Modeling of Isothermal Dissolution of Precipitates in a 6061 Aluminum Alloy Sheet during Solution Heat Treatment
Abstract
:1. Introduction
2. Models and Methods
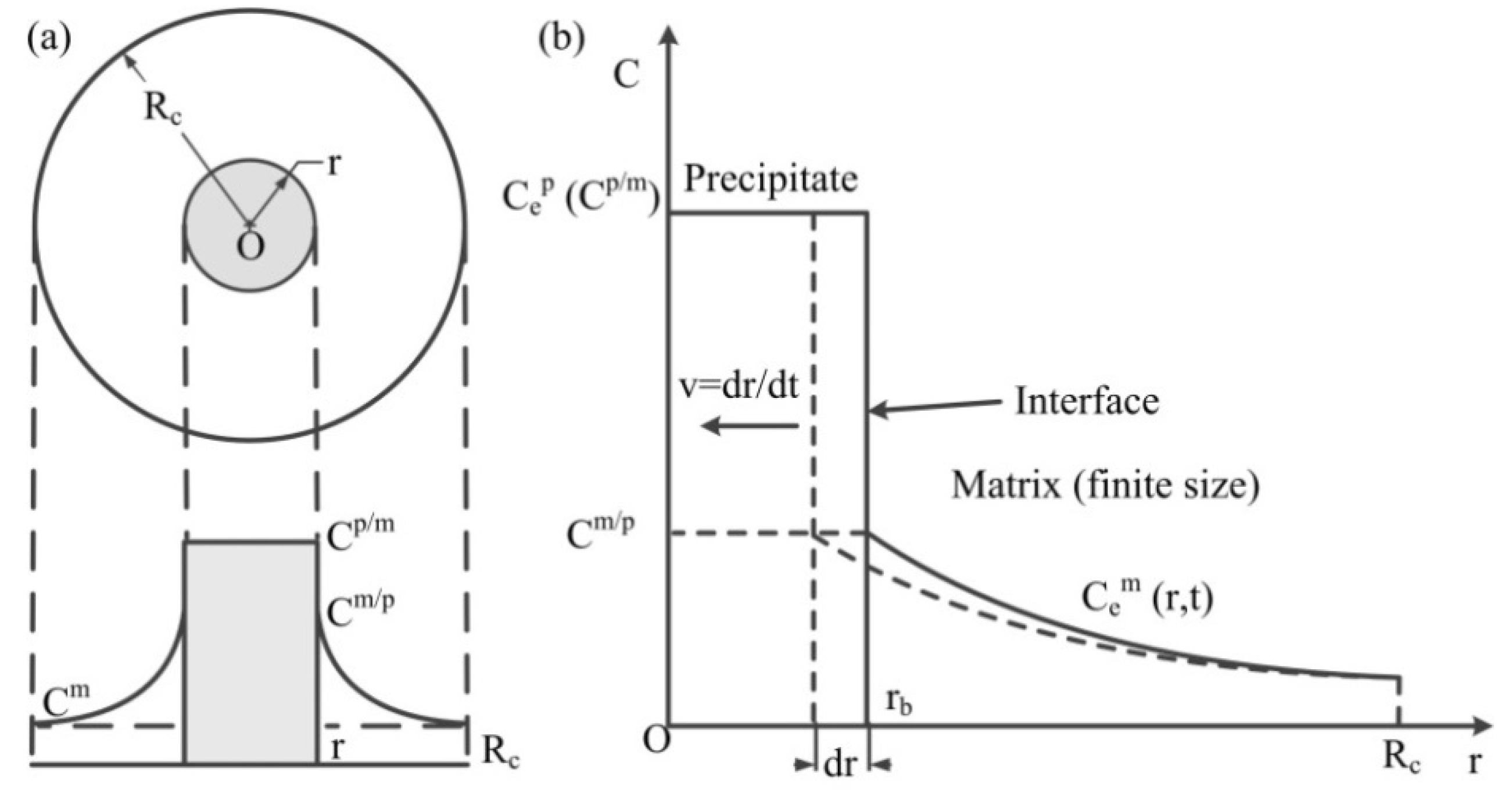
2.1. Dissolution Model
- The model was derived on the following assumptions:
- The precipitates are all spherical, with the same size, and uniformly distributed in the matrix.
- The composition and proportion of elements of the precipitates remain unchanged during the whole dissolution process.
- The solubility and diffusion coefficient of each element are not affected by other elements, concentration, and distance.
- All the precipitates are located within the grains, regardless of the dissolution of the precipitates at the grain boundary and the diffusion across the grain boundary.
- The dissolution process is isothermal.
- The precipitate dissolves in a spherical space, and there is only one precipitate in this space.
