Experimental Investigation on Tensile Properties and Yield Strength Modeling of T5 Heat-Treated Counter Pressure Cast A356 Aluminum Alloys
Abstract
:1. Introduction
2. Experiments
2.1. Material and Heat Treatment
2.2. Mechanical Properties
2.3. Microstructure Observation
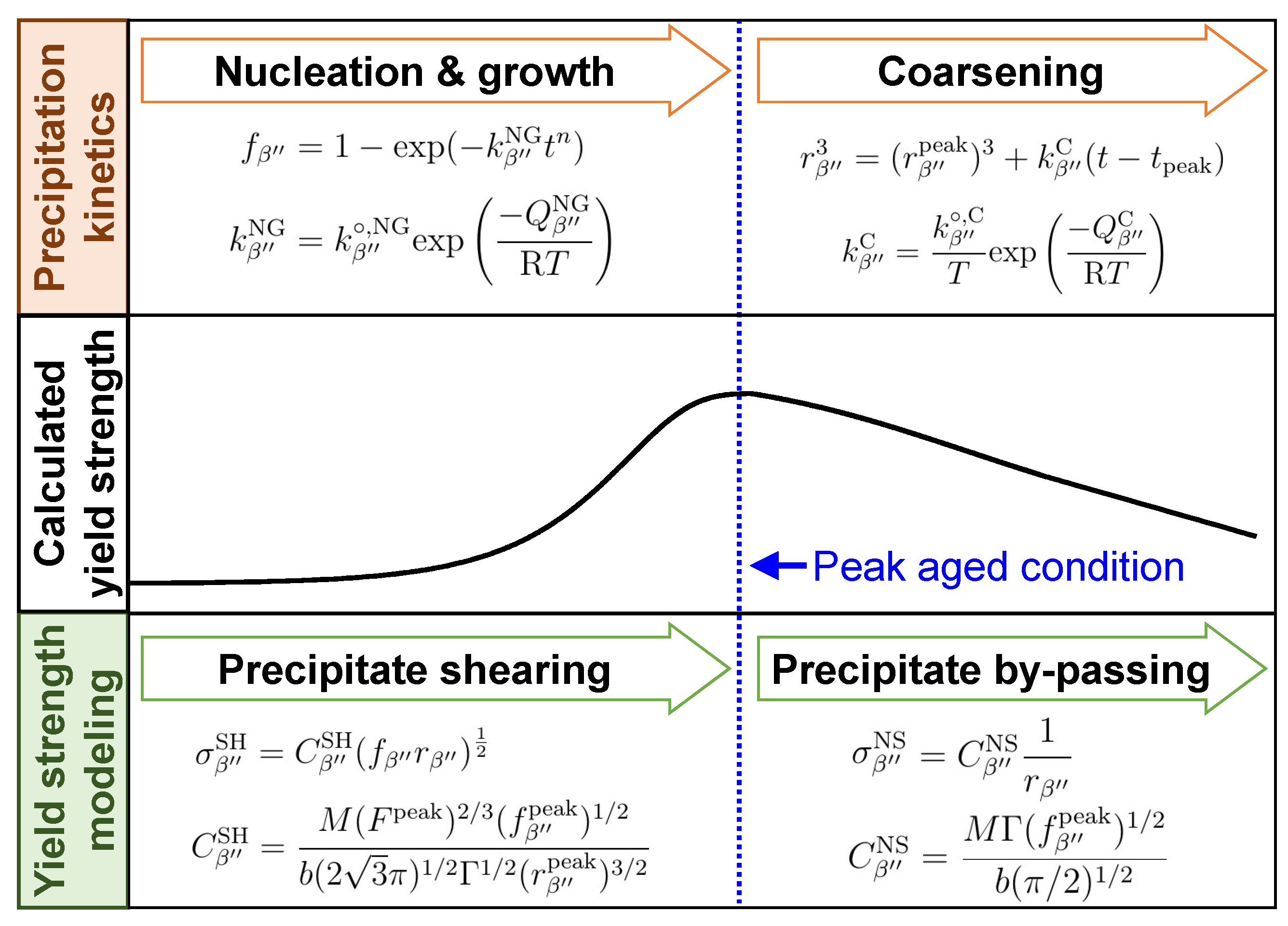
3. Yield Strength Modeling of T5 Heat-Treated CPC A356 Alloy
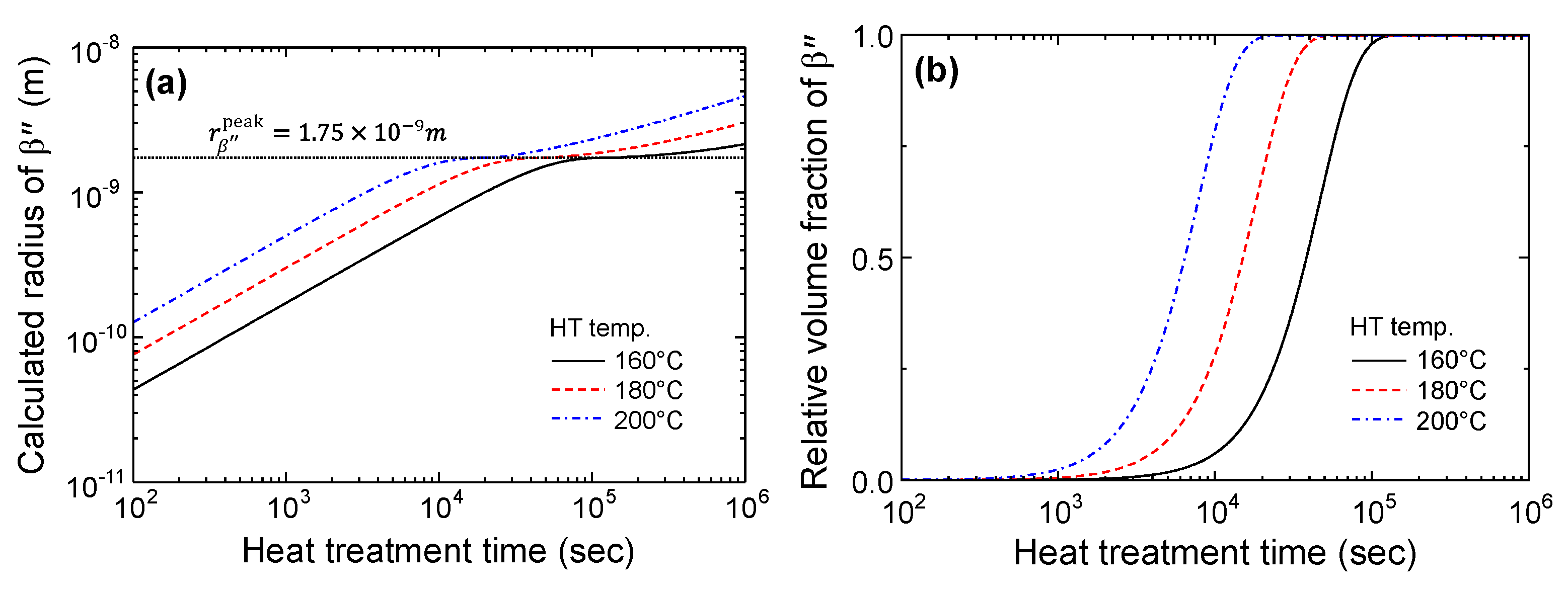
3.1. Precipitation Kinetics Model
- Precipitation kinetics of the needle-like are not much different from those of a spherical precipitate with similar volume so that the size of the needle-like could be expressed as equivalent radius ;
- Precipitation kinetics of are dominantly controlled by nucleation and the growth mechanism until its radius reaches to ;
- After the peak aging condition, coarsening of existing precipitates occur rather than simultaneous nucleation and growth of .
