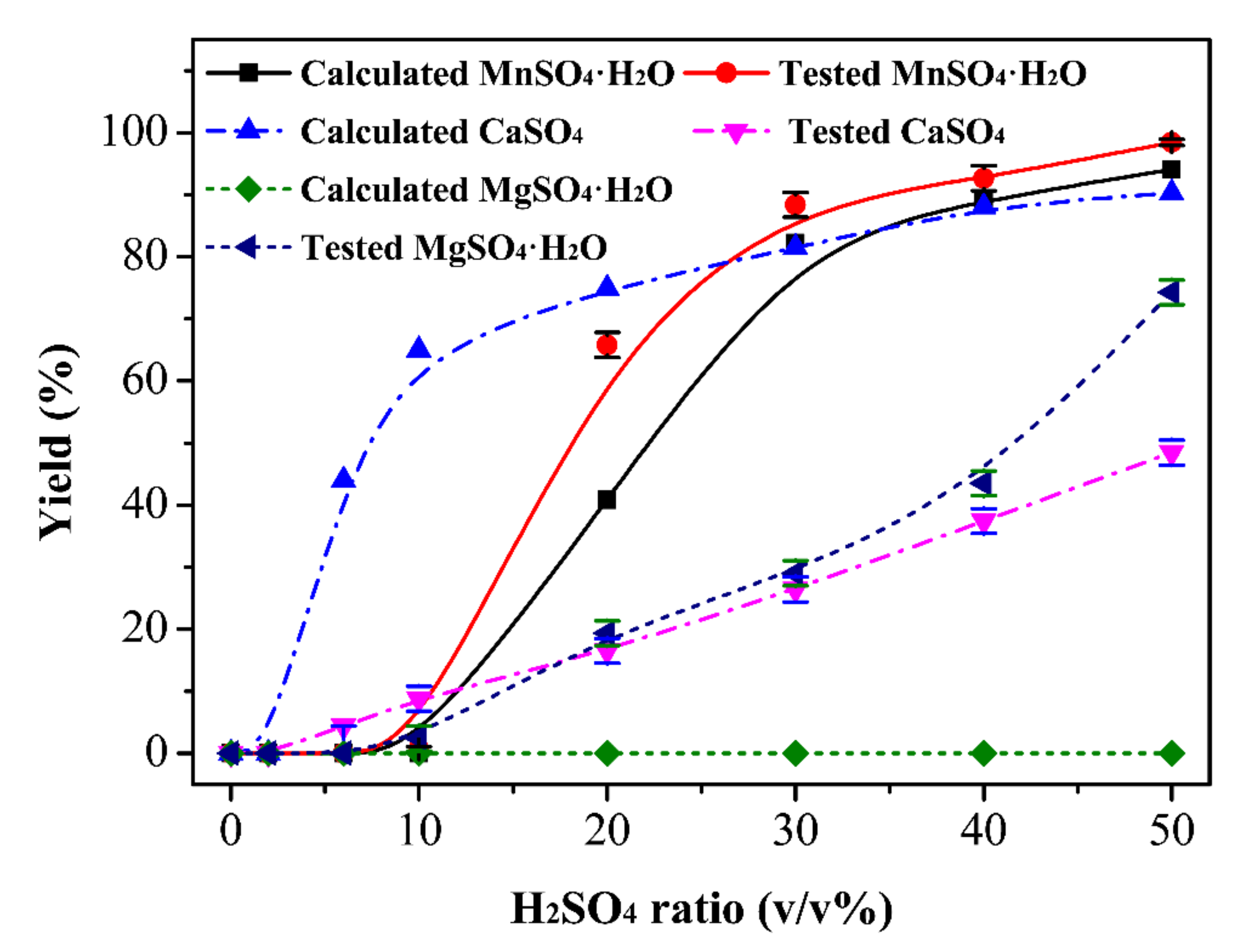
3.2. Calculated and Tested Yield of Different Components
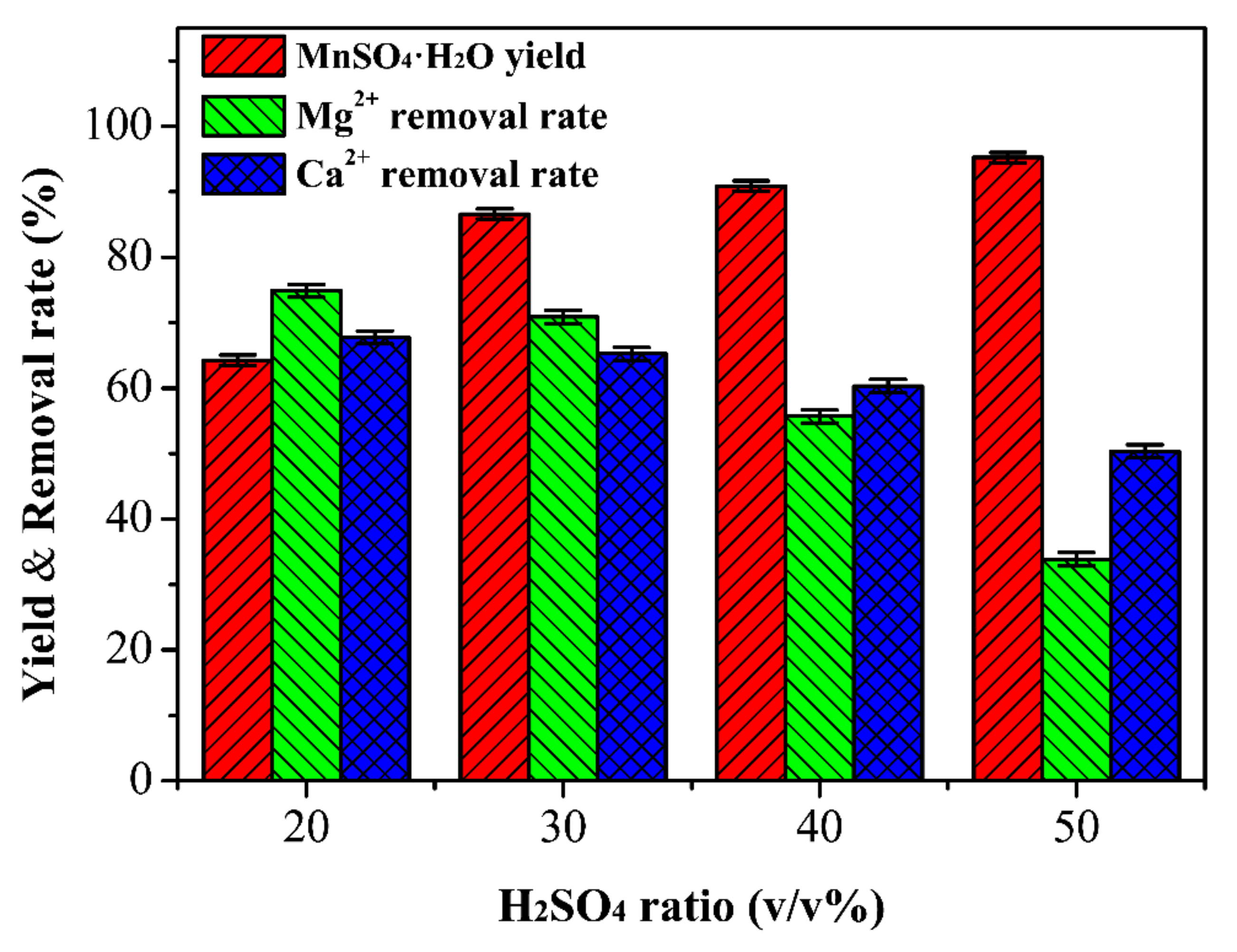
3.3. Determination of the Optimal Ratio of H2SO4
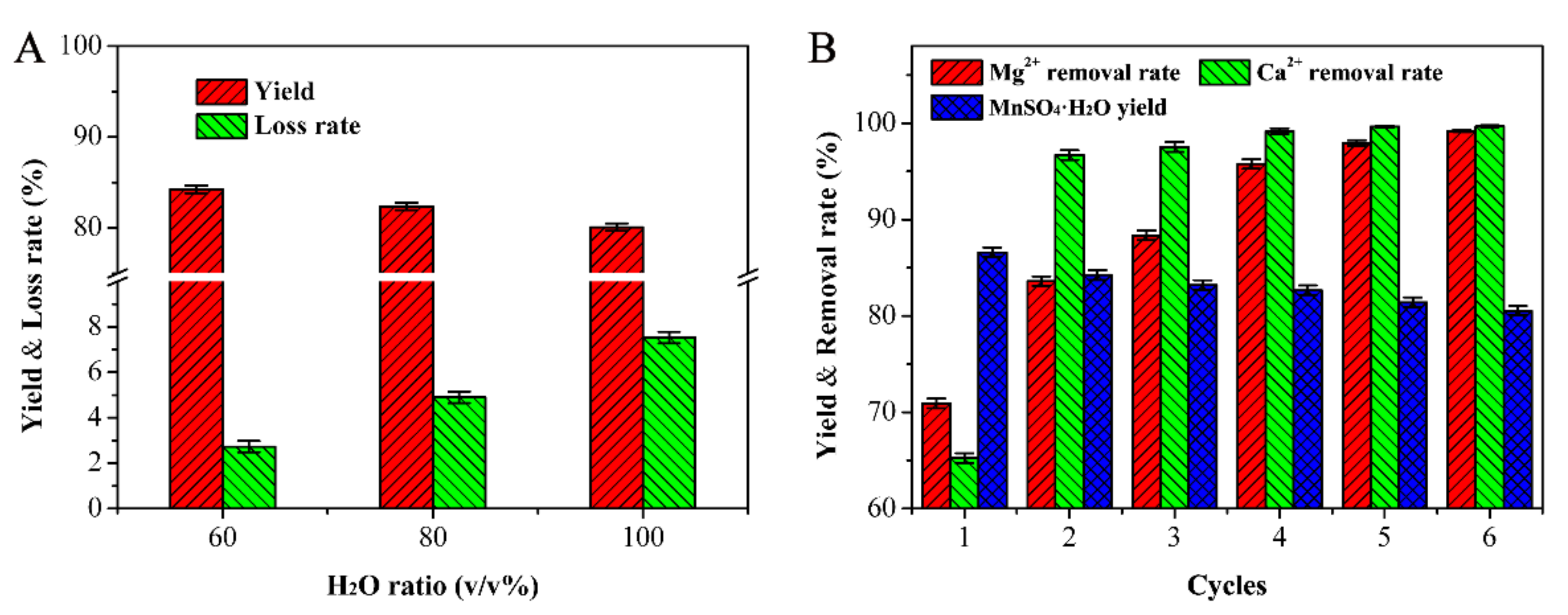
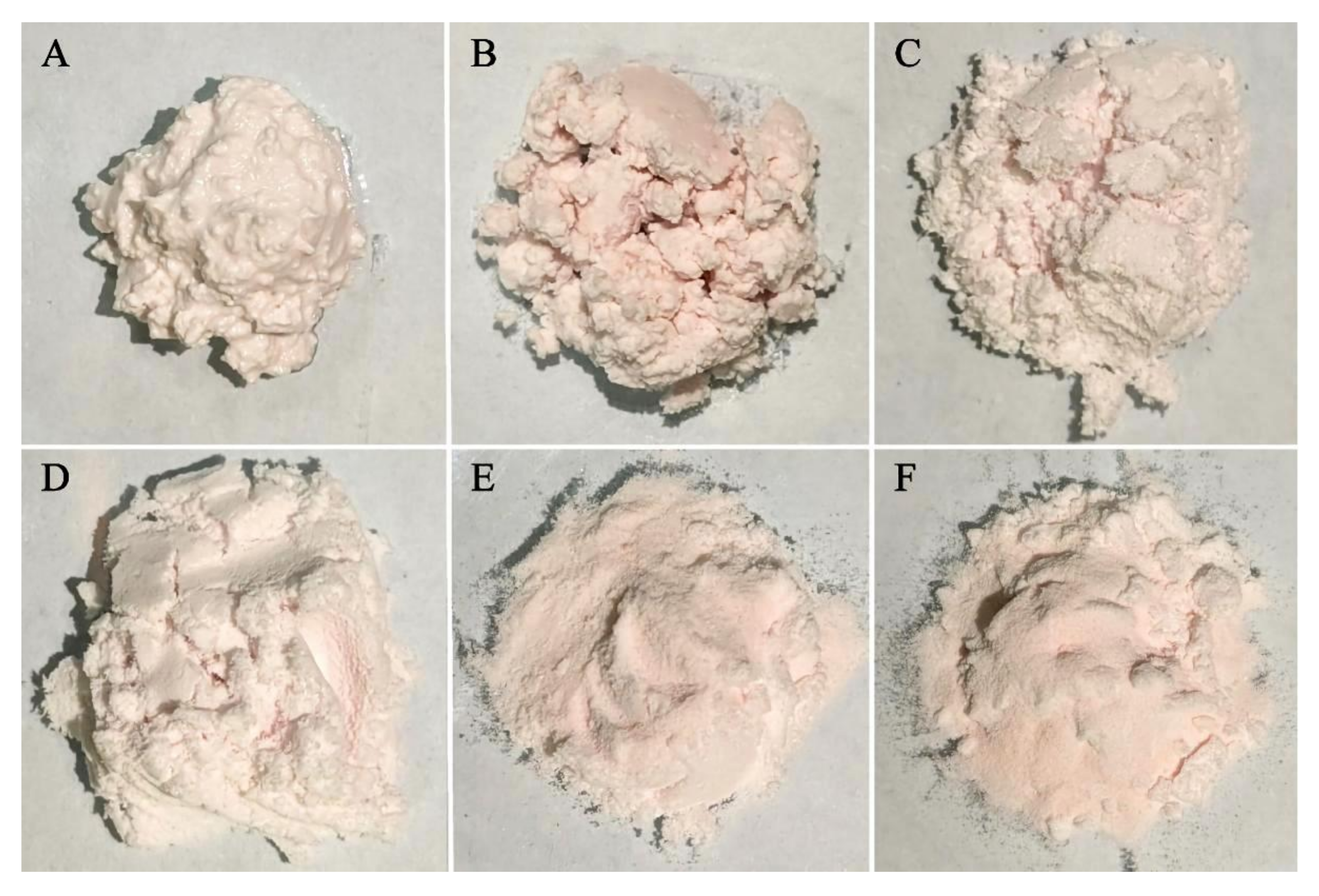
3.4. Recrystallization Experiment
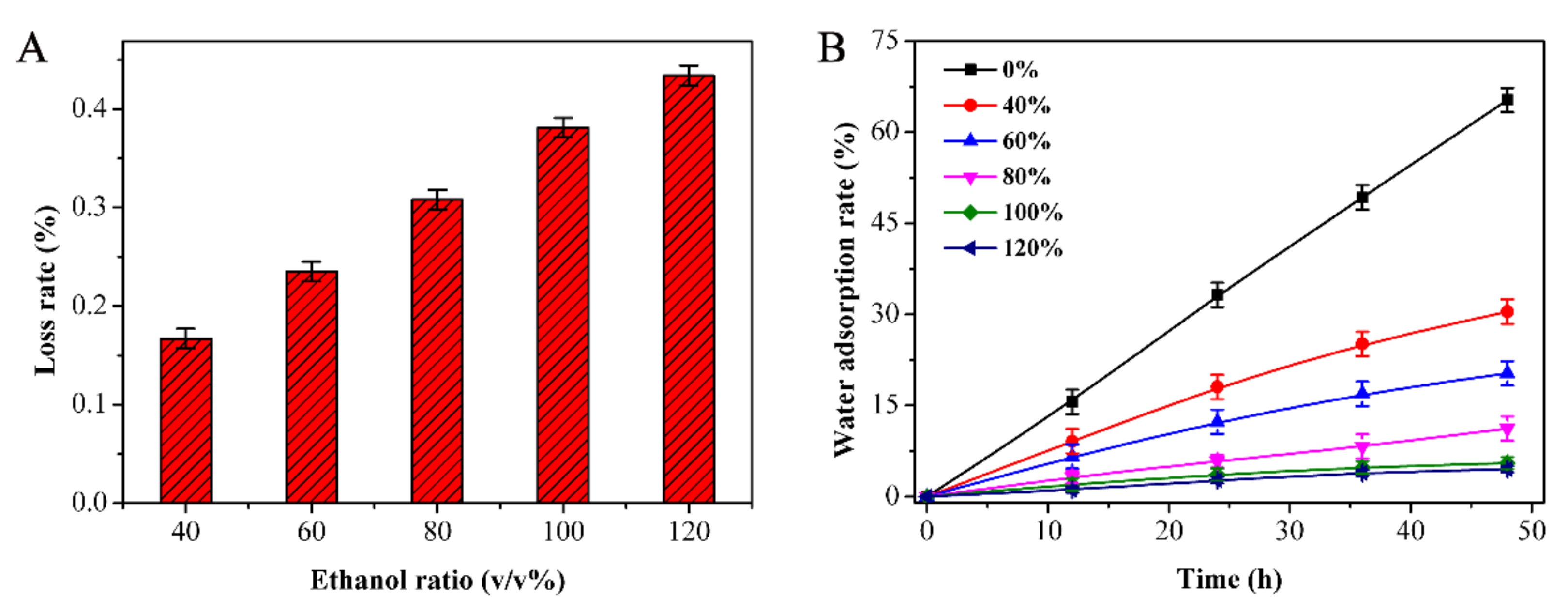
3.5. Determination of the Optimal Ratio of Ethanol
3.6. High-Purity MnSO4·H2O Test Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, Y.; Makhlouf, M. Stabilizing the strengthening precipitates in aluminum-manganese alloys by the addition of tungsten. Mater. Sci. Eng. A 2017, 691, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Z.; Spasova, M.; Yelsukova, A.E.; Lu, S.; Farle, M.; Wiedwald, U.; Gökce, B. Formation Mechanism of Laser-Synthesized Iron-Manganese Alloy Nanoparticles, Manganese Oxide Nanosheets and Nanofibers. Part. Part. Syst. Charact. 2017, 34, 1600225. [Google Scholar] [CrossRef]
- Tan, L.; Hu, H.; Liao, J.; Wang, C.; Li, C. Preparation of High-purity Manganese Sulfate for Battery by Solvent Extraction. Nonferrous Met. (Extr. Metall.) 2014, 9, 62–65. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, K.; Chen, F.; Jiang, Q. Application Prospect and Development of High Pure Manganese Sulfate Monohydrate in Battery Level. China’s Manganese Ind. 2014, 32, 6–8. [Google Scholar] [CrossRef]