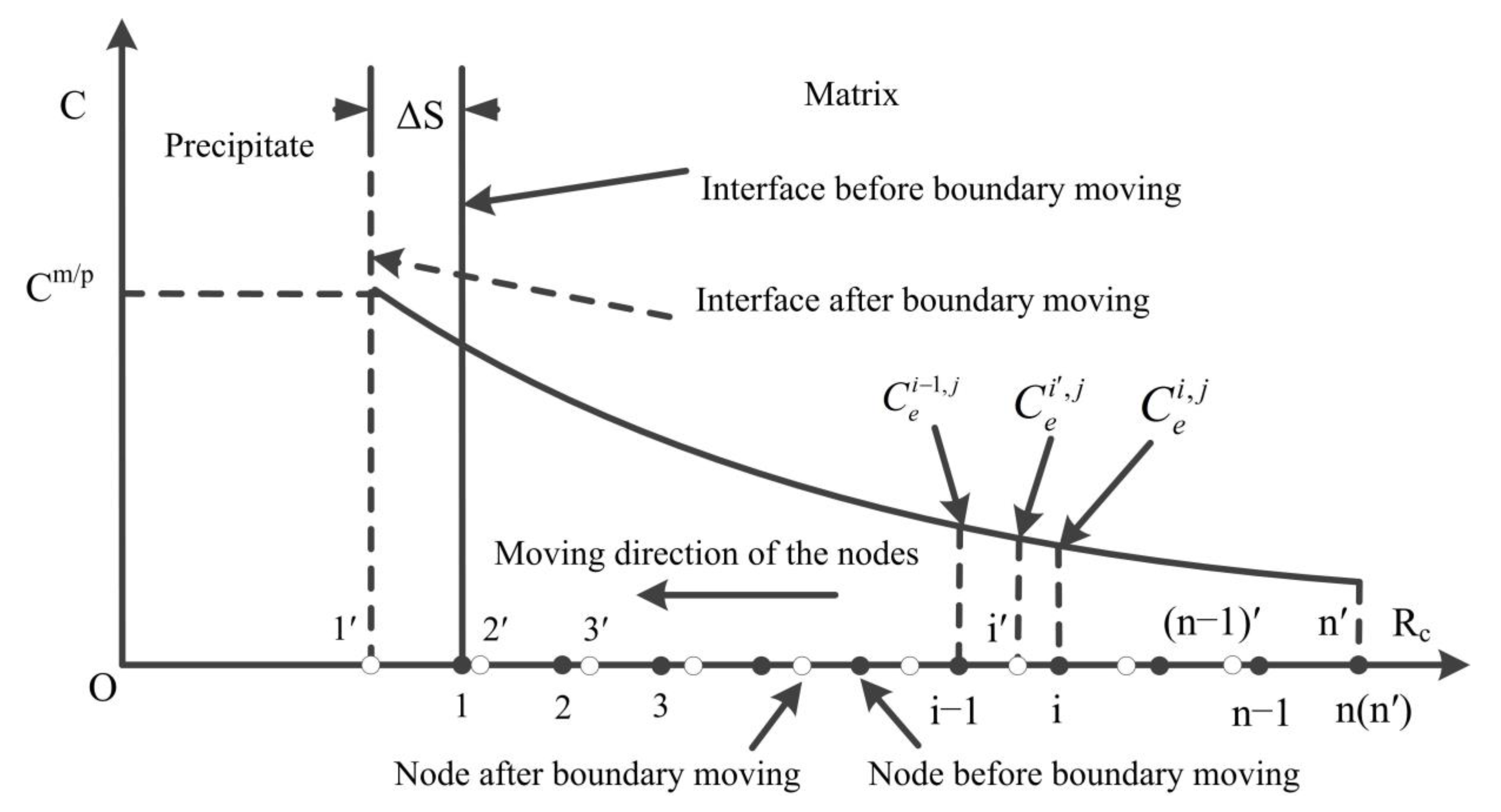
2.2. Model Discretization and Mobile Nodes Method
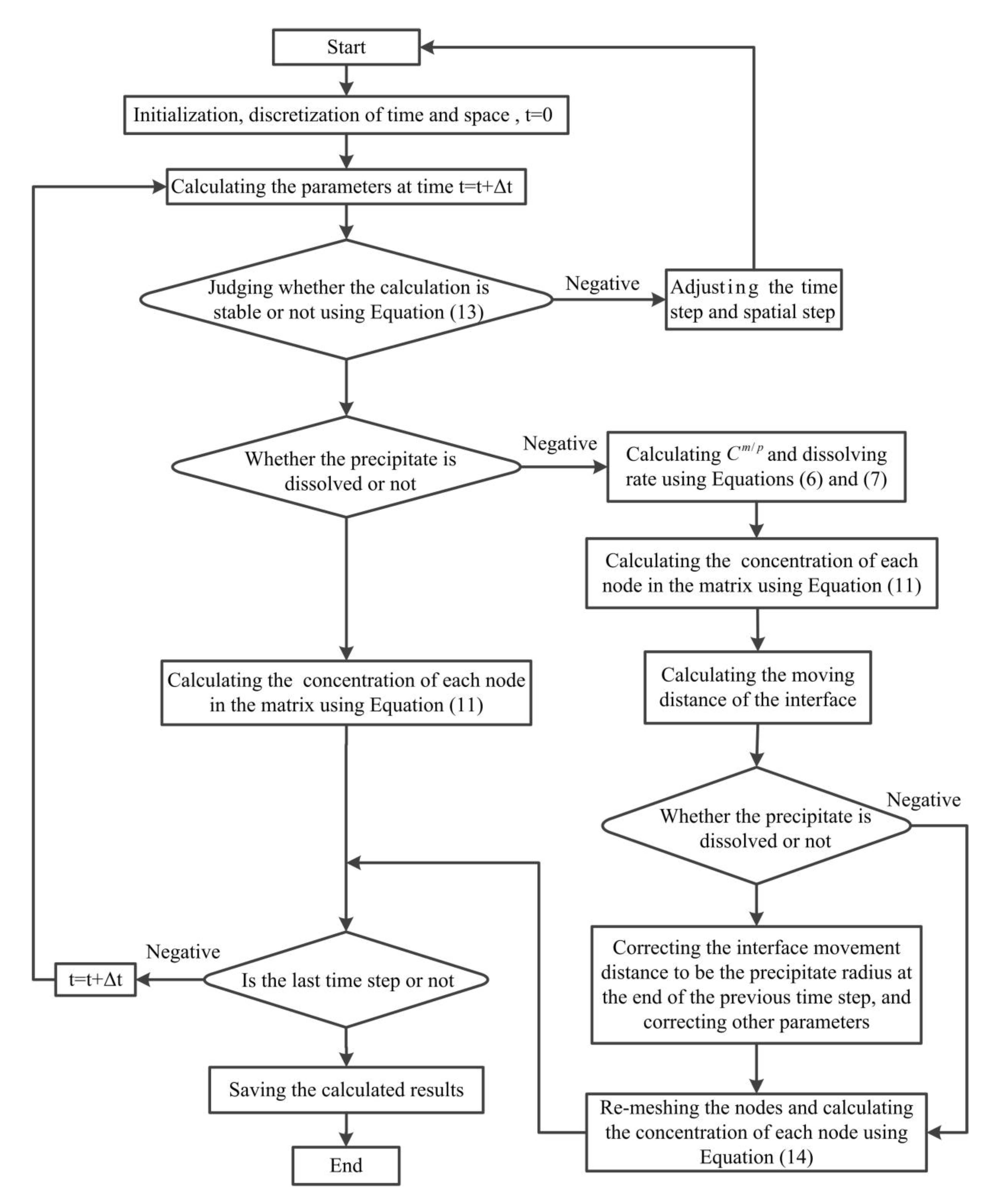
2.3. Calculation Process
3. Simulations and Experiments
3.1. Simulation Parameters
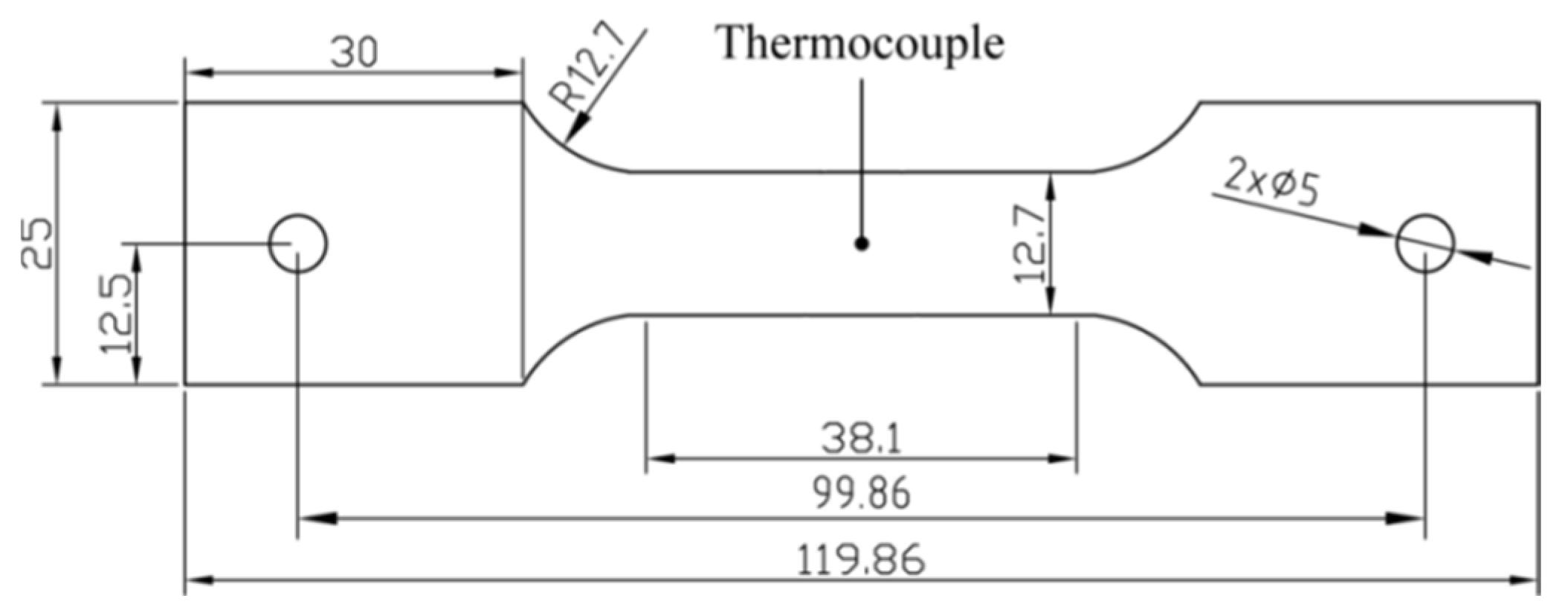
3.2. Verification Experiments
4. Results and Discussion
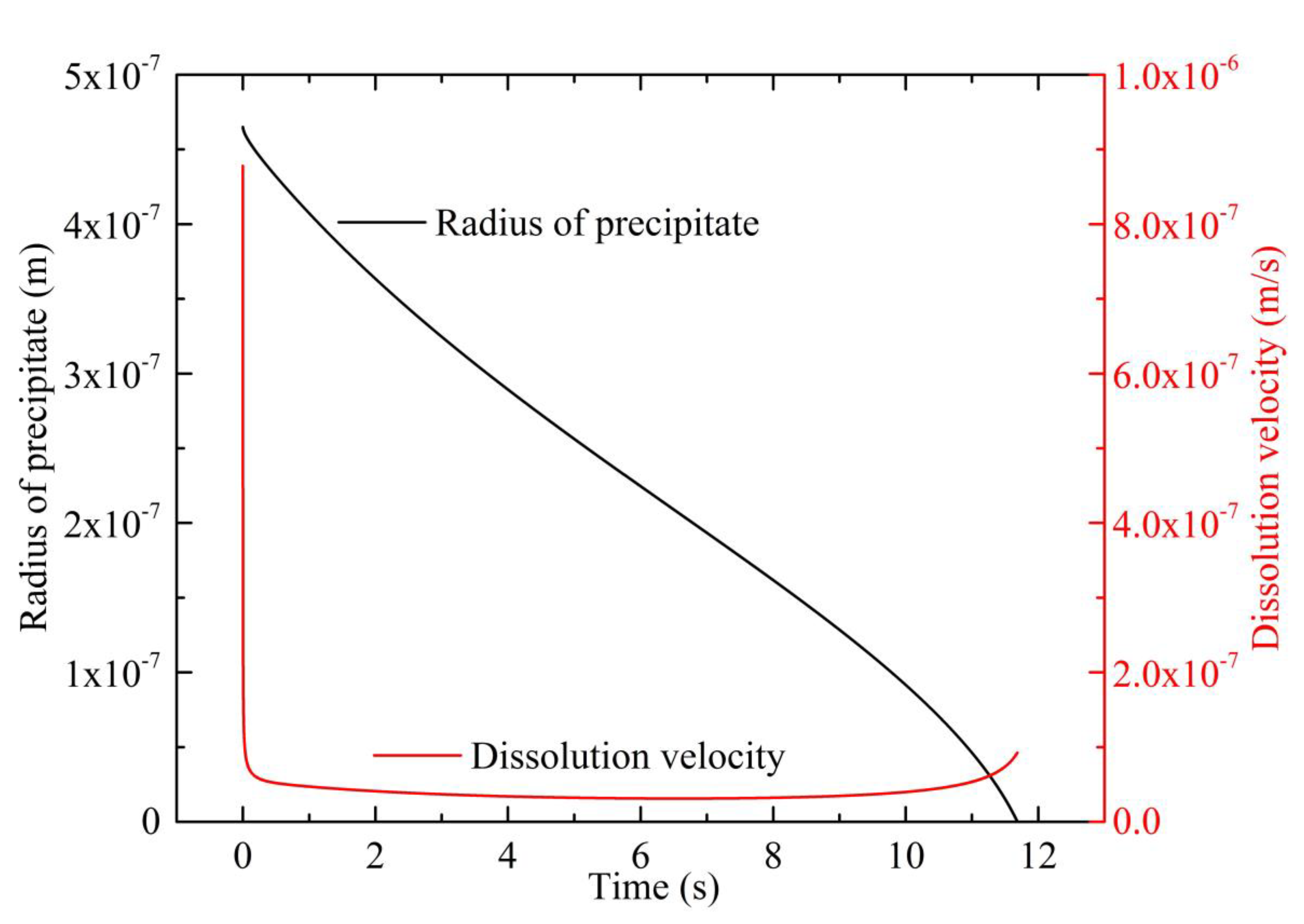
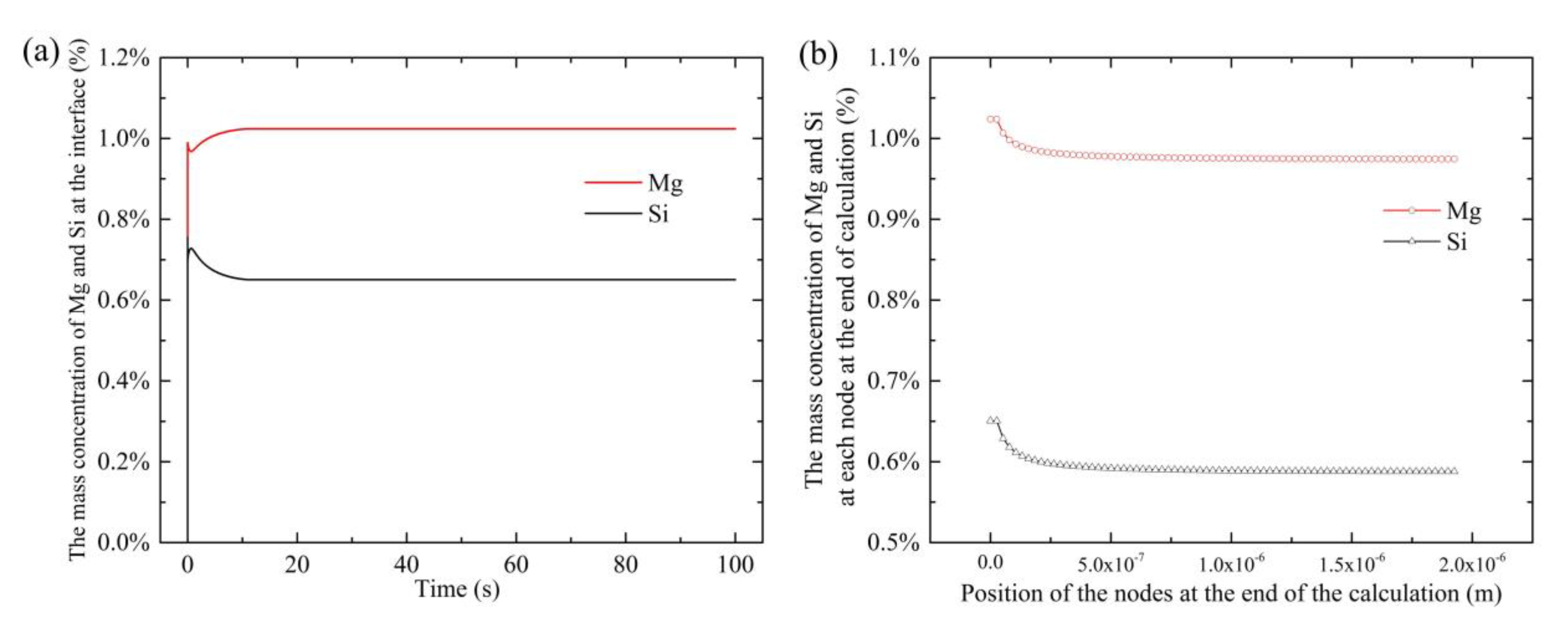
4.1. Modeling of Precipitates’ Dissolution
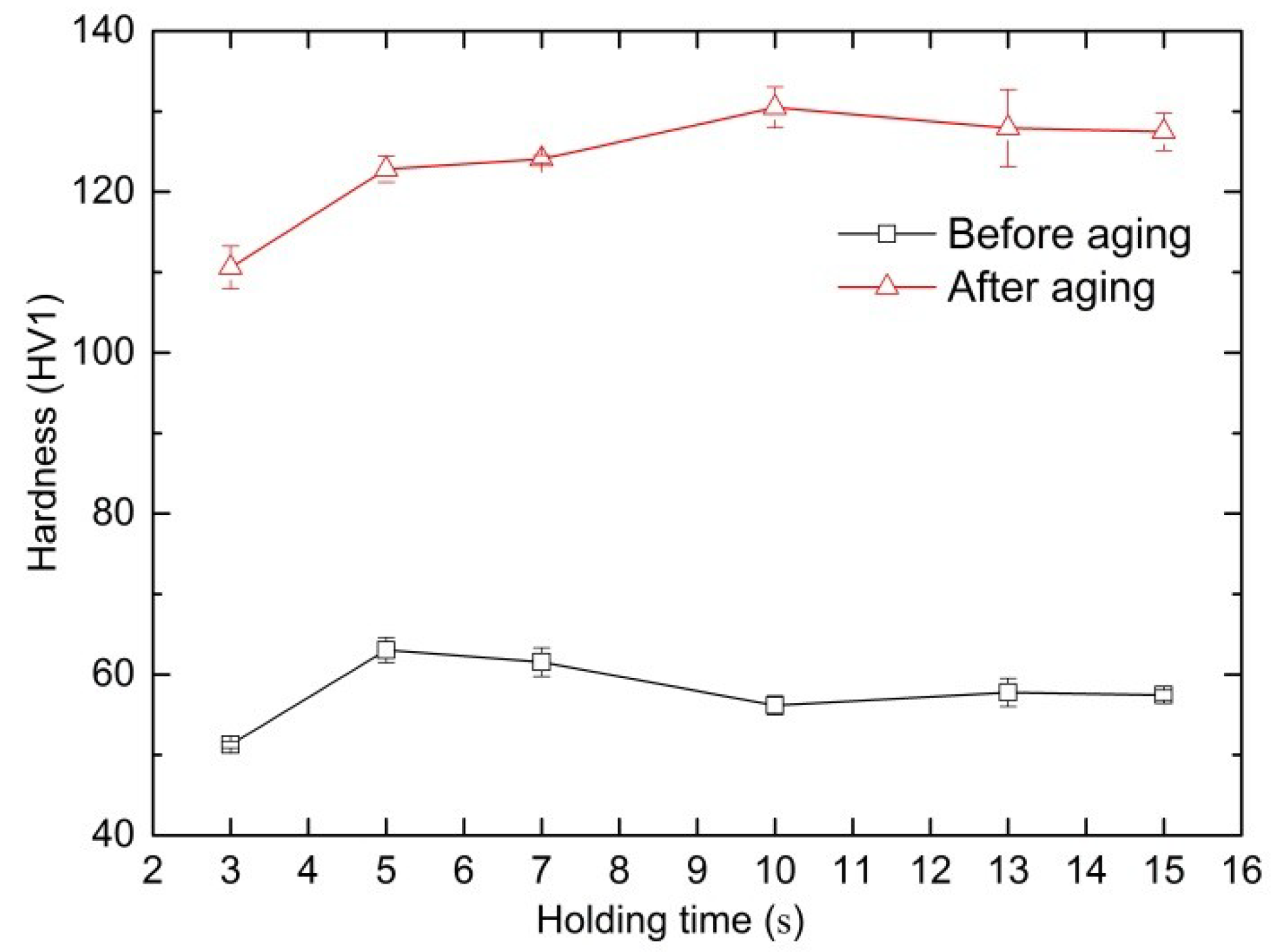
4.2. Verification Experimental Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandla, N.K.; Kant, S.; Goud, M.M. Mechanical, tribological and microstructural characterization of stir cast Al-6061 metal/matrix composites—A comprehensive review. Sādhanā 2021, 46, 47. [Google Scholar] [CrossRef]
- Ghiotti, A.; Simonetto, E.; Bruschi, S. Influence of process parameters on friction behaviour of AA7075 in hot stamping. Wear 2019, 426–427, 348–356. [Google Scholar] [CrossRef]
- Raugei, M.; El Fakir, O.; Wang, L.L.; Lin, J.G.; Morrey, D. Life cycle assessment of the potential environmental benefits of a novel hot forming process in automotive manufacturing. J. Clean. Prod. 2014, 83, 80–86. [Google Scholar] [CrossRef]
