The Effect of Immersion Corrosion Time on Electrochemical Corrosion Behavior and the Corrosion Mechanism of EH47 Ship Steel in Seawater
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Electrochemical Test
2.3. Immersion Test
3. Results
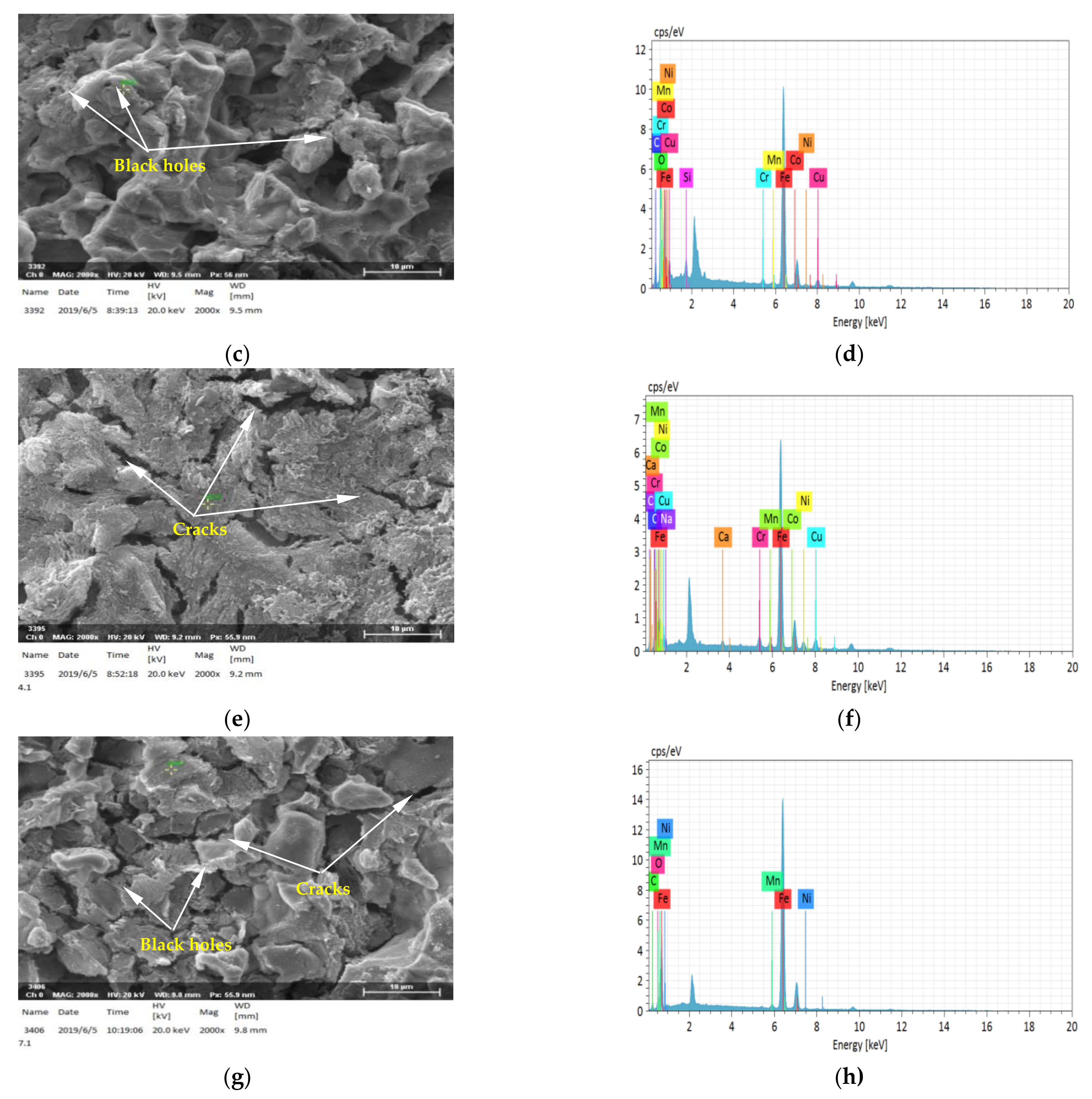
3.1. Energy Spectrum Analysis of Steel
3.2. Electrochemical Characteristics of EH47 in Seawater
3.2.1. Open Circuit Potential of EH47
3.2.2. Potentiodynamic Polarization Tests
3.2.3. EIS Measurements
3.3. The Corrosion Mechanism of EH47
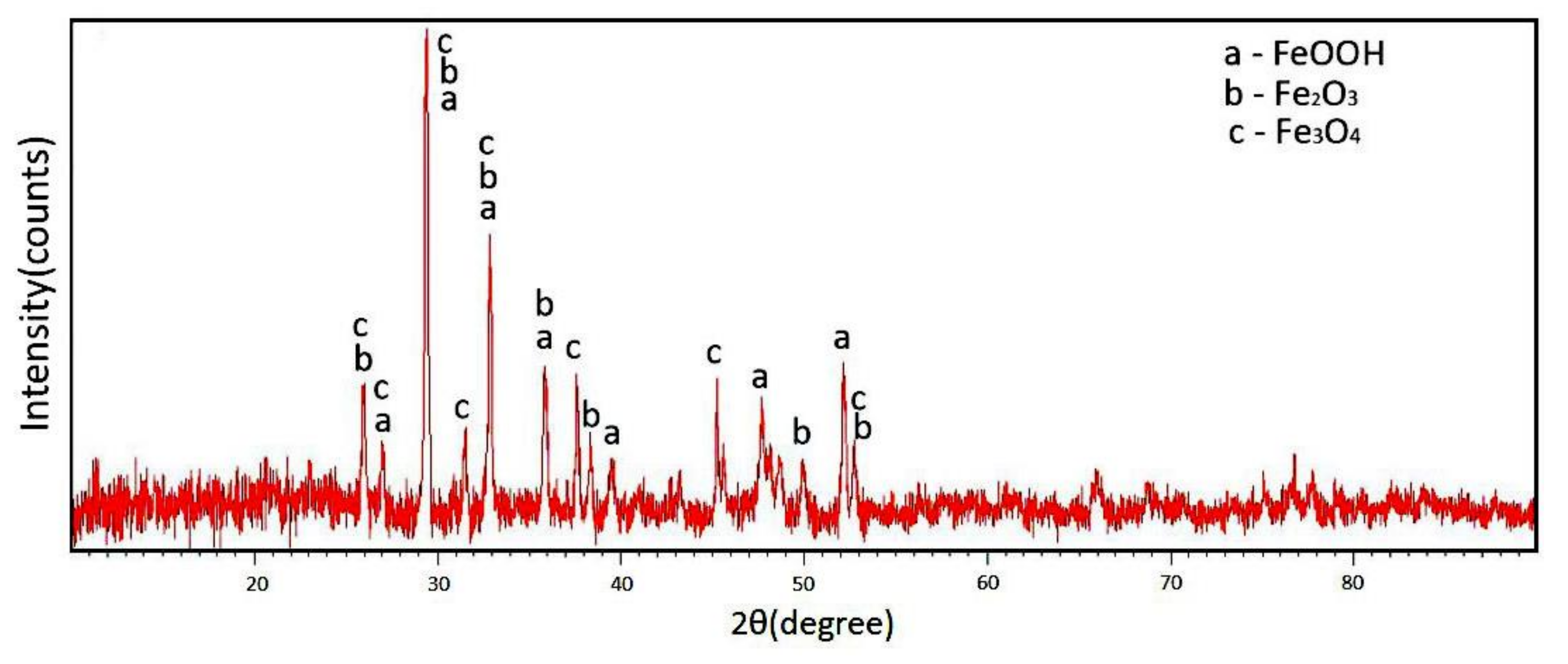
3.3.1. The Phase Composition of the Corrosion Products for EH47
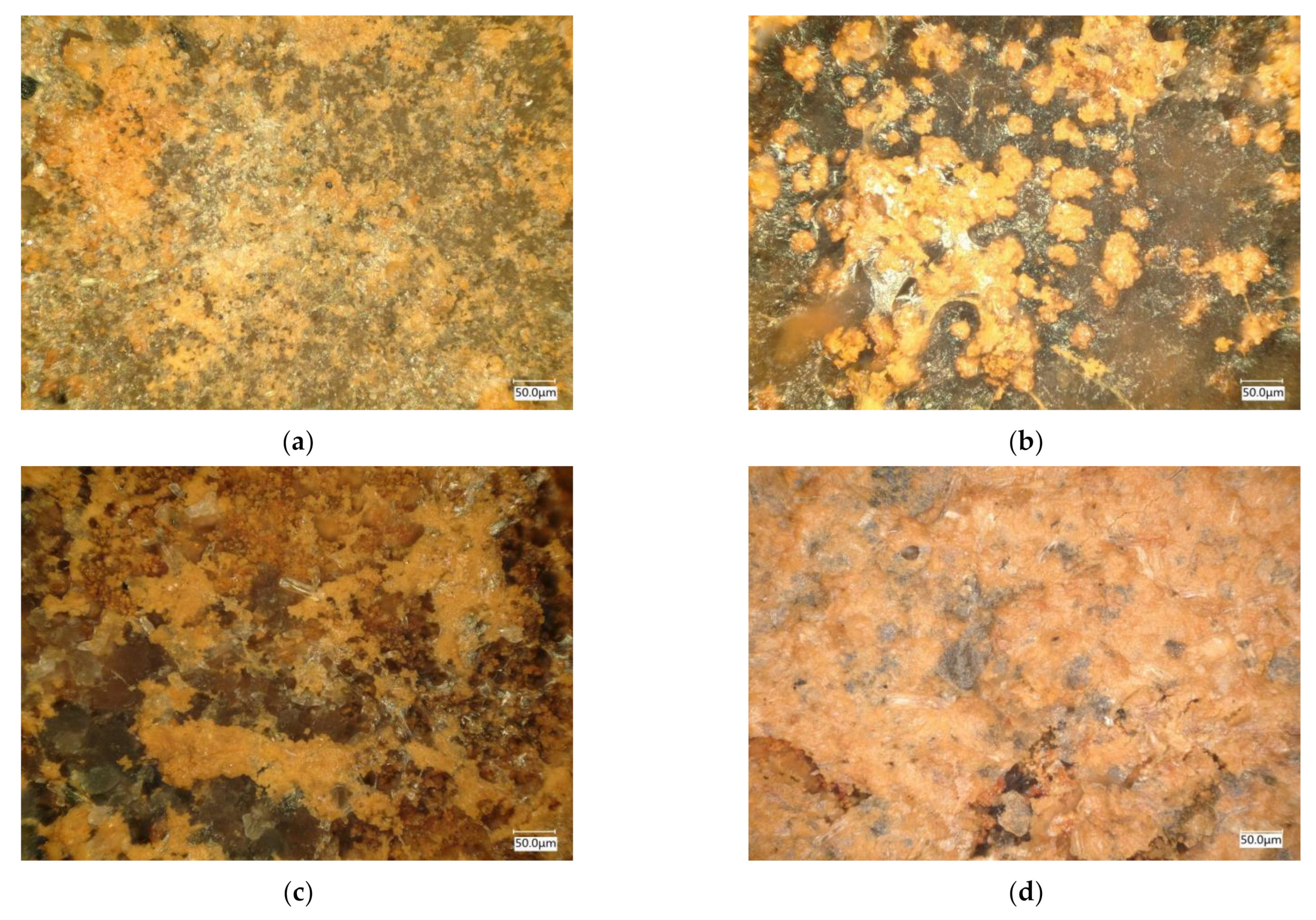
3.3.2. The Microstructure of Corrosion Products of EH47 Formed on the Sample Surface under Different Full Immersion Corrosion Times
3.3.3. The Mechanism of Immersion Corrosion of EH47
4. Conclusions
- With an increase in immersion time, the open circuit potential of EH47 decreases and then increases, with the minimum at 28 days of immersion.
- With an extension in immersion time, the corrosion current density (Icorr) of EH47 steel first decreases and then increases, with the minimum at 28 days of immersion. Furthermore, the arc radius of the 28 days of immersion sample is the largest, which indicates that the impedance is the largest, the corrosion rate is the smallest and the corrosion resistance is good.