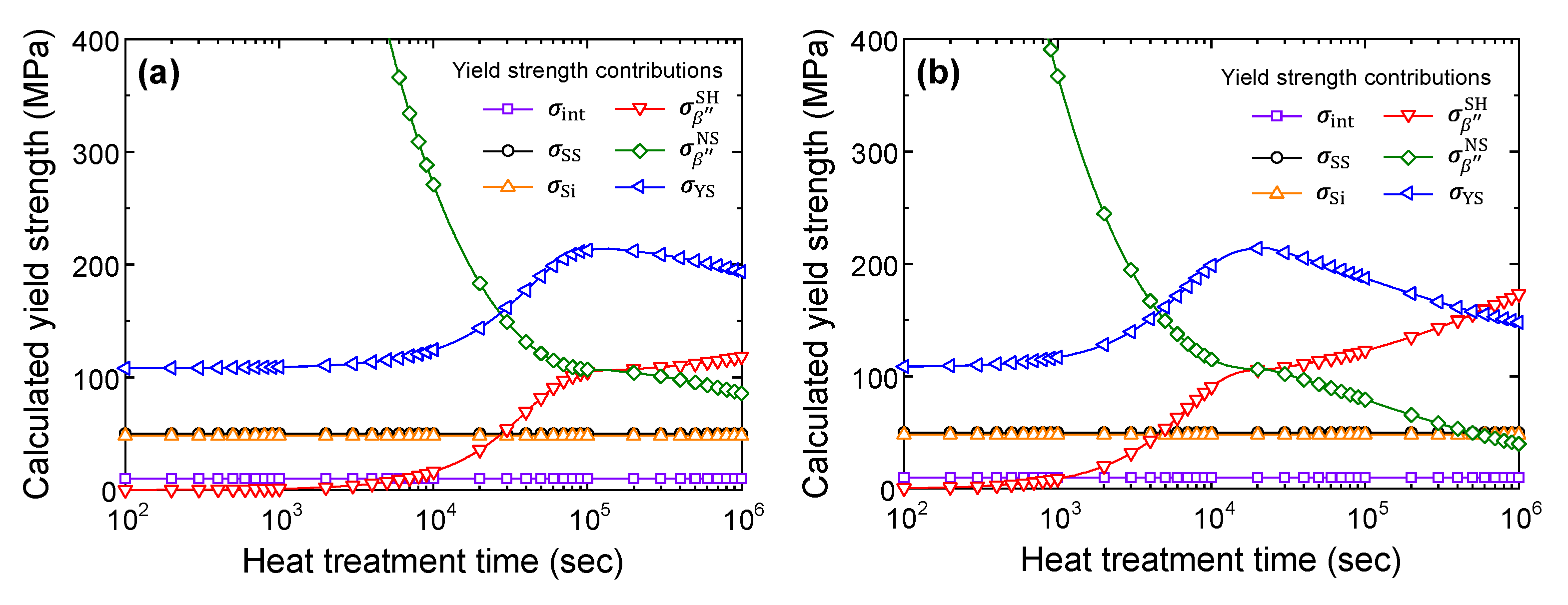
3.2. Yield Strength Model
4. Results and Discussion
4.1. Experimental Results
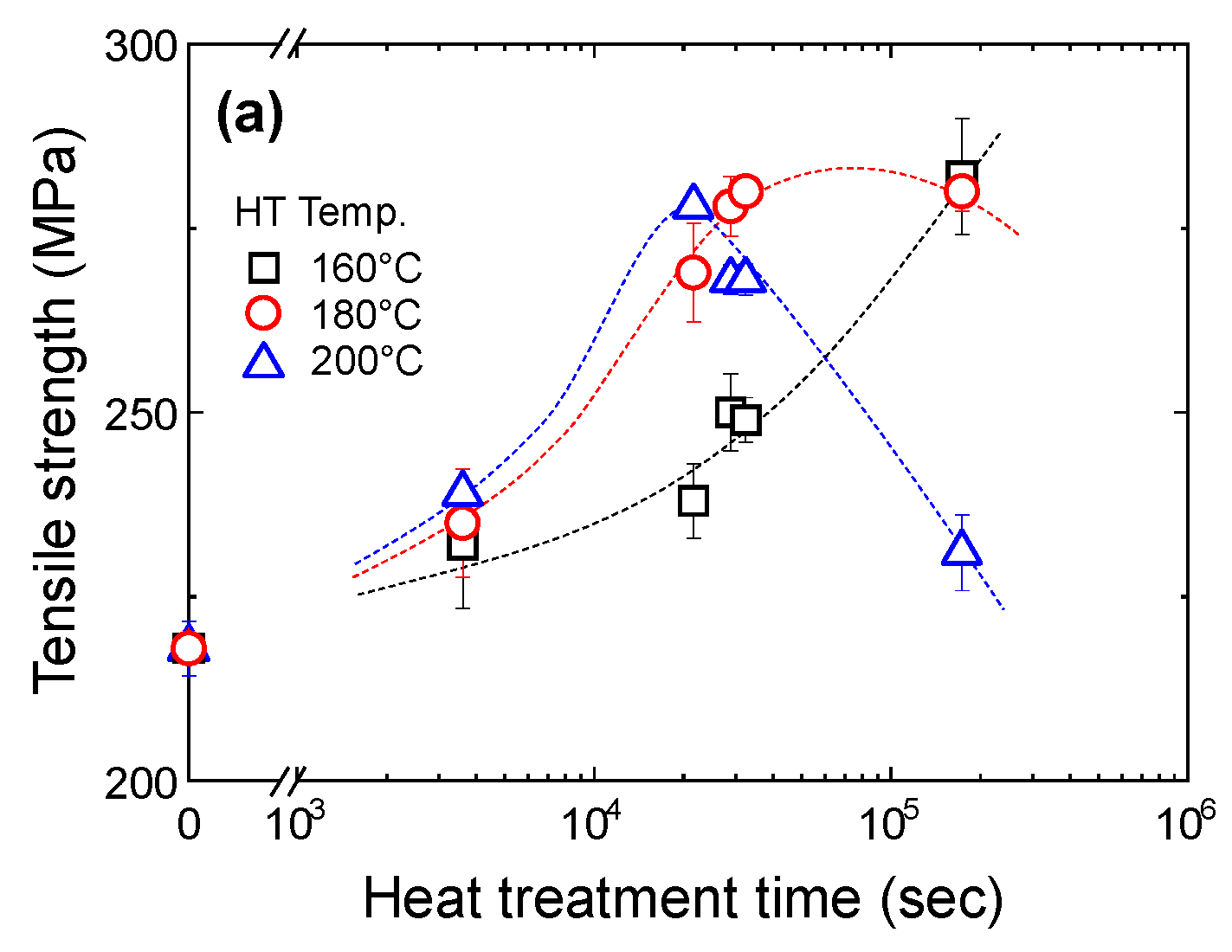
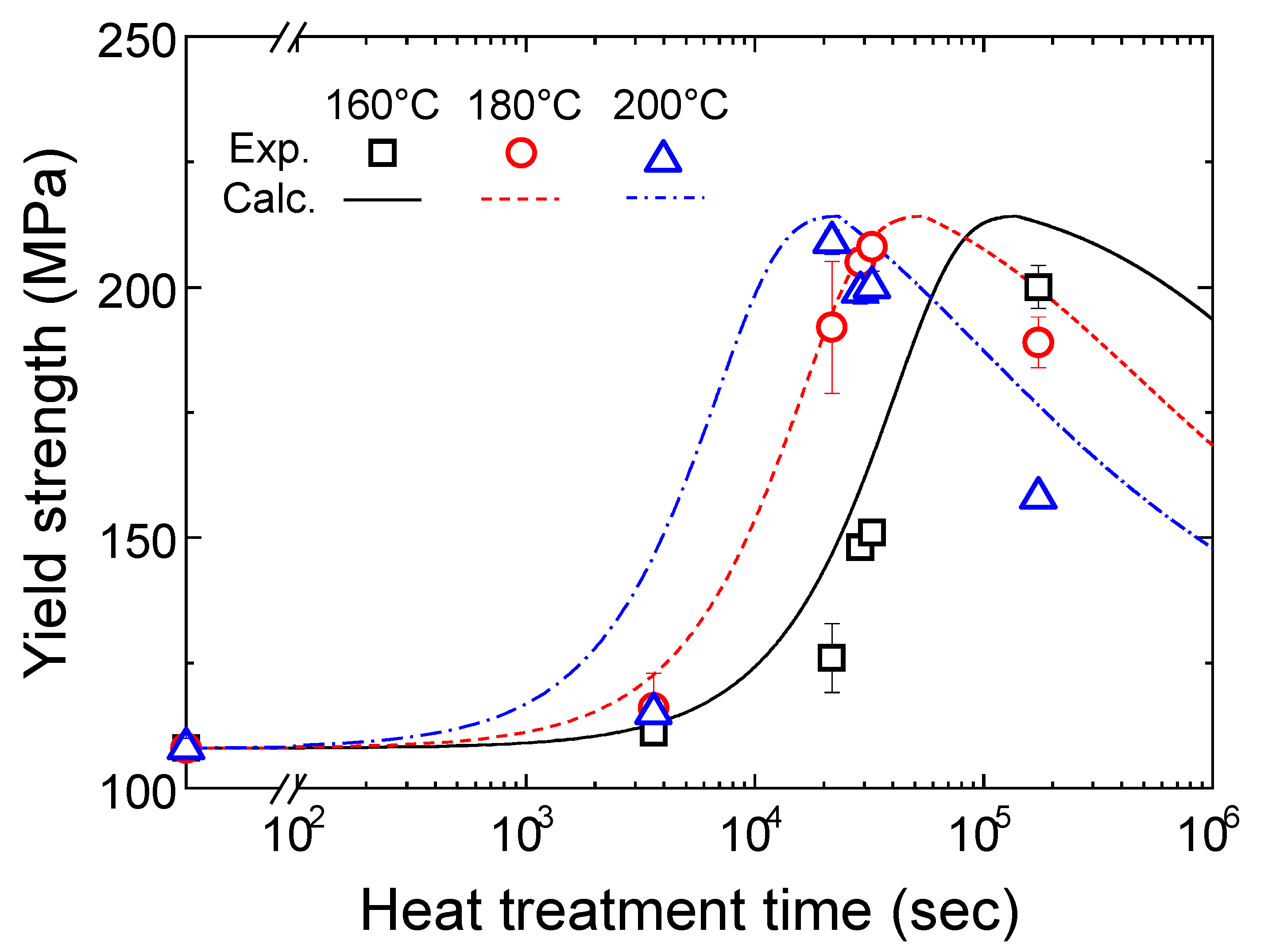
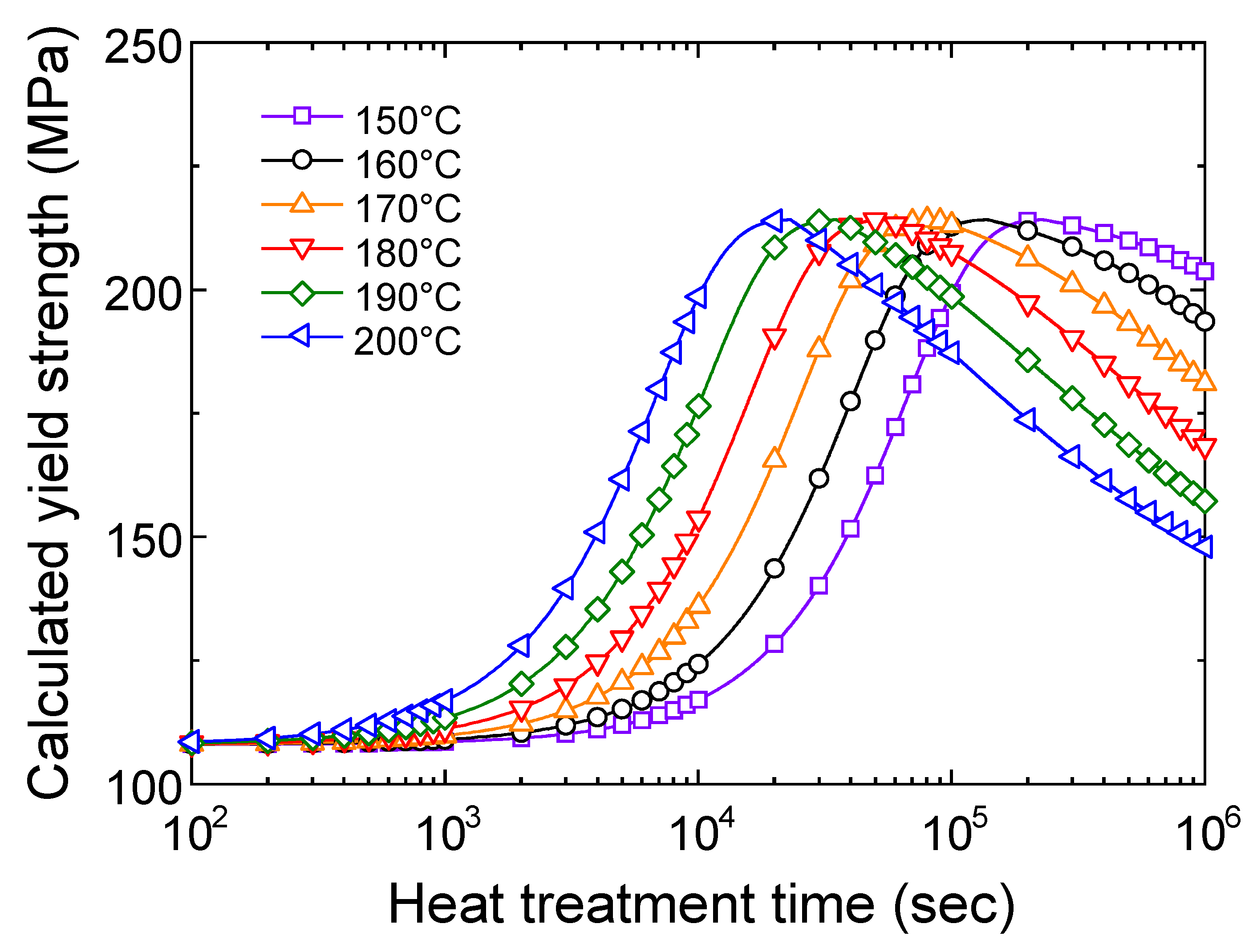
4.1.1. Tensile Properties of the T5 Heat-Treated CPC A356 Alloy
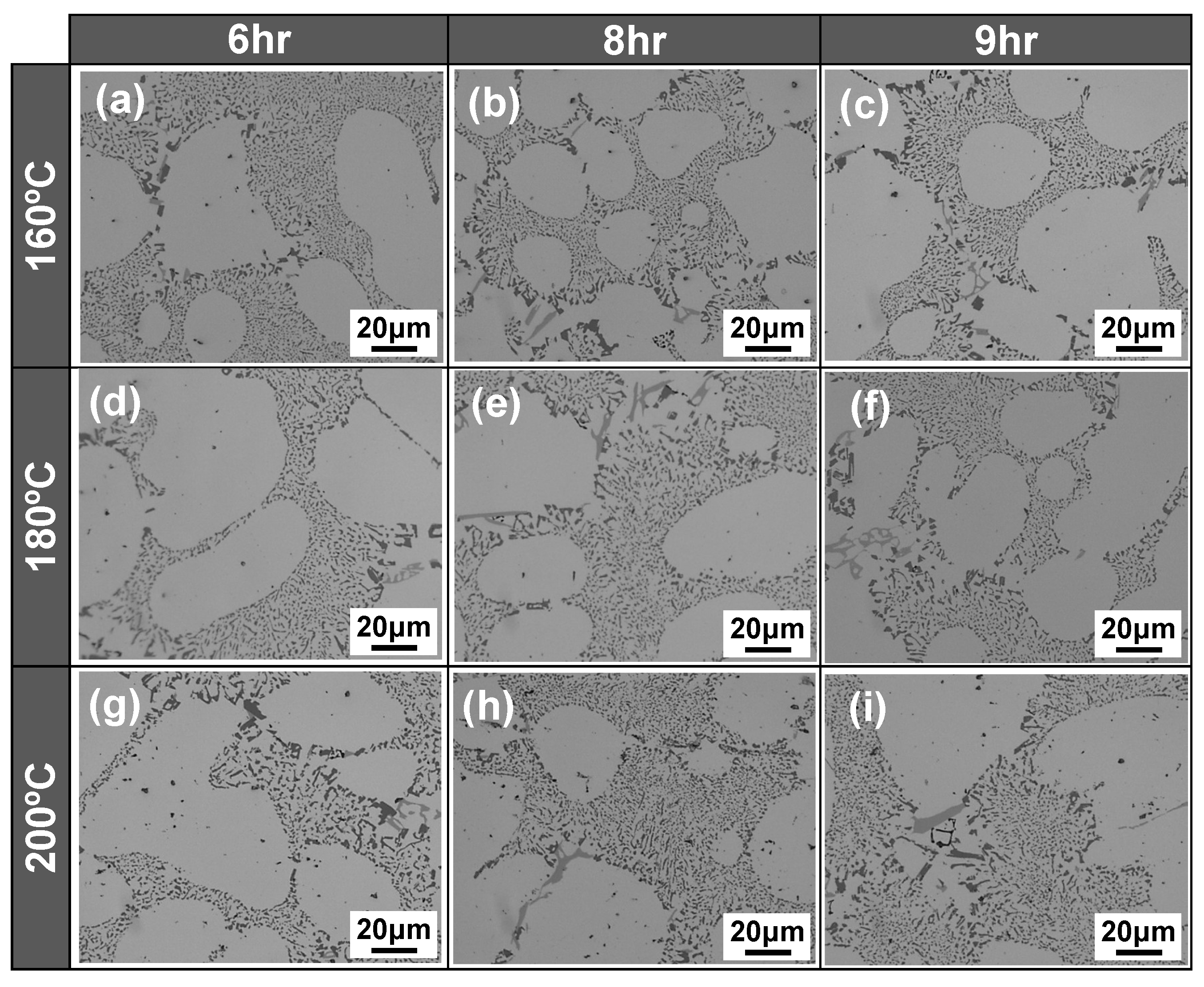
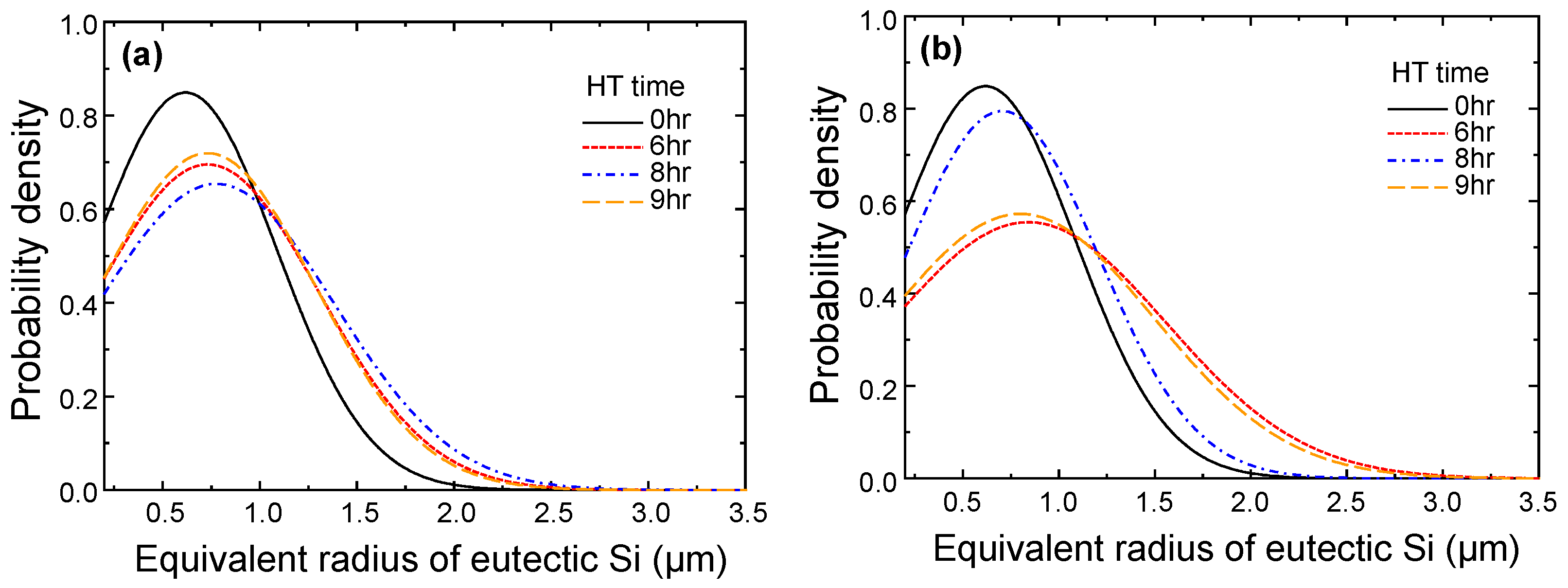
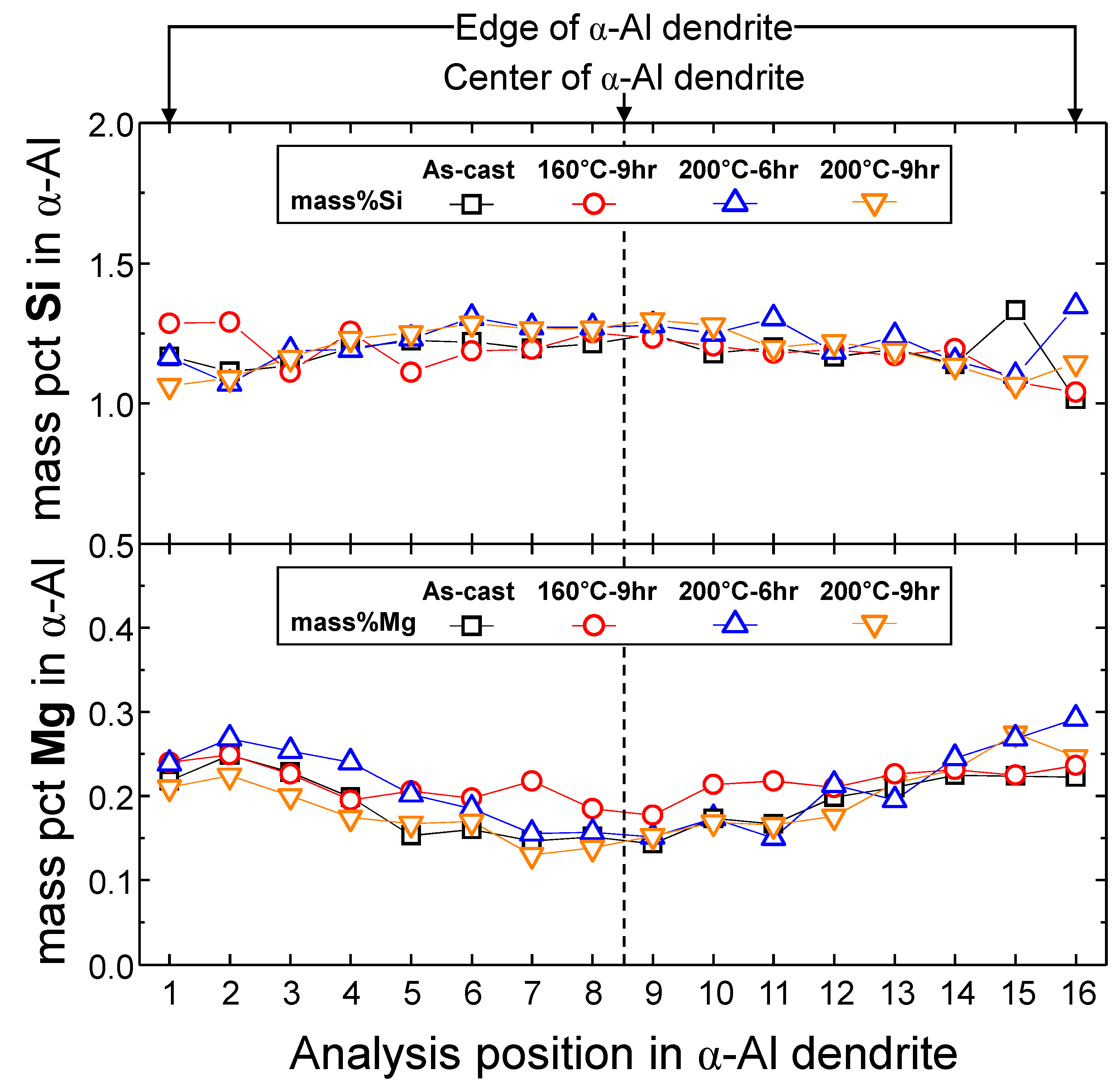
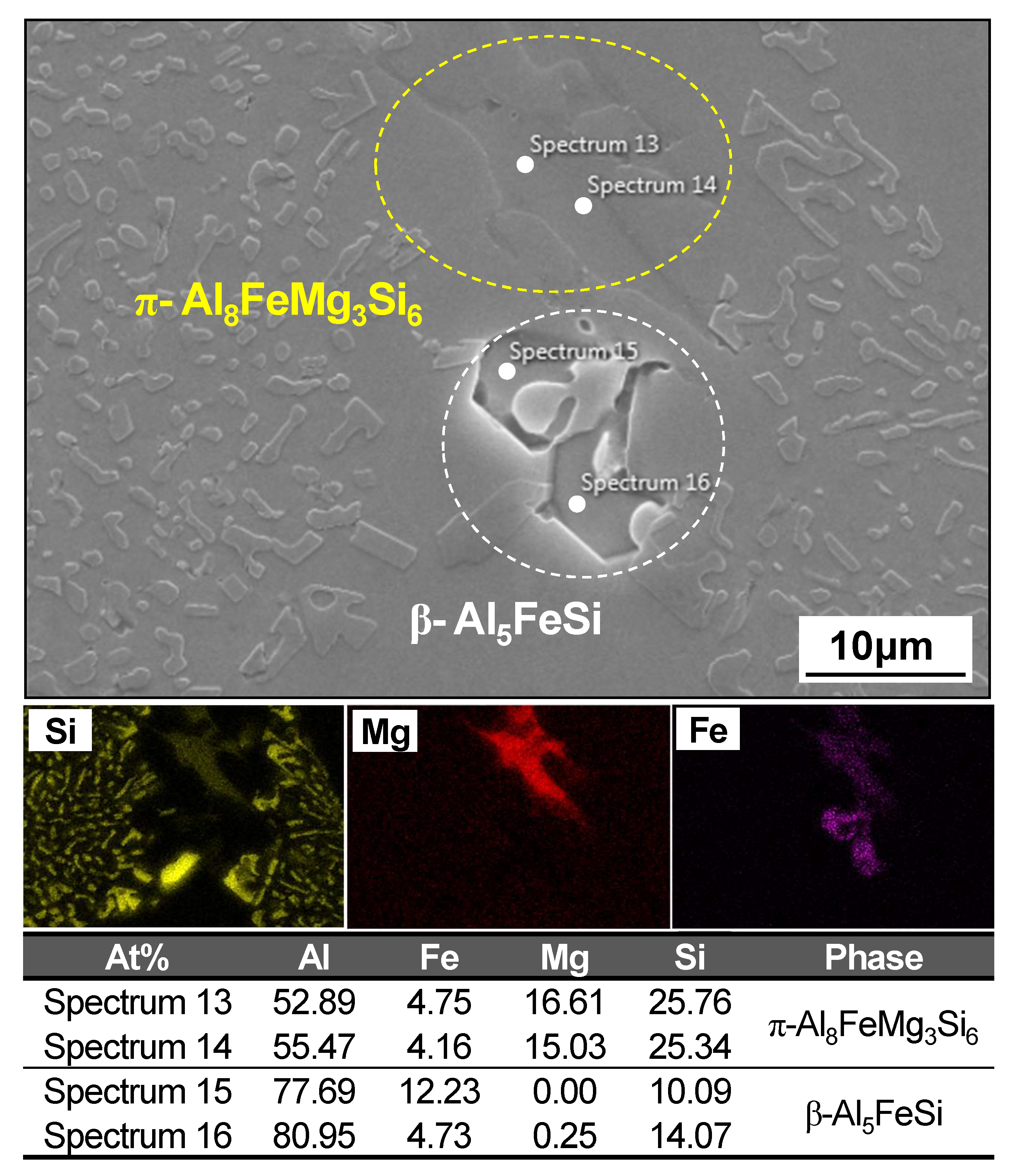
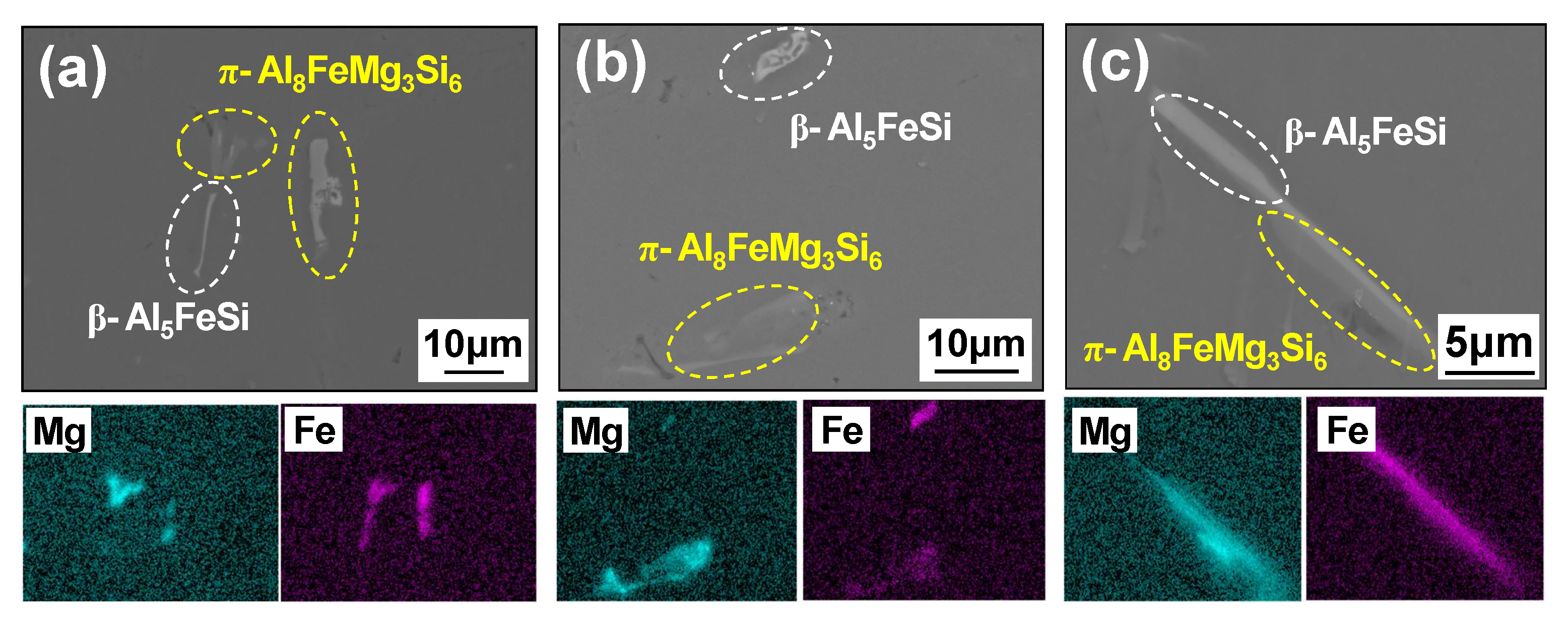
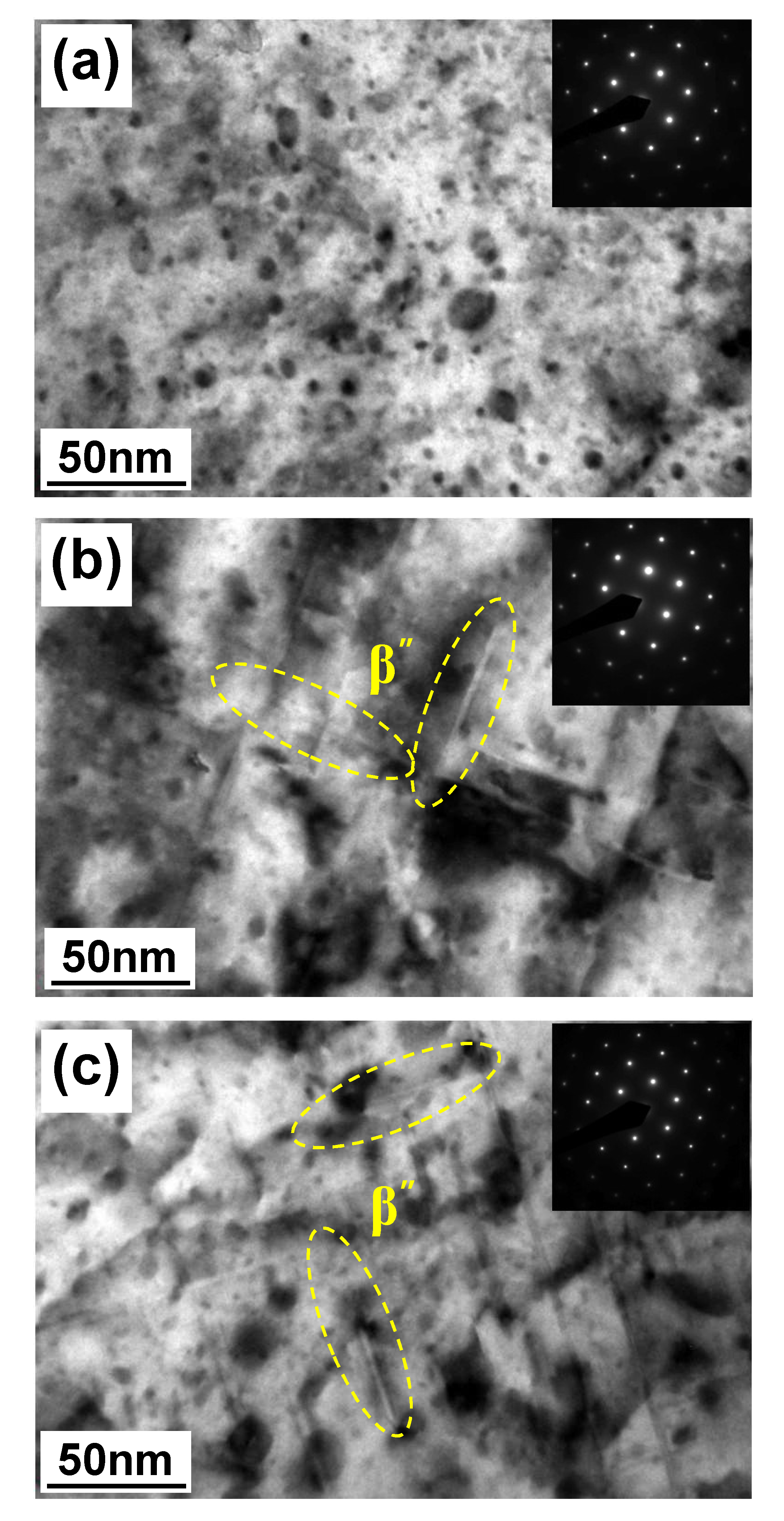
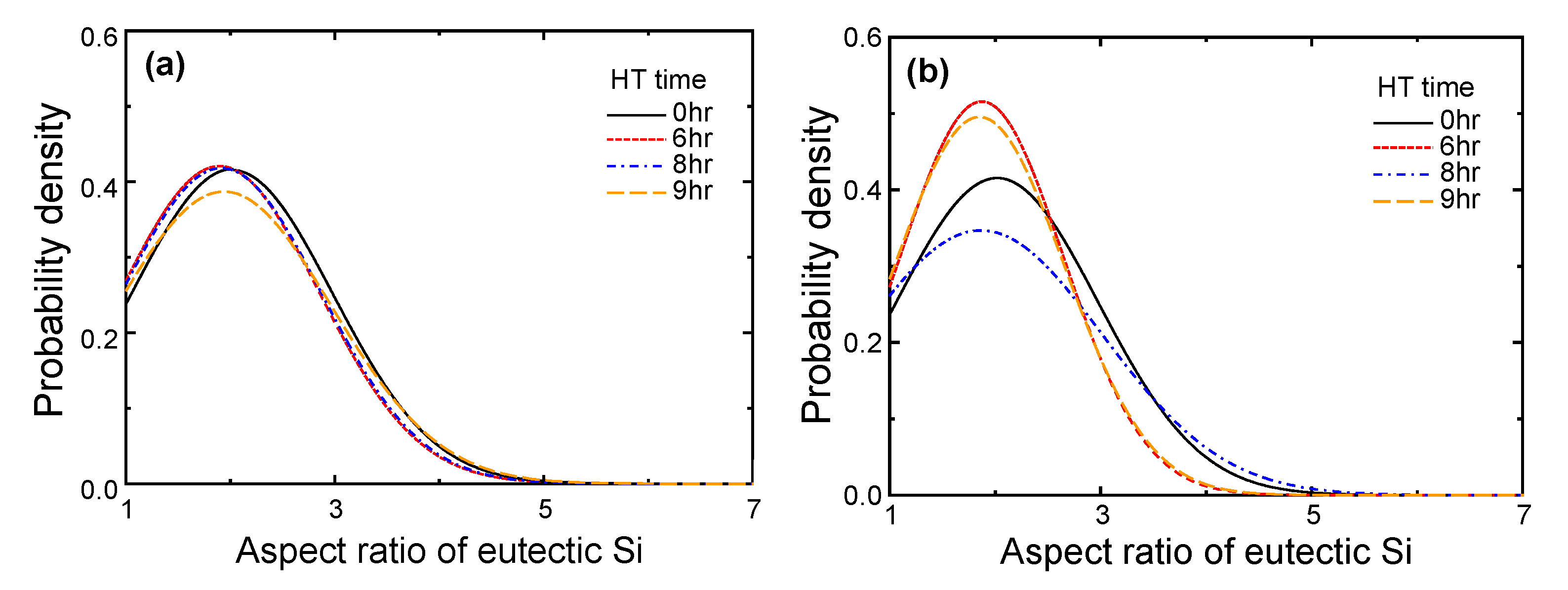
4.1.2. Microstructural Analysis in T5-Heat-Treated CPC A356 Alloys
4.1.3. Relationship between Tensile Properties and Microstructures of T5 Heat-Treated CPC A356 Alloys
4.2. Calculation Results of Yield Strength Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, J.R. ASM Specialty Handbook: Aluminum abd Aluminum Alloys; ASM International: Materials Park, OH, USA, 1993; pp. 718–719. [Google Scholar]
- Lampman, S. Permanent Mold Casting of Aluminum Alloys. In ASM Handbook, Volume 2A, Aluminum Science and Technology; Anderson, K., Weritz, J., Kaufman, J.G., Eds.; ASM International: Materials Park, OH, USA, 2018; pp. 215–216. [Google Scholar]
- Jiang, W.; Fan, Z.; Liu, D.; Liao, D.; Dong, X.; Zong, X. Correlation of microstructure with mechanical properties and fracture behavior of A356-T6 aluminum alloy fabricated by expendable pattern shell casting with vacuum and low-pressure, gravity casting and lost foam casting. Mater. Sci. Eng. A 2013, 560, 396–403. [Google Scholar] [CrossRef]