- Xie, Y.; Ou, Y.; Hu, B.; Ling, Z. Study on production process for high-purity manganese sulfate with waste sulphuric acid of titanium white-pyrolusite-pyrite. Inorg. Chem. Ind. 2013, 45, 49–51. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, R.; Hu, P.; Jiang, Y.; Jiang, Y. Study on Impurity Characteristics and Purification Process in High Purity Manganese Sulfate. World Nonferrous Metals 2020, 186–188. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.; Li, L.; Wang, Y.; Wang, J.; Xu, J.; Si, H.; Peng, B.; Zhu, Z. Study on the process of preparing high-purity manganese sulfate from low-grade manganese carbonate ore. Inorg. Chem. Ind. 2014, 46, 35–38. [Google Scholar]
- Wang, Y.; Wang, W.; Liu, Y.; Liu, F.; Chen, X. Removal of Heavy Metals from manganese sulfate solution and preparation of Battery-level manganese sulfate. Nonferrous Metals 2020, 79–84. [Google Scholar] [CrossRef]
- Bao, X.; Wang, Z.; Liu, J.; Su, Z.; Tian, S.; Zhou, D.; Wang, H. Experimental study on the removal of calcium, magnesium and iron from the depth of industrial manganese sulfate. Min. Metall. Eng. 2013, 33, 90–93. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, Y.; Xie, X.; Nong, Y. Removal of Calcium and Magnesium from Manganese Sulfate by Manganese Fluoride. Technol. Dev. Chem. Ind. 2020, 49, 9–11. [Google Scholar] [CrossRef]
