The Effect of Powder Composition on the Microstructure and Corrosion Resistance of Laser Cladding 60NiTi Alloy Coatings on SS 316L
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
3.1. Phase Structures
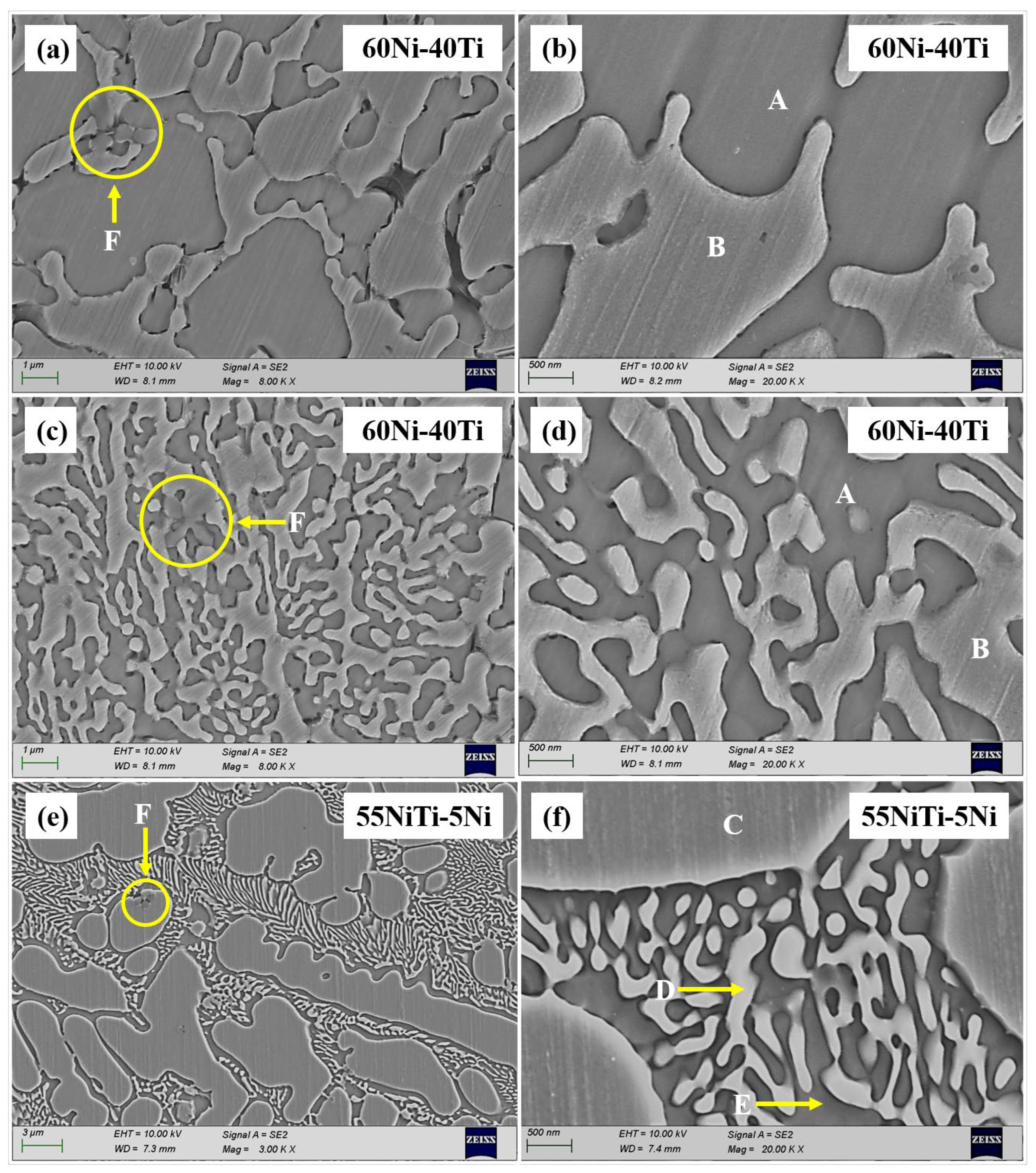
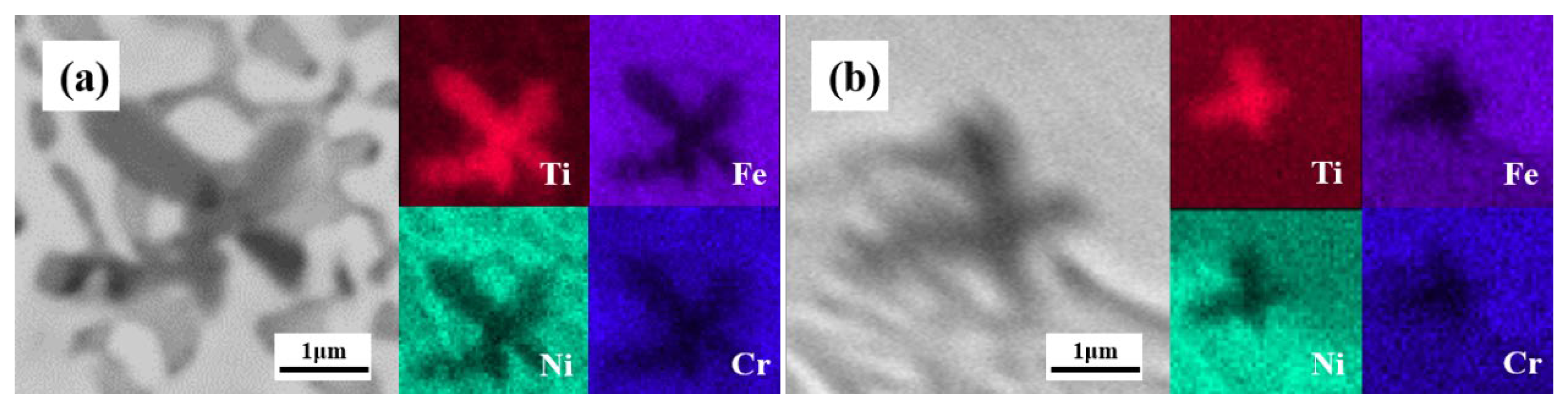