- Youn, S.W.; Kang, C.G. Characterization of age-hardening behavior of eutectic region in squeeze-cast A356-T5 alloy using nanoindenter and atomic force microscope. Mater. Sci. Eng. A 2006, 425, 28–35. [Google Scholar] [CrossRef]
- Rosso, M.; Peter, I.; Villa, R. Effects of T5 and T6 heat treatments applied to rheocast A356 parts for automotive applications. Solid State Phenom. 2008, 141–143, 237–242. [Google Scholar] [CrossRef]
- Maeng, H.; Choi, Y.; Lee, S.J. Model of Precipitation Hardening of Al–Mg–Si Alloys Under Aging. Met. Sci. Heat Treat. 2019, 61, 455–460. [Google Scholar] [CrossRef]
- Kim, D.; Maeng, H.; Choi, Y.; Choi, H.; Lee, S.J. Constitutive Model of Triple-Step-Aged Al–Mg–Si Alloy Incorporating Precipitation Kinetics. Met. Mater. Int. 2020, in press. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, J.; Seo, N.; Son, S.B.; Lee, S.J. Effect of Heating Rate on Microstructure and Mechanical Properties in Al 7055. Met. Mater. Int. 2021, 27, 449–455. [Google Scholar] [CrossRef]
- Choi, S.; Kim, G.; Kim, J.P.; Kim, S.H.; Son, S.B.; Lee, S.J. Probability-Dependent Precipitation Strengthening Effect of Anisotropic Precipitate in Al-Mg-Si Alloy Produced by T6 Heat Treatment. Korean J. Met. Mater. 2021, 59, 1–9. [Google Scholar] [CrossRef]