- The corrosion products of EH47 steel after immersion in seawater are mainly composed of FeOOH, Fe3O4 and Fe2O3 phases.
- The corrosion mechanism of EH47 steel in the initial stage of corrosion is mainly pitting corrosion, and furthermore, the corrosion mechanism is mainly pitting corrosion with a small amount of crevice corrosion with increased corrosion time.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, Q.Y.; Wu, X.G. Corrosion and Protection Technology of Ships; Harbin Engineering University Press: Harbin, China, 2012; pp. 102–133. [Google Scholar]
- Ping, Y. Research on the evaluation method and requirements of the corrosion state of ship structure. Ships 2010, 4, 16–19. [Google Scholar]
- Sun, B.L.; Cao, J.; Si, T.Z.; Zhang, Q.A. Electrochemical corrosion behavior of rusted NSB steel in Simulated Seawater. Anhui Univ. Technol. 2013, 30, 124–128. [Google Scholar]
- Zou, Y.; Wang, J.; Zheng, Y.Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Wang, H.W.; Zhou, P.; Huang, S.W.; Chi, Y. Corrosion mechanism of low alloy steel in NaCl solution with CO2 and H2S. Int. J. Electrochem. Sci. 2016, 11, 1293–1309. [Google Scholar]
- Han, E.H.; Chen, J.M.; Su, Y.J. Corrosion and Protection of Marine Engineering Materials and Structures; Chemical Industry Press: Beijing, China, 2017; pp. 304–382. [Google Scholar]