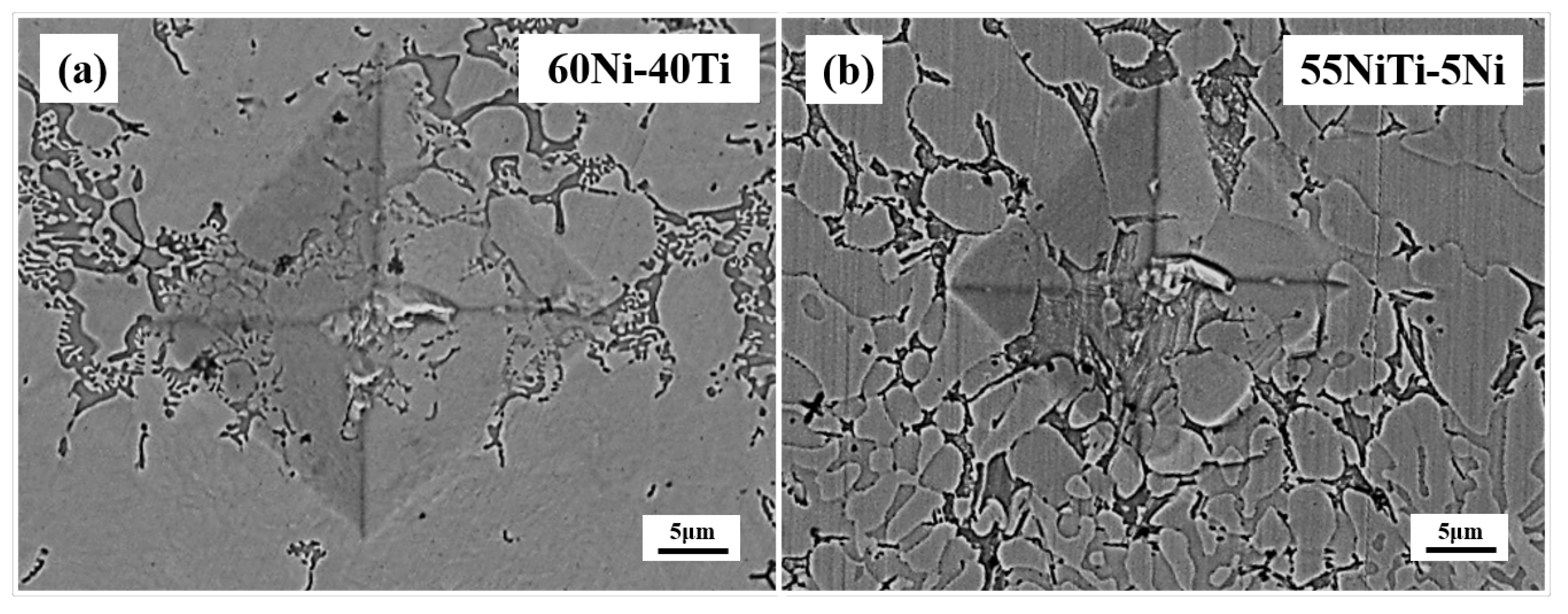
3.2. Microstructural and Compositional Analysis
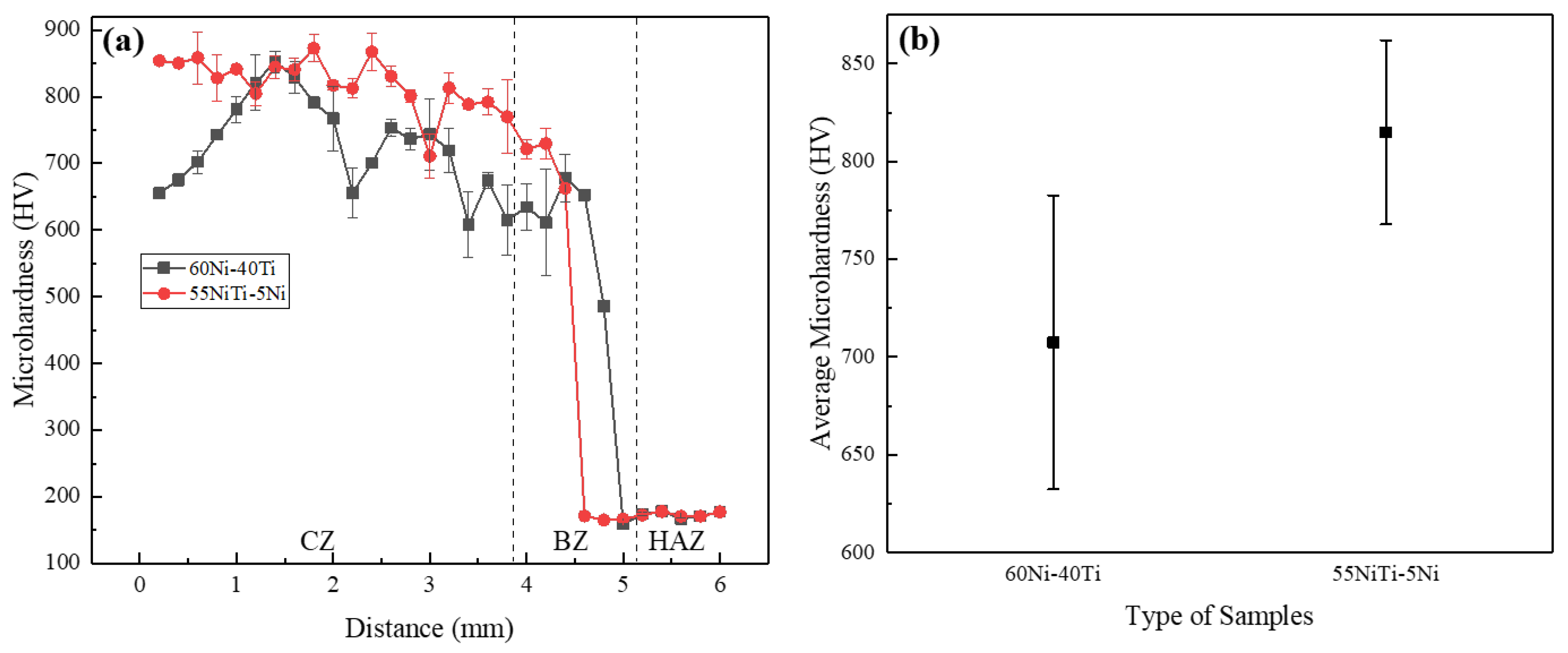