- Dutta, I.; Allen, S.M. A calorimetric study of precipitation in commercial aluminum alloy 6061. J. Mater. Sci. Lett. 1991, 10, 323–326. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al–Mg–Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K.; Saga, M.; Kikuchi, M. Atom probe studies on the early stages of precipitation in Al–Mg–Si alloys. Mater. Sci. Eng. A 1998, 250, 127–132. [Google Scholar] [CrossRef]
- Zhen, L.; Fei, W.D.; Kang, S.B.; Kim, H.W. Precipitation behaviour of Al–Mg–Si alloys with high silicon content. J. Mater. Sci. 1997, 32, 1895–1902. [Google Scholar] [CrossRef]
- Johnson, W.A.; Mehl, K.F. Reaction kinetics in process of nucleation and growth. Trans. AIME 1939, 135, 416. [Google Scholar]
- Avrami, M. Kinetics of phase change. I: General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Kolmogorov, A.N. Statistical theory of nucleation processes. Izu. Akad. Nauk SSSR 1937, 3, 355–366. [Google Scholar]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Wagner, C. Theorie der Alterung von Niederschlägen durch Umlösen (Ostwald-Reifung). Z. Elektrochem 1961, 65, 581–591. [Google Scholar]
- Esmaeili, S.; Lloyd, D.J.; Poole, W.J. A yield strength model for the Al–Mg–Si–Cu alloy AA6111. Acta Mater. 2003, 51, 2243–2257. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Guo, H.; Xia, Z.; Wu, Q.; Liu, B. Correlation of solidification microstructure refining scale, Mg composition and heat treatment conditions with mechanical properties in Al–7Si–Mg cast aluminum alloys. Mater. Sci. Eng. A 2017, 685, 391–402. [Google Scholar] [CrossRef]