- He, T.; Qian, L.; Cui, J.; Shu, H. Deep Removal of Ca and Mg from Industrial Manganese Sulfate with Fluorination Precipitation. Nonferrous Met. (Extr. Metall.) 2018, 5–8. [Google Scholar] [CrossRef]
- Ang, H.M.; Reyhani, M.; Tad, M.O.; Safaeefar, P. Growth Kinetics of Manganese Sulphate from Heating and Salting-out Batch Crystallisation. Dev. Chem. Eng. Miner. Process. 2006, 14, 303–312. [Google Scholar] [CrossRef]
- Li, X.; Ji, D.; Su, H.; Pan, L.; Li, J.; Li, J.; Wen, Y. Study on production of manganese sulphate by high-temperature crystallizing process. Inorg. Chem. Ind. 2010, 42, 16–19. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Cao, J.; Kou, X. Purification of calcium and magnesium from industrial manganese sulfate liquid by fluoride precipitant. Ind. Water Treat. 2019, 39, 77–81. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, L.; Zhang, G.; Guan, W.; Sun, Z.; Zhang, D.; Qing, J. A novel process on separation of manganese from calcium and magnesium using synergistic solvent extraction system. Hydrometallurgy 2019, 185, 55–60. [Google Scholar] [CrossRef]
- Dan, D.; Liu, Z.; Sun, L.; Tang, L.; Zou, X. Removal of Ca and Mg Ions from Industrial Manganese Sulfate Solution by Solvent Extraction. J. Jishou Univ. 2016, 37, 55–62. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, G. Study on removal of calcium and magnesium from Leaching solution of manganese ore by solvent extraction. China’s Manganese Ind. 2008, 26, 34–37. [Google Scholar] [CrossRef]