3.3. Microhardness
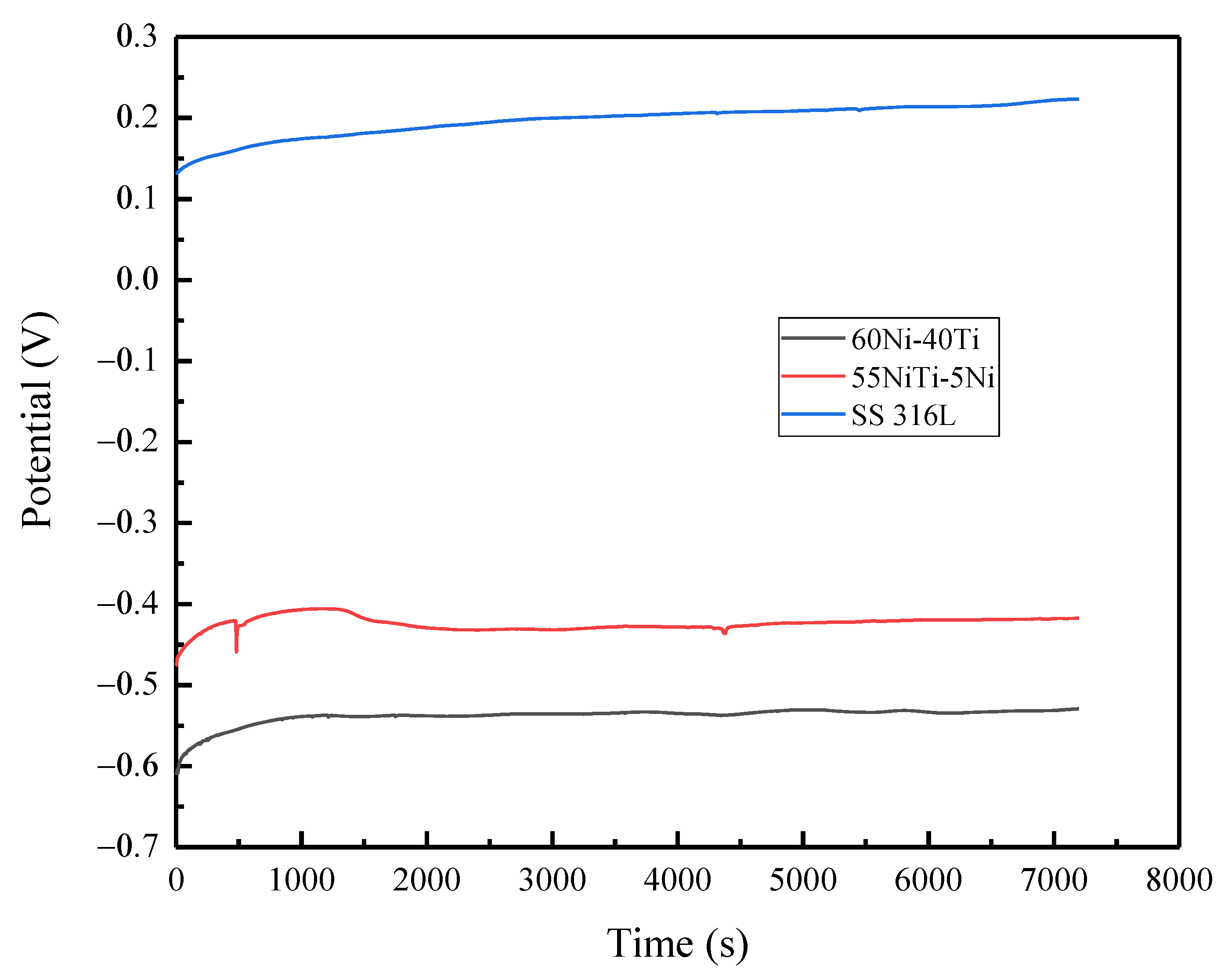
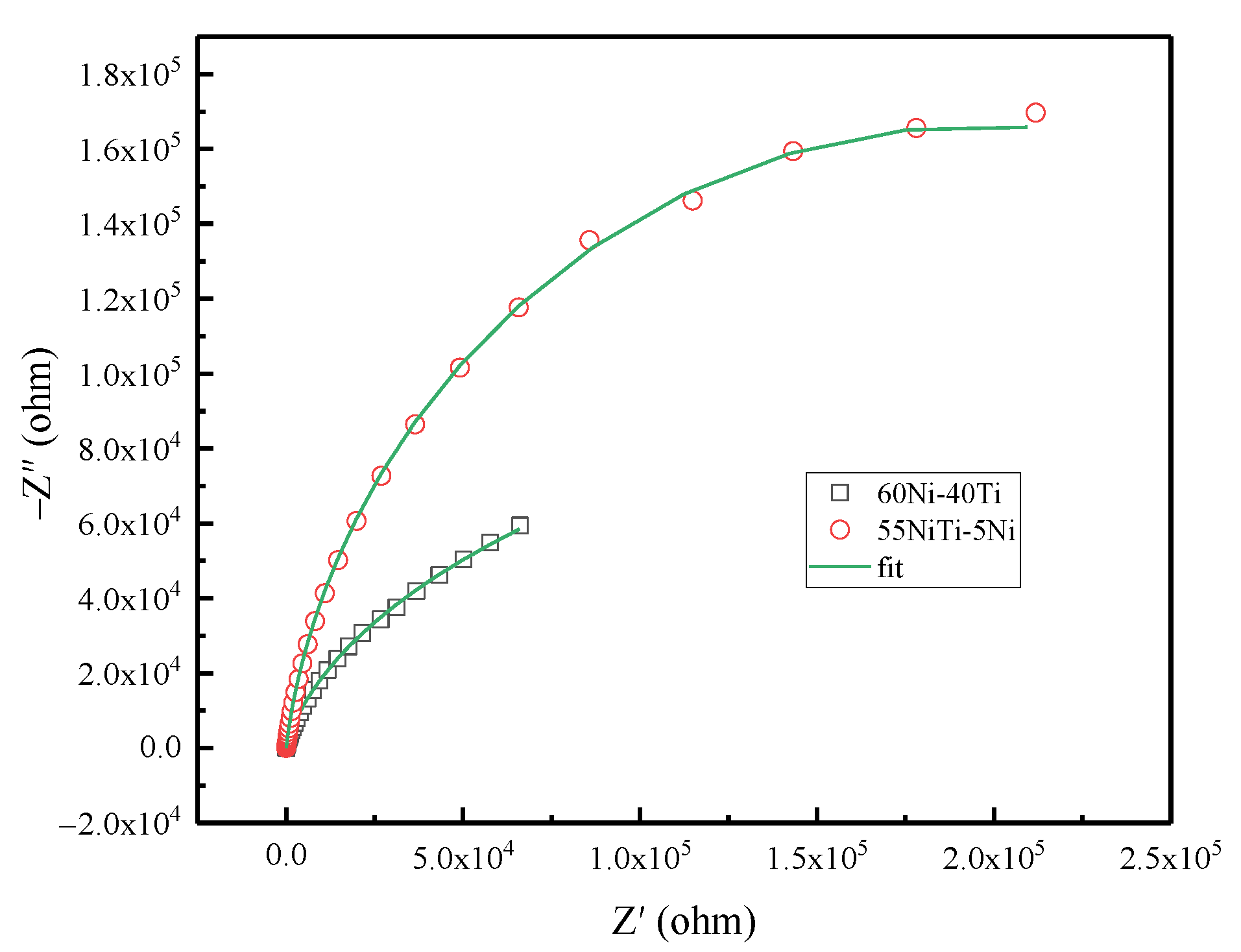