- Colley, L.J.; Wells, M.A.; Poole, W.J. Microstructure–strength models for heat treatment of Al–Si–Mg casting alloys I: Microstructure evolution and precipitation kinetics. Can. Metall. Q. 2014, 53, 125–137. [Google Scholar] [CrossRef]
- Elsharkawi, E.A.; Samuel, E.; Samuel, A.M.; Samuel, F.H. Effects of Mg, Fe, Be additions and solution heat treatment on the π-AlMgFeSi iron intermetallic phase in Al–7Si–Mg alloys. J. Mater. Sci. 2010, 45, 1528–1539. [Google Scholar] [CrossRef]
- Zhen, L.; Kang, S.B. Effect of predeformation on microstructure and tensile properties of Al–Mg–Si alloys with high silicon content. Mater. Sci. Technol. 1998, 14, 317–321. [Google Scholar] [CrossRef]
- Takeda, M.; Ohkubo, F.; Shirai, T.; Fukui, K. Stability of metastable phases and microstructures in the ageing process of Al–Mg–Si ternary alloys. J. Mater. Sci. 1998, 33, 2385–2390. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Jansen, J.; Zandbergen, H.W. Atomic model for GP-zones in a 6082 Al–Mg–Si system. Acta Mater. 2001, 49, 321–328. [Google Scholar] [CrossRef]
- Andersen, S.J.; Zandbergen, H.W.; Jansen, J.; TrÆholt, C.; Tundal, U.; Reiso, O. The crystal structure of the β″ phase in Al–Mg–Si alloys. Acta Mater. 1998, 46, 3283–3298. [Google Scholar] [CrossRef]