- Ilea, P.; Popescu, I.C.; Urdǎ, M.; Oniciu, L. The electrodeposition of manganese from aqueous solutions of MnSO4. IV: Electrowinning by galvanostatic electrolysis. Hydrometallurgy 1997, 46, 149–156. [Google Scholar] [CrossRef]
- Farrah, H.E.; Lawrance, G.A.; Wanless, E.J. Solubility of calcium sulfate salts in acidic manganese sulfate solutions from 30 to 105℃. Hydrometallurgy 2007, 86, 13–21. [Google Scholar] [CrossRef]
- He, Y.; Li, F.; Luo, Z.; Luo, K. Preparation of Battery-Grade Manganese Sulfate by Purification of Industrial-Grade Manganese Sulfate with High-Temperature Crystallization Method. Min. Metall. Eng. 2019, 39, 85–88. [Google Scholar] [CrossRef]
- Yuan, F.; Feng, Y.; Li, H.; Yi, A.; Li, H.; Wu, M.; Guo, C. Research on Separation of Manganese and Manganese From Manganese Sulfate Solution Containing Magnesium by Evaporation Crystallization. Hydrometall. China 2015, 34, 225–228. [Google Scholar] [CrossRef]
- Giulietti, M.; Seckler, M.M.; Derenzo, S.; Ré, M.I.; Cekinski, E. Industrial Crystallization and Precipitation from Solutions: State of the Technique. Braz. J. Chem. Eng. 2001, 18, 423–440. [Google Scholar] [CrossRef]
- Zou, X. Present Situation of Production Technology of High Purity Manganese Sulfate. China’s Manganese Ind. 2018, 36, 4–6. [Google Scholar] [CrossRef]