- Prakashaiah, B.G.; Vinaya Kumara, D.; Anup Pandith, A.; Nityananda Shetty, A.; Amitha Rani, B.E. Corrosion inhibition of 2024-T3 aluminum alloy in 3.5% NaCl by thiosemicarbazone derivatives. Corros. Sci. 2018, 136, 326–338. [Google Scholar] [CrossRef]
- Lebrini, M.; Lagrenée, M.; Vezin, H.; Gengembre, L.; Bentiss, F. Electrochemical and quantum chemical studies of new thiadiazole derivatives adsorption on mild steel in normal hydrochloric acid medium. Corros. Sci. 2005, 47, 485–505. [Google Scholar] [CrossRef]
- Chen, X.Q.; Chang, W.S.; Chen, D.B. Study on the law and characteristics of pitting corrosion induced by inclusions in carbon steel. J. Nav. Acad. Eng. 2004, 16, 30–36. [Google Scholar]
- Sazou, D.; Pavlidou, M.; Pagitsas, M. Potential oscillations induced by localized corrosion of the passivity on iron in halide-containing sulfuric acid media as a probe for a comparative study of the halide effect. J. Electroanal. Chem. 2012, 675, 54–67. [Google Scholar] [CrossRef]
- Usman, B.J.; Umoren, S.A.; Gasem, Z.M. Inhibition of API 5L X60 steel corrosion in CO2 -saturated 3.5% NaCl solution by tannic acid and synergistic effect of KI additive. J. Mol. Liq. 2017, 237, 146–156. [Google Scholar] [CrossRef]
- Deyab, M.; Mohamed, N.H.; Moustafa, Y. Corrosion protection of petroleum pipelines in NaCl solution by microcrystalline waxes from waste materials: Electrochemical studies. Corros. Sci. 2017, 122, 74–79. [Google Scholar] [CrossRef]