- Matsuda, K.; Gamada, H.; Fujii, K.; Uetani, Y.; Sato, T.; Kamio, A.; Ikeno, S. Microscopy on the structure of Guinier-Preston zones in an Al-1.6 mass Pct Mg2Si alloy. Metall. Mater. Trans. A 1998, 29, 1161–1167. [Google Scholar] [CrossRef]
- Zandbergen, H.W.; Anderson, S.J.; Jansen, J. Structure Determination of Mg5Si6 Particles in Al by Dynamic Electron Diffraction Studies. Science 1997, 277, 1221–1225. [Google Scholar] [CrossRef]
- Cayron, C.; Buffat, P.A. Transmission electron microscopy study of the β′ phase (Al–Mg–Si alloys) and QC phase (Al–Cu–Mg–Si alloys): Ordering mechanism and crystallographic structure. Acta Mater. 2000, 48, 2639–2653. [Google Scholar] [CrossRef]
- Geisler, A.H.; Hill, J.K. Analyses and interpretations of X-ray diffraction effects in patterns of aged alloys. Acta Crystallogr. 1948, 1, 238–252. [Google Scholar] [CrossRef]
- Ninive, P.H.; Strandlie, A.; Gulbrandsen-Dahl, S.; Lefebvre, W.; Marioara, C.D.; Andersen, S.J.; Friis, J.; Holmestad, R.; Løvvik, O.M. Detailed atomistic insight into the β″ phase in Al–Mg–Si alloys. Acta Mater. 2014, 69, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Haghdadi, N.; Zarei-Hanzaki, A.; Roostaei, A.A.; Hemmati, A.R. Evaluating the mechanical properties of a thermomechanically processed unmodified A356 Al alloy employing shear punch testing method. Mater. Des. 2013, 43, 419–425. [Google Scholar] [CrossRef]