- Jakovljevic, B.; Bourget, C.; Nucciarone, D. CYANEX (R) 301 binary extractant systems in cobalt/nickel recovery from acidic chloride solutions. Hydrometallurgy 2004, 75, 25–36. [Google Scholar] [CrossRef]
- Pakarinen, J.; Paatero, E. Effect of temperature on Mn-Ca selectivity with organophosphorus acid extractants. Hydrometallurgy 2011, 106, 159–164. [Google Scholar] [CrossRef]
- Benrath, V.A.; Blankenstein, A. Uber mischkristalle in der vitriolreihe I. Z. Furanorgaische Und All Gem. Chem Emio 1933, 216, 41–47. [Google Scholar] [CrossRef]
- Yang, S.; Ren, B.; Wang, C. The Study of Magnesium Removing by Ethanol Recycling for the Technology of Electrolytic Manganese Processing. China’s Manganese Ind. 2011, 29, 15–17. [Google Scholar] [CrossRef]
- Li, W.; Jia, J.; Zhao, X.; Ji, Y. Simulation and analysis of produced water in Anhui oil production plant with software OLI ANALYZER STUDIO. Comput. Appl. Chem. 2013, 30, 179–182. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Feng, J.; Yan, W. A Review of Fabrication and Purification of Manganese Sulphate. China’s Manganese Ind. 2017, 35, 114–118. [Google Scholar] [CrossRef]
- Yuan, M.; Qiu, G. Study on the removing of MgSO4 from MnSO4 solution based on the aqueous system phase diagram. Cent. South Univ. Technol. 2000, 212–214. [Google Scholar] [CrossRef]