- Yang, S.C.; Bi, H.J.; Niu, S.R.; Jin, S.T.; Shen, K. Effect of temperature and Cl~-mass fraction on pitting corrosion resistance of 304 stainless steel. J. Lanzhou Univ. Technol. 2010, 36, 5–9. [Google Scholar]
- Xia, J.M.; Li, Z.Y.; Jiang, J.C.; Wang, X.Q.; Zhang, X.D. Effect of Flow Rates on erosion corrosion behavior of hull steel in real seawater. Int. J. Electrochem. Sci. 2021, 16, 1–13. [Google Scholar]
- Jia, C.Y.; Niu, J.Q.; Liu, D.S. Research on marine atmospheric corrosion resistance of ship plate steel. Shanghai Met. 2013, 35, 26–30. [Google Scholar]
- Melchers, R.E.; Jeffrey, R. Early corrosion of mild steel in seawater. Corros. Sci. 2005, 47, 1678–1693. [Google Scholar] [CrossRef]
- Soares, C.G.; Garbatov Zayed, Y. Effect of environmental factors on steel plate corrosion under marine immersion conditions. Corros. Eng. Sci. Technol. 2011, 46, 524–541. [Google Scholar] [CrossRef]
- Panayotova, M.; Garbatov, Y.; Guedes, S.C. Factors Influencing Atmospheric Corrosion and Corrosion in Closed Spaces of Marine Steel Structural. In Proceedings of the 7th International Conference on Marine Science and Technology, Varna, Bulgaria, 7–9 October 2004; Union of Scientists of Varna: Varna, Bulgaria, 2004. [Google Scholar]
- Li, G.M.; Chang, W.S.; Chen, X.Q. Study on simulated pitting corrosion test method of carbon steel and low alloy steel. Mater. Dev. Appl. 2008, 3, 40–42. [Google Scholar]