- Myhr, O.R.; Grong, Ø.; Andersen, S.J. Modelling of the age hardening behaviour of Al-Mg-Si alloys. Acta Mater. 2001, 49, 65–75. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Guo, H.; Xia, Z.; Wu, Q.; Liu, B. Modeling the precipitation kinetics and tensile properties in Al-7Si-Mg cast aluminum alloys. Mater. Sci. Eng. A 2017, 685, 403–416. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Alkahtani, S.A.; Abuhasel, K.A.; Samuel, F.H. Effect of intermetallics on the microstructure and tensile properties of aluminum based alloys: Role of Sr, Mg and Be addition. Mater. Des. 2015, 86, 30–40. [Google Scholar] [CrossRef]


| Element | Si | Mg | Ti | Fe | Sr | Al |
|---|---|---|---|---|---|---|
| Content | 7.01 | 0.40 | 0.12 | 0.11 | 0.0057 | Balance |
| Parameter | Unit | Value | Remark |
|---|---|---|---|
| n | - | 1.8 | Optimized in the present study |
| m | 1.75 × 10 | From the literature [19] | |
| - | 0.0072 | From the literature [19] | |
| 1/sec | 4.65644 × 10 | Optimized in the present study | |
| kJ/mol | 181.384 | Optimized in the present study | |
| m/sec | 8 × 10 | Optimized in the present study | |
| kJ/mol | 125 | Optimized in the present study |
| Parameter | Unit | Value | Remark |
|---|---|---|---|
| MPa | 10 | From the literature [19] | |
| MPa | 50 | From the literature [19] | |
| MPa | 48 | Optimized in the present study | |
| MPa/ | 131.384 | Optimized in the present study |
| Phase | GP Zone | |||
|---|---|---|---|---|
| Shape | fine plate [27] | Needle-like [28] | Rod-like [29] | Platelets [30] |
| Composition | MgSi [27] | MgSi [28] MgAlSi [31] | MgSi [29] | MgSi [30] |