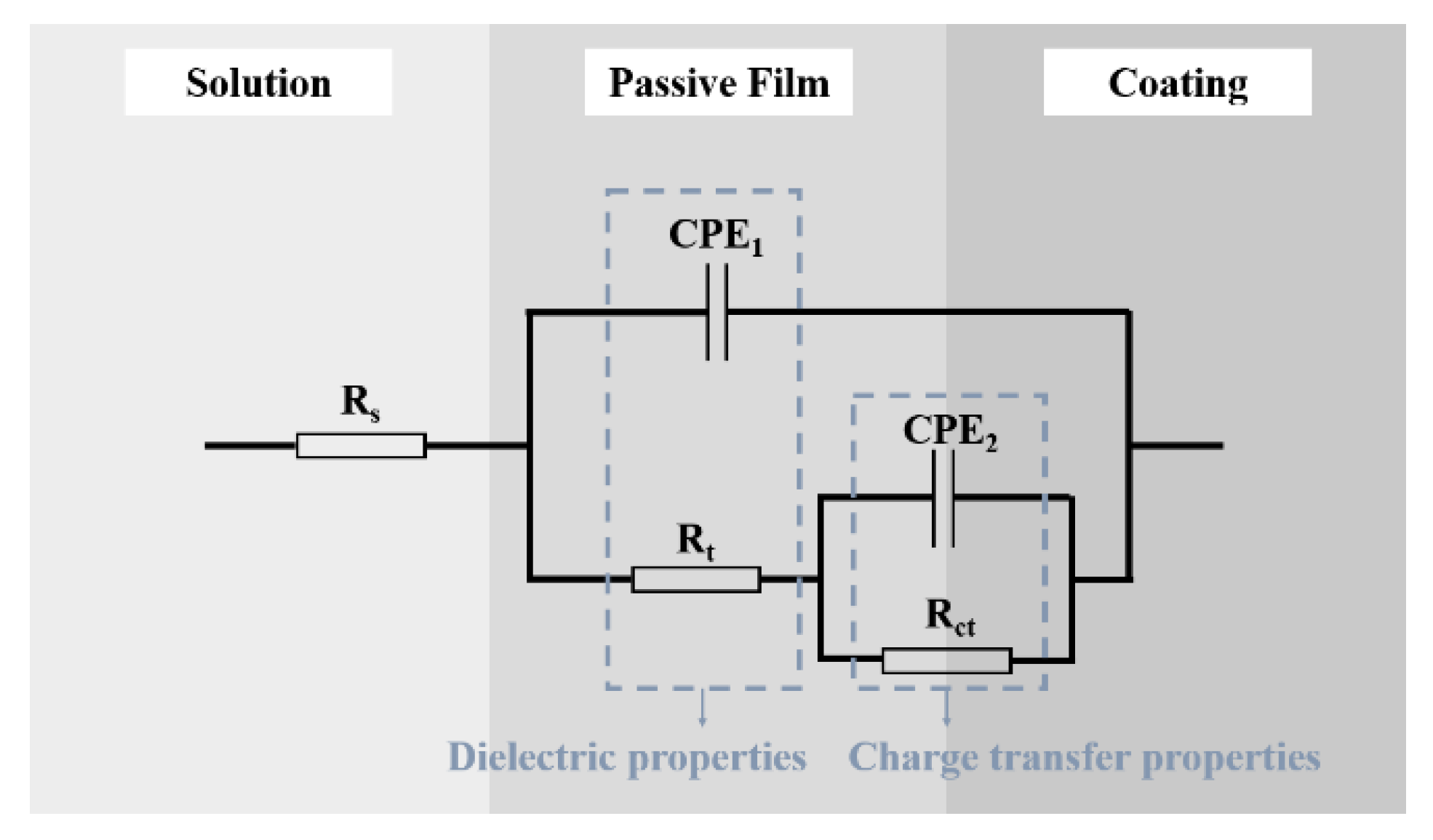
3.4. Electrochemical Behavior
4. Conclusions
- (1).