- Gan, Y.; Li, Y.; Lin, H.C. Laboratory simulation of local corrosion process of low alloy steel in seawater. J. Corros. Protec. 2001, 21, 82–87. [Google Scholar]
- Wang, J.M.; Chen, X.Q.; Li, G.M. Comparison of pitting corrosion resistance of two kinds of marine low alloy steels. J. Corros. Protec. 2005, 25, 356–360. [Google Scholar]
- Cao, G.L.; Li, G.M.; Chen, S.; Chang, W.S.; Chen, X.Q. Comparison of pitting corrosion resistance of Ni and Cr in typical seawater corrosion resistant steels. Acta Metal. Sin. 2010, 46, 748–754. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Chen, X.Q.; Chen, D.W.; Li, G.M.; Pan, R.Y. Pitting induced sensitivity and mechanism of different low carbon steels. J. Corros. Protec. 2001, 21, 265–272. [Google Scholar]
- Liu, W.; Wang, H.T.; Yang, H.Y.; Huang, G.Q. Compilation and standard interpretation of seawater corrosion test method for metallic materials. Environ. Technol. 2016, 34, 57–60. [Google Scholar]
- Song, S.Z. Corrosion Electrochemical Methods; Chemical Industry Press: Beijing, China, 1988; pp. 55–70. [Google Scholar]
- Cao, C.N.; Zhang, J.Q. Electrochemical Impedance Spectroscopy; Science Press: Beijing, China, 2002; pp. 46–88. [Google Scholar]
- Yang, S.W.; Chang, T.J. Corrosion and Protection of Materials; Harbin Engineering University Press: Harbin, China, 2003; pp. 102–108. [Google Scholar]