- Geng, H.; Wang, Y.; Wang, Z.; Zhang, Y. Investigation on Contact Heating of Aluminum Alloy Sheets in Hot Stamping Process. Metals 2019, 9, 1341. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, Z.J.; Wang, Z.J.; Zhu, B.; Wang, Y.L.; Zhang, Y.S. Flow and friction behaviors of 6061 aluminum alloy at elevated temperatures and hot stamping of a B-pillar. Int. J. Adv. Manuf. Technol. 2018, 96, 4063–4083. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, M.H.; Wang, N.; Sun, J.W. Hot forming process with synchronous cooling for AA2024 aluminum alloy and its application. Int. J. Adv. Manuf. Technol. 2016, 86, 133–139. [Google Scholar] [CrossRef]
- Sarafoglou, P.I.; Serafeim, A.; Fanikos, I.A.; Aristeidakis, J.S.; Haidemenopoulos, G.N. Modeling of Microsegregation and Homogenization of 6xxx Al-Alloys Including Precipitation and Strengthening During Homogenization Cooling. Materials 2019, 12, 1421. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.C.; Chen, Y.P.; Xu, G.L.; Wang, J. Dynamic mechanical properties and failure behavior on 6061 aluminum alloy annular forgings. Forg. Stamp. Technol. 2021, 46, 179–185. (In Chinese) [Google Scholar] [CrossRef]