| Structure | fcc based cubic [27] | Monoclinic (C2/m) [28] | Hexagonal (P6/m) [29] | Cubic (CaF) [30] |
| Lattice Parameters | a = 4.05 Å [27] | a = 15.16 Å b = 4.05Å c = 6.74 Å = 105.3 [28] | a = 7.1 Å c = 4.05 Å [29] | a = 6.39Å [30] |
| Size | 1–3 nm [25,26] | 4 × 4 × 35–50 nm [25,26] | 20 × 20 × 500 nm [26] | 10–20 nm diameter [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-W.; Lee, S.-J.; Kim, D.-U.; Kim, M.-S. Experimental Investigation on Tensile Properties and Yield Strength Modeling of T5 Heat-Treated Counter Pressure Cast A356 Aluminum Alloys. Metals 2021, 11, 1192. https://doi.org/10.3390/met11081192
Kim S-W, Lee S-J, Kim D-U, Kim M-S. Experimental Investigation on Tensile Properties and Yield Strength Modeling of T5 Heat-Treated Counter Pressure Cast A356 Aluminum Alloys. Metals. 2021; 11(8):1192. https://doi.org/10.3390/met11081192
Chicago/Turabian StyleKim, Sang-Won, Seok-Jae Lee, Dae-Up Kim, and Min-Su Kim. 2021. "Experimental Investigation on Tensile Properties and Yield Strength Modeling of T5 Heat-Treated Counter Pressure Cast A356 Aluminum Alloys" Metals 11, no. 8: 1192. https://doi.org/10.3390/met11081192
APA StyleKim, S.-W., Lee, S.-J., Kim, D.-U., & Kim, M.-S. (2021). Experimental Investigation on Tensile Properties and Yield Strength Modeling of T5 Heat-Treated Counter Pressure Cast A356 Aluminum Alloys. Metals, 11(8), 1192. https://doi.org/10.3390/met11081192