- Wu, H.; Li, Y.; Lu, Y.; Li, Z.; Cheng, X.W.; Hasan, M.; Zhang, H.M.; Jiang, Z.Y. Influences of load and microstructure on tribocorrosion behaviour of high strength hull steel in saline solution. Tribol Lett. 2019, 67, 1–12. [Google Scholar] [CrossRef]
- Sun, B.L. Electrochemical Corrosion Characteristics of Low-Carbon Alloy Steel under NaCl Solution. Master’s Thesis, Anhui University of Technology, Anhui, China, 2013. [Google Scholar]
- Zheng, L.Y.; Cao, F.H.; Liu, W.J. Electrochemical study on failure behavior of Q235 steel in simulated natural environment. Equip. Environ. Eng. 2011, 8, 8–15. [Google Scholar]
- Liang, C.F.; Hou, W.T. Study on atmospheric exposure corrosion of low carbon alloy steel and low alloy steel for 16 years. J. Corros. Protec. 2005, 25, 2–6. [Google Scholar]
- Sherif El-Sayed, M.; Almajid, A.A. Electrochemical corrosion behavior of API X-70 5L grade steel in 4.0 wt.% sodium chloride solutions after different immersion periods of time. Int. J. Electrochem. Sci. 2015, 10, 34–45. [Google Scholar]
- Han, P.; Han, P.J.; Xie, R.Z.; Bin, H.; Bai, X.H. Study of the electrochemical corrosion behaviour of X70 steel in H2SO4 contaminated silty soil. Int. J. Electrochem. Sci. 2018, 13, 8694–8710. [Google Scholar] [CrossRef]