- Gussev, M.N.; Sridharan, N.; Babu, S.S.; Terrani, K.A. Influence of neutron irradiation on Al-6061 alloy produced via ultrasonic additive manufacturing. J. Nucl. Mater. 2021, 550, 152939. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, B.; Wang, Y.L.; Li, S.Q.; Zhang, Y.S. Fast solution heat treatment of high strength aluminum alloy sheets in radiant heating furnace during hot stamping. Int. J. Lightweight Mater. Manuf. 2020, 3, 20–25. [Google Scholar] [CrossRef]
- Zhang, X.K.; Guo, M.X.; Zhang, J.S.; Zhuang, L.Z. Dissolution of Precipitates During Solution Treatment of Al-Mg-Si-Cu Alloys. Metall. Mater. Trans. B. 2016, 47, 608–620. [Google Scholar] [CrossRef]
- Whelan, M.J. On the kinetics of precipitate dissolution. Metal Sci. J. 1969, 3, 95–97. [Google Scholar] [CrossRef]
- Brown, L.C. Diffusion-controlled dissolution of planar, cylindrical, and spherical precipitates. J. Appl. Phys. 1976, 47, 449–458. [Google Scholar] [CrossRef]
- Cheng, L.M.; Hawbolt, E.B.; Meadowcroft, T.R. Modeling of dissolution, growth, and coarsening of aluminum nitride in low-carbon steels. Metall. Mater. Trans. A 2000, 31, 1907–1916. [Google Scholar] [CrossRef]