- 55NiTi-5Ni coating has a lower dilution rate than 60Ni-40Ti, which means a better cladding quality.
- (2).
- 55NiTi-5Ni coating shows a typical dendritic eutectic structure, which is composed of a dendritic and interdendritic eutectic structure, but 60Ni-40Ti tends to form a eutectic network structure composed of a coarse network structure with refined areas. XRD results show that the main phases in both coatings are (Ni, Fe)Ti and (Ni, Fe)3Ti, but the proportion of the (Ni, Fe)Ti phase is higher in 55NiTi-5Ni along with more (Ni, Fe)3Ti in 60Ni-40Ti.
- (3).
- The average microhardness of 60Ni-40Ti and 55NiTi-5Ni is 815.0 and 707.6 HV, respectively, which is considerably higher than substrate SS 316L, which is attributed to grain refinement and nickel–titanium particle agglomeration. Moreover, the microhardness of 55NiTi-5Ni is slightly higher than that of 60Ni-40Ti, which is related to its uniform and fine eutectic structure.
- (4).
- Compared with SS 316L, the corrosion resistance of NiTi coatings is improved dramatically. The 55NiTi-5Ni coating shows better corrosion resistance because its uniform microstructure and fine eutectic structure facilitate the formation of the dense oxide film. In addition, its lower proportion of the (Ni, Fe)3Ti phase might induce the formation of fewer micro-cells on the surface and fewer pitting initiation points.
Author Contributions
Funding
Conflicts of Interest
References
- Buehler, W.J.; Gilfrich, J.V.; Wiley, R.C. Effect of low-temperature phase changes on the mechanical properties of alloys near composition TiNi. J. Appl. Phys. 1963, 34, 1475–1477. [Google Scholar] [CrossRef]
- Chaudhari, R.; Vora, J.J.; Patel, V.; Lacalle, L.N.L.; Parikh, D.M. Effect of WEDM process parameters on surface morphology of nitinol shape memory alloy. Materials 2020, 13, 4943. [Google Scholar] [CrossRef] [PubMed]
- Rathi, P.; Ghiya, R.; Shah, H.; Srivastava, P.; Patel, S.; Chaudhari, R.; Vora, J. Multi-response optimization of Ni55.8Ti shape memory alloy using Taguchi–Grey relational analysis approach. In Recent Advances in Mechanical Infrastructure; Springer: Singapore, 19 October 2019; pp. 13–23. [Google Scholar]
- Khanlari, K.; Ramezani, M.; Kelly, P. 60NiTi: A review of recent research findings, potential for structural and mechanical applications, and areas of continued investigations. Trans. Indian Inst. Met. 2017, 71, 781–799. [Google Scholar] [CrossRef]
- Khanlari, K.; Ramezani, M.; Kelly, P.; Neitzert, T. Effect of hafnium addition in 60NiTi alloy hardened under open atmosphere conditions. Metallogr. Microstruct. Anal. 2018, 7, 476–486. [Google Scholar] [CrossRef]
- Qin, Q.; Wen, Y.; Wang, G.; Zhang, L. Effects of solution and aging treatments on corrosion resistance of as-cast 60NiTi alloy. J. Mater. Eng. Perform. 2016, 25, 5167–5172. [Google Scholar] [CrossRef]
- Miller, C.; Choudhury, D.; Zou, M. The Effects of surface roughness on the durability of polydopamine/PTFE solid lubricant coatings on NiTiNOL 60. Tribol. Trans. 2019, 62, 919–929. [Google Scholar] [CrossRef]
- Chen, Z.; Khanlari, K.; Ramezani, M.; Hayat, M.; Piaras, K.; Cao, P.; Neitzert, T.; Pasang, T.; Lau, K.T.; Zhu, M. Microstructural and mechanical properties of porous 60NiTi prepared by conventional press-and-sinter method. MATEC Web Conf. 2017, 109, 01002. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Gisario, A.; Brotzu, A.; Natali, S. Laser welding of NiTi shape memory sheets using a diode laser. Opt. Laser Technol. 2018, 108, 142–149. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Qin, Q.; Fan, Q.; Bao, S.; Wen, Y. Effects of annealing on hardness and corrosion resistance of 60NiTi film deposited by magnetron sputtering. J. Alloy. Compd. 2018, 746, 45–53. [Google Scholar] [CrossRef]