- Peng, X. Corrosion Electrochemical Behavior and Correlative Parameters of Rusty Carbon Steels in Seawater Environment. Master’s Thesis, Ocean University of China, Qingdao, China, 2013. [Google Scholar]
- Dai, N.; Zhang, L.C.; Zhang, J.X.; Chen, Q.M.; Wu, M.L. Distinction in corrosion resistance of selective laser melted Ti-6Al-4V alloy on different planes. Corros. Sci. 2016, 111, 703–720. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.W.; Yuan, X.J.; Tan, Z.J.; Tang, M.Q.; Feng, Z.Q. Effect of in situ ion nitridetreatment on the corrosion behavior of titanium. Int. J. Electrochem. Sci. 2018, 13, 353–361. [Google Scholar] [CrossRef]
- Sun, L.L. Mechanism of Q235 Steel and Stainless Steel in Seawater. Master’s Thesis, Shandong University, Shandong, China, 2010. [Google Scholar]
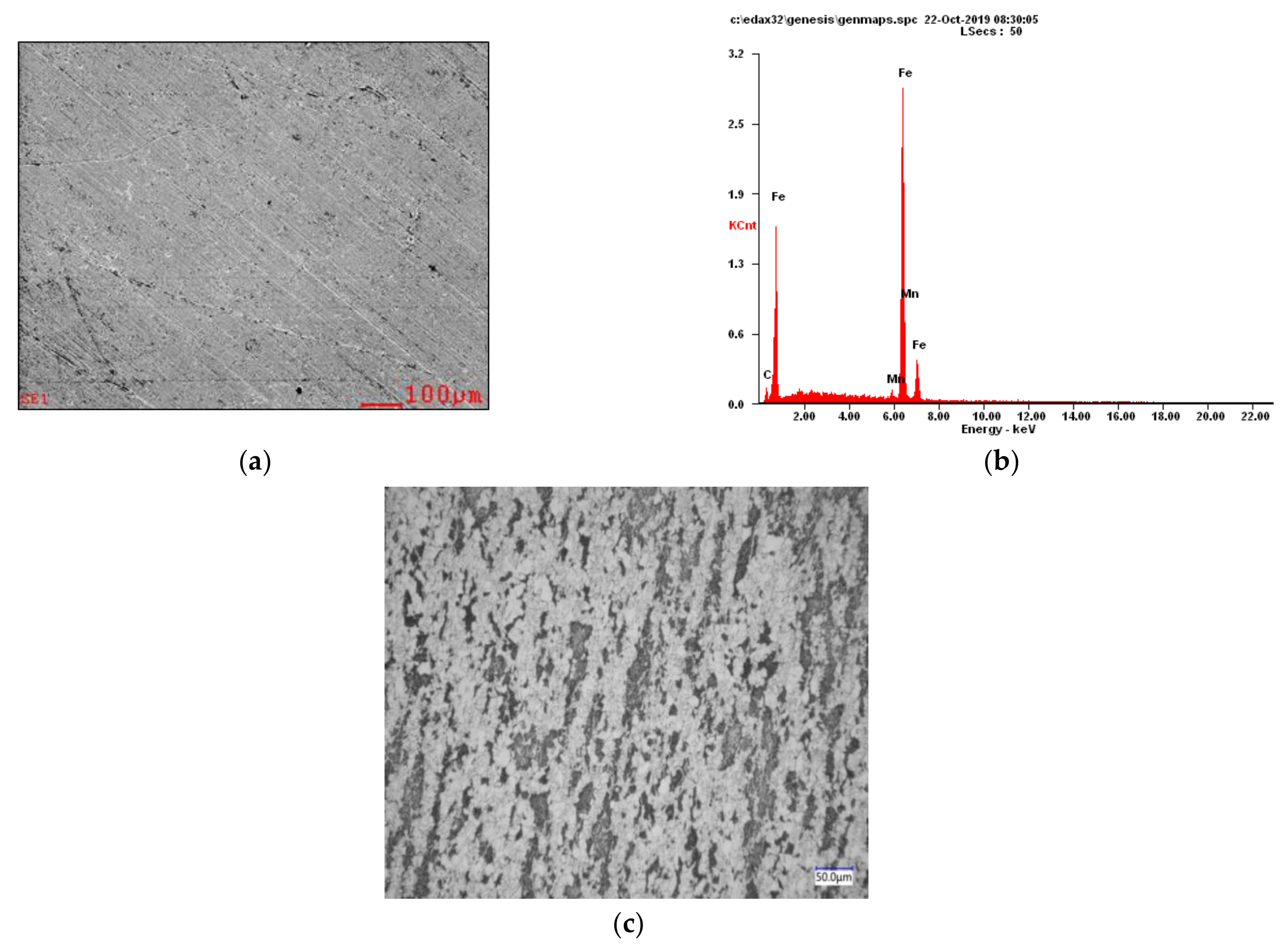

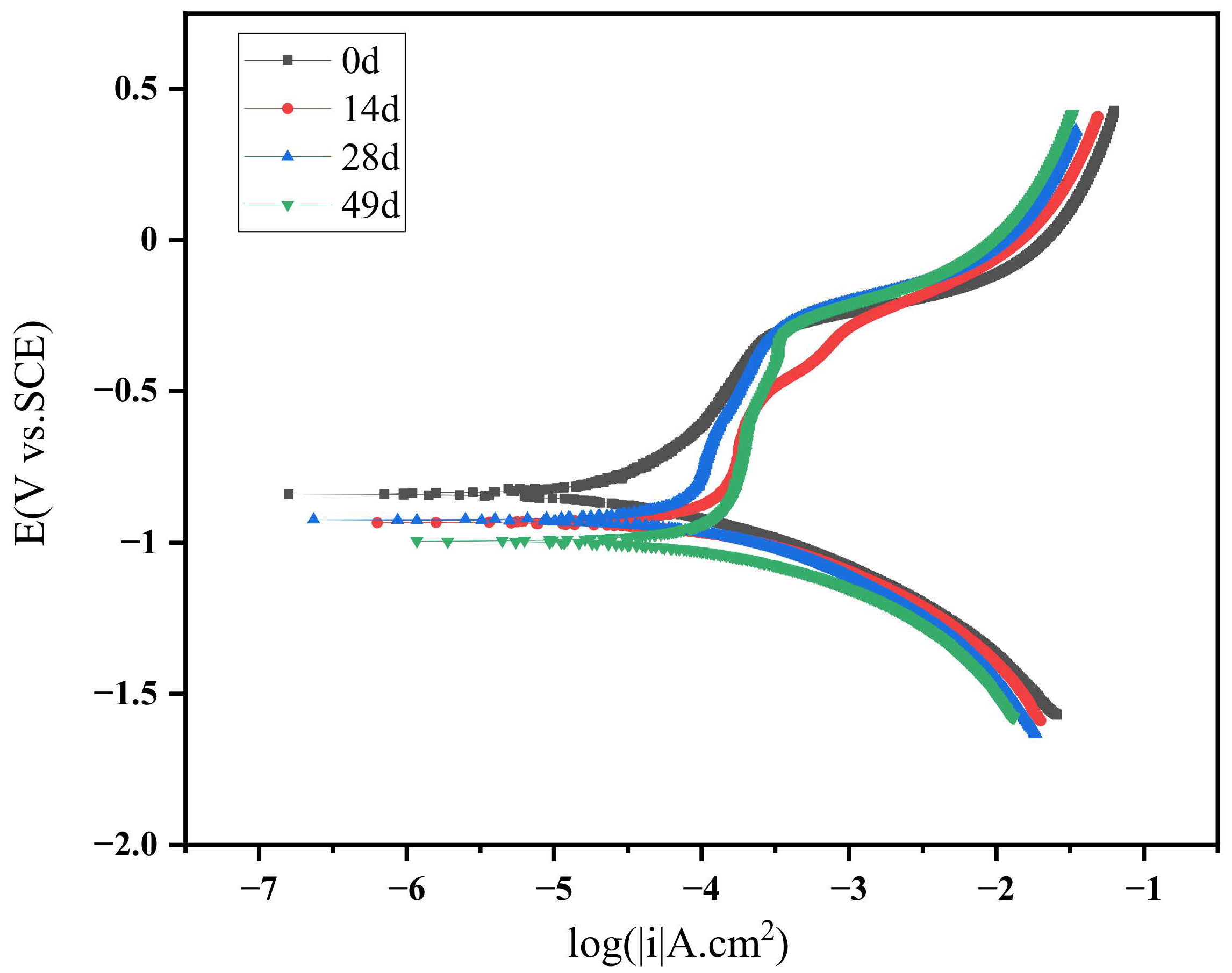
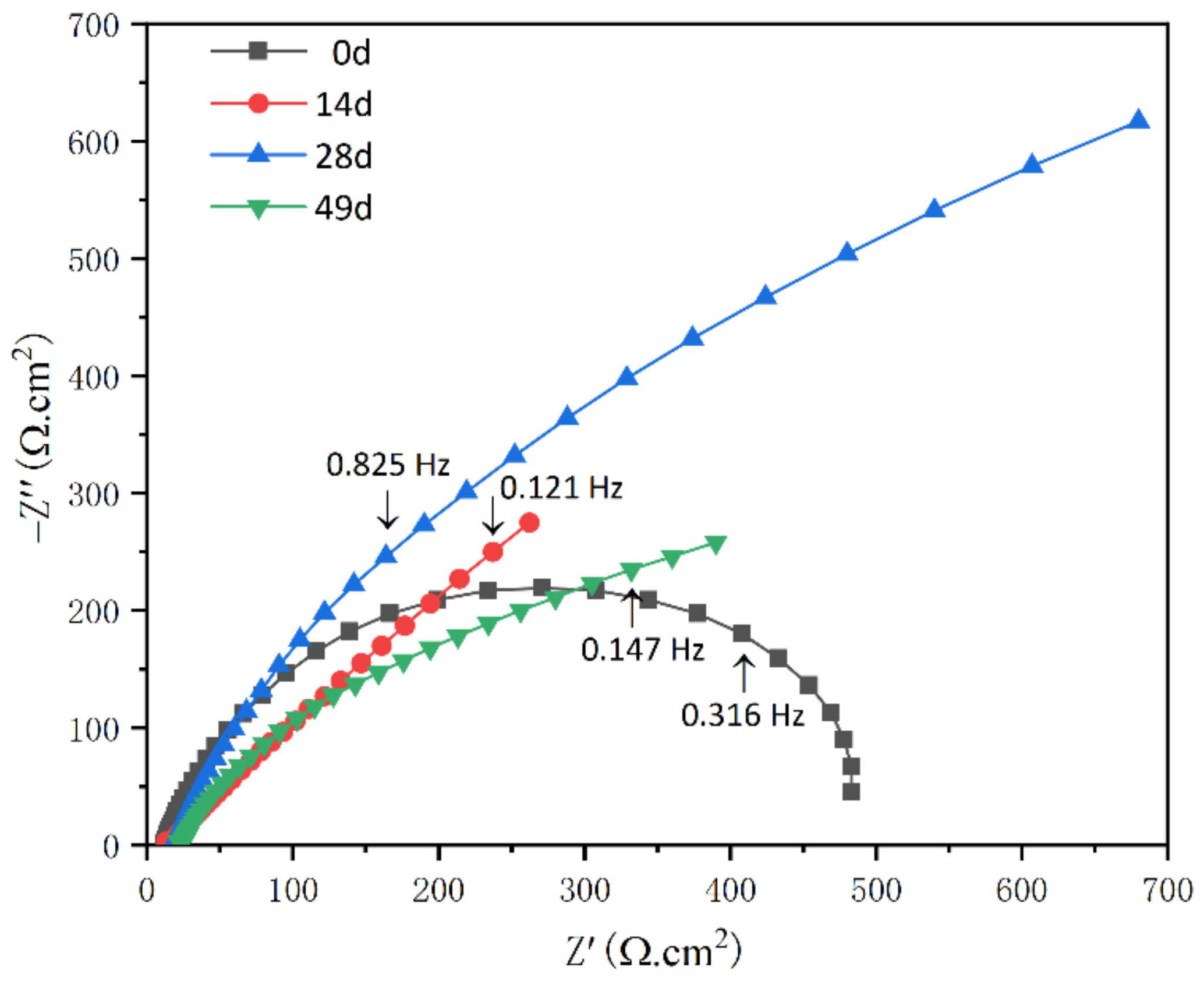

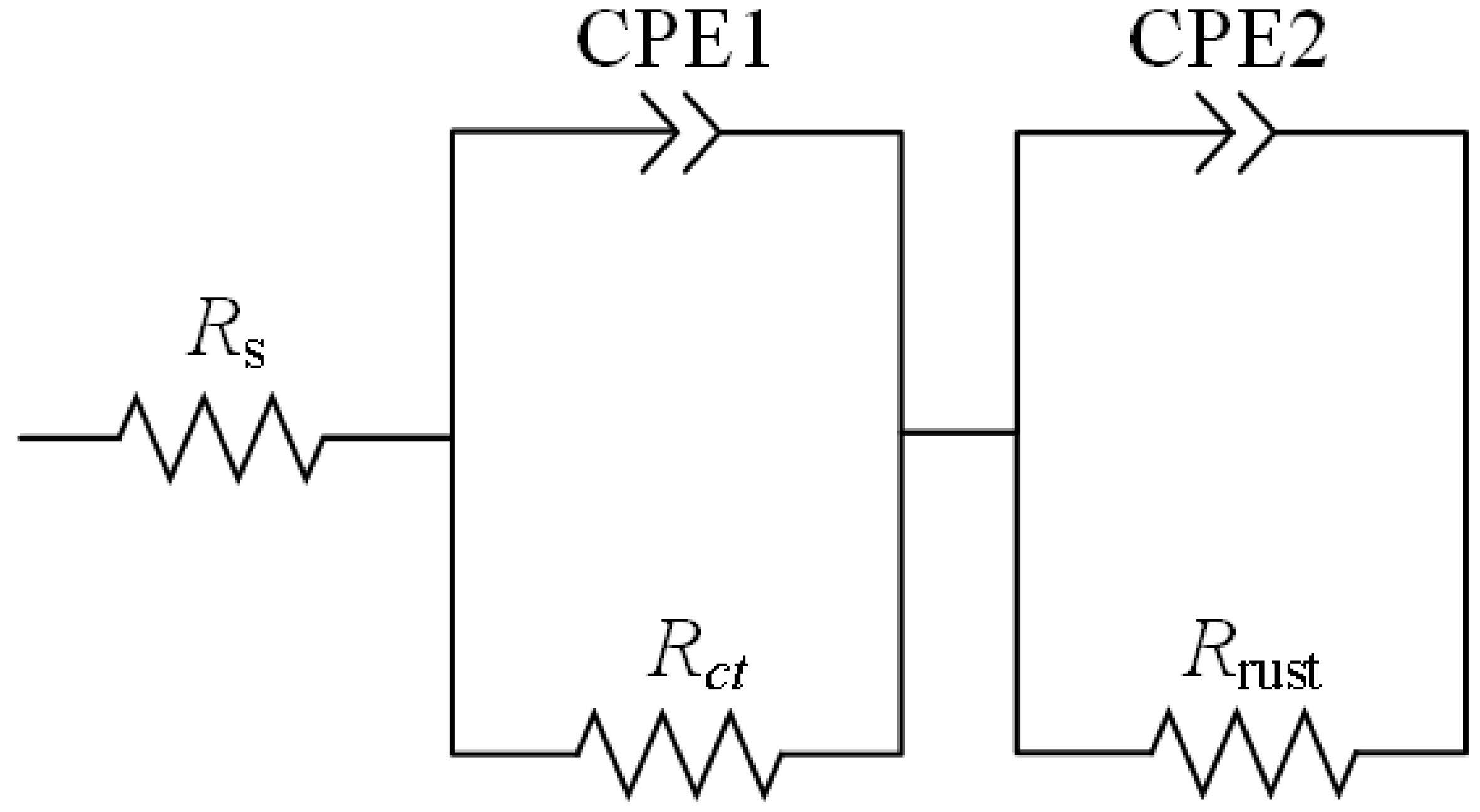


| Corrosion Time (d) | Corrosion Potential, vs. SCE | Corrosion Current Density (A/cm2) | Passive Domain, vs. SCE |
|---|---|---|---|
| 0 | −0.915 | 1.444 × 10−4 | −0.8~−0.2 |
| 14 | −0.934 | 1.107 × 10−4 | −0.8~−0.5 |
| 28 | −0.924 | 8.093 × 10−5 | −0.8~−0.2 |
| 49 | −0.995 | 1.030 × 10−4 | −0.9~−0.2 |
| t(d) | Rs/ (Ω·cm2) | Rct/ (Ω·cm2) | CPE1 | n1 | Rrust/ (Ω·cm2) | CPE2 | n2 | IE% |
|---|---|---|---|---|---|---|---|---|
| Y0/·Ω−1cm−2·s−n | Y0/·Ω−1cm−2·s−n | |||||||