- Zuo, Q.; Liu, F.; Wang, L.; Chen, C.F.; Zhang, Z.H. An analytical model for secondary phase dissolution kinetics. J. Mater. Sci. 2014, 49, 3066–3079. [Google Scholar] [CrossRef]
- Vermolen, F.J.; van der Zwaag, S. A numerical model for the dissolution of spherical particles in binary alloys under mixed mode control. Mat. Sci. Eng. A-Struct. 1996, 220, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Vermolen, F.J.; Javierre, E.; Vuik, C.; Zhao, L.; van der Zwaag, S. A three-dimensional model for particle dissolution in binary alloys. Comp. Mater. Sci. 2007, 39, 767–774. [Google Scholar] [CrossRef]
- Vermolen, F.J.; Vuik, K.; van der Zwaag, S. A mathematical model for the dissolution kinetics of Mg2Si-phases in Al-Mg-Si alloys during homogenisation under industrial conditions. Mat. Sci. Eng. A-Struct. 1998, 254, 13–32. [Google Scholar] [CrossRef]
- Vermolen, F.J.; Vuik, K.; van der Zwaag, S. The dissolution of a stoichiometric second phase in ternary alloys: A numerical analysis. Mat. Sci. Eng. A-Struct. 1998, 246, 93–103. [Google Scholar] [CrossRef]