- Cottrell, F.G. On the Solubility of Manganous Sulphate. J. Phys. Chem. 2002, 4, 637–656. [Google Scholar] [CrossRef][Green Version]
- Matricardi, L.R.; Downing, J.; Staff, U.B. Manganese and Manganese Alloys. Kirk-Othmer Encycl. Chem. Technol. 2012. [Google Scholar] [CrossRef]
- Safaeefar, P.; Ang, H.M.; Kuramochi, H.; Asakuma, Y.; Maeda, K.; Tade, M.O.; Fukui, K. Measurement and correlation of the solubility of MnSO4·H2O in ethanol+water+MgSO4·7H2O solutions. Fluid Phase Equilibria 2006, 250, 64–69. [Google Scholar] [CrossRef]
| Ion | Mn (g/L) | Mg (mg/L) | Ca (mg/L) | Na (mg/L) | K (mg/L) | Ni (mg/L) | Co (mg/L) | Zn (mg/L) |
|---|---|---|---|---|---|---|---|---|
| Concentration | 139.20 ± 1.24 | 1726.39 ± 39.51 | 544.95 ± 8.99 | 93.11 ± 9.13 | 23.62 ± 6.91 | 236.87 ± 6.88 | 64.20 ± 1.02 | 48.20 ± 7.94 |
| Cycle Number | Mg/wt.% | Ca/wt.% |
|---|---|---|
| 1 | 0.11 | 0.042 |
| 2 | 0.062 | 0.0040 |
| 3 | 0.044 | 0.0030 |
| 4 | 0.016 | 0.0010 |
| 5 | 0.0078 | 0.00044 |
| 6 | 0.0031 | 0.00039 |
| Inspected Item | Wt.% | |
|---|---|---|
| Standard | Result | |
| MnSO4·H2O | ≥99 | 99.3 |
| Mn | ≥32 | 32.3 |
| Fe | ≤0.001 | 0.0005 |
| Zn | ≤0.001 | 0.004 |
| Cu | ≤0.001 | <0.001 |
| Pb | ≤0.001 | 0.0005 |
| Cd | ≤0.0005 | 0.0001 |
| K | ≤0.01 | <0.001 |
| Na | ≤0.01 | 0.002 |
| Ca | ≤0.01 | 0.002 |
| Mg | ≤0.01 | 0.004 |
| Ni | ≤0.005 | <0.001 |
| Co | ≤0.005 | 0.001 |
| insoluble residue | ≤0.01 | 0.007 |