| 0 | 12 | 12 | 0.0007 | 0.98 | 380 | 0.0017 | 0.75 | 0 |
| 14 | 8 | 8 | 0.0029 | 0.60 | 1623 | 0.0020 | 0.87 | 50 |
| 28 | 19 | 171 | 0.0015 | 0.75 | 2458 | 0.0016 | 0.93 | 92 |
| 49 | 23 | 90 | 0.0003 | 0.66 | 1103 | 0.0019 | 0.95 | 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yan, L.; Zhu, Y.; Ai, F.; Li, H.; Li, Y.; Jiang, Z. The Effect of Immersion Corrosion Time on Electrochemical Corrosion Behavior and the Corrosion Mechanism of EH47 Ship Steel in Seawater. Metals 2021, 11, 1317. https://doi.org/10.3390/met11081317
Zhang H, Yan L, Zhu Y, Ai F, Li H, Li Y, Jiang Z. The Effect of Immersion Corrosion Time on Electrochemical Corrosion Behavior and the Corrosion Mechanism of EH47 Ship Steel in Seawater. Metals. 2021; 11(8):1317. https://doi.org/10.3390/met11081317
Chicago/Turabian StyleZhang, Hongmei, Ling Yan, Yangyang Zhu, Fangfang Ai, Hongnan Li, Yan Li, and Zhengyi Jiang. 2021. "The Effect of Immersion Corrosion Time on Electrochemical Corrosion Behavior and the Corrosion Mechanism of EH47 Ship Steel in Seawater" Metals 11, no. 8: 1317. https://doi.org/10.3390/met11081317
APA StyleZhang, H., Yan, L., Zhu, Y., Ai, F., Li, H., Li, Y., & Jiang, Z. (2021). The Effect of Immersion Corrosion Time on Electrochemical Corrosion Behavior and the Corrosion Mechanism of EH47 Ship Steel in Seawater. Metals, 11(8), 1317. https://doi.org/10.3390/met11081317