- Chen, S.P.; Vossenberg, M.S.; Vermolen, F.J.; van de Langkruis, J.; van der Zwaag, S. Dissolution of β particles in an Al-Mg-Si alloy during DSC runs. Mat. Sci. Eng. A-Struct. 1999, 272, 250–256. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, L.; Du, Y. Diffusivities in liquid and fcc Al-Mg-Si alloys and their application to the simulation of solidification and dissolution processes. Calphad 2015, 49, 58–66. [Google Scholar] [CrossRef]
- Mohamed, M.; Foster, A.; Lin, J.G. Solution heat treatment in HFQ process. In Proceedings of the 12th International Conference on Metal Forming, METAL FORMING 2008, Krakow, Poland, 21–24 September 2008. [Google Scholar]
- Van de Langkruis, J.; Kuijpers, N.C.W.; Kool, W.H.; Vermolen, F.J.; van der Zwaag, S. Modelling Mg2Si Dissolution in an AA6063 Alloy During Pre-heating to the Extrusion Temperature. In Proceedings of the 7th International Aluminum ExtrusionTechnology Seminar, Aluminum Extruders Council, Chicago, IL, USA, 16–19 May 2000; Volume 1, pp. 119–124. [Google Scholar]
- Gui, Z.X.; Liang, W.K.; Liu, Y.; Zhang, Y.S. Thermo-mechanical behavior of the Al–Si alloy coated hot stamping boron steel. Mater. Design 2014, 60, 26–33. [Google Scholar] [CrossRef]