| pH (100 g/L, 25 °C) | 4.0–6.5 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, K.; Ming, X.; Zhan, F.; Muhammad, Y.; Wei, Y.; Li, W.; Zhan, H. The Efficient Removal of Calcium and Magnesium Ions from Industrial Manganese Sulfate Solution through the Integrated Application of Concentrated Sulfuric Acid and Ethanol. Metals 2021, 11, 1339. https://doi.org/10.3390/met11091339
Chen H, Wang K, Ming X, Zhan F, Muhammad Y, Wei Y, Li W, Zhan H. The Efficient Removal of Calcium and Magnesium Ions from Industrial Manganese Sulfate Solution through the Integrated Application of Concentrated Sulfuric Acid and Ethanol. Metals. 2021; 11(9):1339. https://doi.org/10.3390/met11091339
Chicago/Turabian StyleChen, Houyang, Kaituo Wang, Xianquan Ming, Feng Zhan, Yaseen Muhammad, Yuezhou Wei, Weijian Li, and Haiqing Zhan. 2021. "The Efficient Removal of Calcium and Magnesium Ions from Industrial Manganese Sulfate Solution through the Integrated Application of Concentrated Sulfuric Acid and Ethanol" Metals 11, no. 9: 1339. https://doi.org/10.3390/met11091339
APA StyleChen, H., Wang, K., Ming, X., Zhan, F., Muhammad, Y., Wei, Y., Li, W., & Zhan, H. (2021). The Efficient Removal of Calcium and Magnesium Ions from Industrial Manganese Sulfate Solution through the Integrated Application of Concentrated Sulfuric Acid and Ethanol. Metals, 11(9), 1339. https://doi.org/10.3390/met11091339