- Österreicher, J.A.; Kumar, M.; Schiffl, A.; Schwarz, S.; Hillebrand, D.; Bourret, G.R. Sample preparation methods for scanning electron microscopy of homogenized Al-Mg-Si billets: A comparative study. Mater. Charact. 2016, 122, 63–69. [Google Scholar] [CrossRef]
- Vissers, R.; Van Huis, M.A.; Jansen, J.; Zandbergen, H.W.; Marioara, C.D.; Andersen, S.J. The crystal structure of the β′ phase in Al-Mg-Si alloys. Acta Mater. 2007, 55, 3815–3823. [Google Scholar] [CrossRef]
- Rometsch, P.A.; Arnberg, L.; Zhang, D.L. Modelling dissolution of Mg2Si and homogenisation in Al-Si-Mg casting alloys. Int. J. Cast Metal. Res. 1999, 12, 1–8. [Google Scholar] [CrossRef]
- Sadeghi, I.; Wells, M.A.; Esmaeili, S. Modeling homogenization behavior of Al-Si-Cu-Mg aluminum alloy. Mater. Design 2017, 128, 241–249. [Google Scholar] [CrossRef]
- Maeno, T.; Mori, K.; Yachi, R. Hot stamping of high-strength aluminium alloy aircraft parts using quick heating. CIRP Ann.-Manuf. Technol. 2017, 66, 269–272. [Google Scholar] [CrossRef]

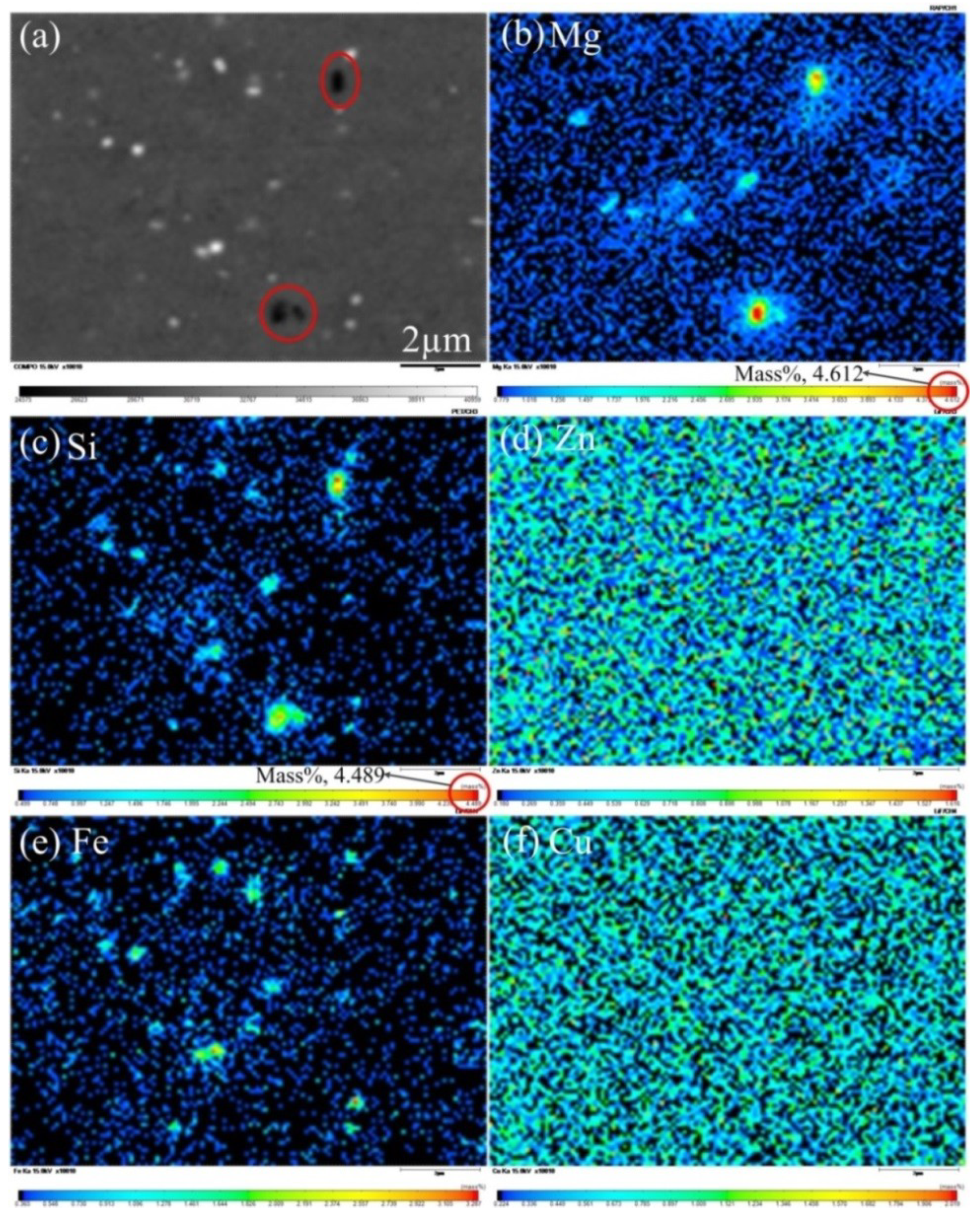
| Mg | Si | Mn | Cu | Fe | Cr | Ni | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| 1.104 | 0.676 | 0.078 | 0.150 | 0.423 | 0.165 | 0.006 | 0.210 | 0.027 | Remain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fang, D.; Zhu, B.; Wang, Y.; Li, S.; Zhang, Y. Modeling of Isothermal Dissolution of Precipitates in a 6061 Aluminum Alloy Sheet during Solution Heat Treatment. Metals 2021, 11, 1234. https://doi.org/10.3390/met11081234
Liu Y, Fang D, Zhu B, Wang Y, Li S, Zhang Y. Modeling of Isothermal Dissolution of Precipitates in a 6061 Aluminum Alloy Sheet during Solution Heat Treatment. Metals. 2021; 11(8):1234. https://doi.org/10.3390/met11081234
Chicago/Turabian StyleLiu, Yong, Dongyu Fang, Bin Zhu, Yilin Wang, Shiqi Li, and Yisheng Zhang. 2021. "Modeling of Isothermal Dissolution of Precipitates in a 6061 Aluminum Alloy Sheet during Solution Heat Treatment" Metals 11, no. 8: 1234. https://doi.org/10.3390/met11081234
APA StyleLiu, Y., Fang, D., Zhu, B., Wang, Y., Li, S., & Zhang, Y. (2021). Modeling of Isothermal Dissolution of Precipitates in a 6061 Aluminum Alloy Sheet during Solution Heat Treatment. Metals, 11(8), 1234. https://doi.org/10.3390/met11081234