- Liu, B.; Jin, W.; Lu, A.; Liu, K.; Wang, C.; Mi, G. Optimal design for dual laser beam butt welding process parameter using artificial neural networks and genetic algorithm for SUS316L austenitic stainless steel. Opt. Laser Technol. 2020, 125. [Google Scholar] [CrossRef]
- Goodarzi, D.M.; Pekkarinen, J.; Salminen, A. Analysis of laser cladding process parameter influence on the clad bead geometry. Weld. World 2017, 61, 883–891. [Google Scholar] [CrossRef]
- Riveiro, A.; del Val, J.; Comesaña, R.; Lusquiños, F.; Quintero, F.; Boutinguiza, M.; Pou, J. Laser additive manufacturing processes for near net shape components. In Near Net Shape Manufacturing Processes; Springer International Publishing: Cham, Switzerland, 2019; pp. 105–141. [Google Scholar]
- Chen, T.; Wu, W.; Li, W.; Liu, D. Laser cladding of nanoparticle TiC ceramic powder: Effects of process parameters on the quality characteristics of the coatings and its prediction model. Opt. Laser Technol. 2019, 116, 345–355. [Google Scholar] [CrossRef]
- Calleja, A.; Tabernero, I.; Ealo, J.A.; Campa, F.J.; Lamikiz, A.; de Lacalle, L.N.L. Feed rate calculation algorithm for the homogeneous material deposition of blisk blades by 5-axis laser cladding. Int. J. Adv. Manuf. Technol. 2014, 74, 1219–1228. [Google Scholar] [CrossRef]
- Liu, F.; Mao, Y.; Lin, X.; Zhou, B.; Qian, T. Microstructure and high temperature oxidation resistance of Ti-Ni gradient coating on TA2 titanium alloy fabricated by laser cladding. Opt. Laser Technol. 2016, 83, 140–147. [Google Scholar] [CrossRef]
- Fu, B.; Feng, K.; Li, Z. Study on the effect of Cu addition on the microstructure and properties of NiTi alloy fabricated by laser cladding. Mater. Lett. 2018, 220, 148–151. [Google Scholar] [CrossRef]
- Norhafzan, B.; Aqida, S.N.; Chikarakara, E.; Brabazon, D. Surface modification of AISI H13 tool steel by laser cladding with NiTi powder. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Cheng, F.T.; Lo, K.H.; Man, H.C. A preliminary study of laser cladding of AISI 316 stainless steel using preplaced NiTi wire. Mater. Sci. Eng. A 2004, 380, 20–29. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Cheng, F.T.; Man, H.C. Corrosion behavior of AISI 316L stainless steel surface-modified with NiTi. Surf. Coat. Technol. 2006, 200, 6054–6061. [Google Scholar] [CrossRef]
- Ingole, S. 60NiTi Alloy for tribological and biomedical surface engineering applications. JOM 2013, 65, 792–798. [Google Scholar] [CrossRef]
- Waghmare, D.T.; Kumar Padhee, C.; Prasad, R.; Masanta, M. NiTi coating on Ti-6Al-4V alloy by TIG cladding process for improvement of wear resistance: Microstructure evolution and mechanical performances. J. Mater. Process. Technol. 2018, 262, 551–561. [Google Scholar] [CrossRef]
- American Society for Testing Materials. Standard Test Method for Vickers Hardness of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2003; p. e2. Available online: https://www.astm.org/DATABASE.CART/HISTORICAL/E92-82R03.htm (accessed on 15 May 2021).
- Han, T.; Xiao, M.; Zhang, J.; Feng, X.; Shen, Y. Laser cladding composite coating on mild steel using Ni–Cr–Ti–B4C powder. Surf. Eng. 2018, 36, 1278–1284. [Google Scholar] [CrossRef]
- Shi, Z.-P.; Wang, Z.-B.; Wang, J.-Q.; Qiao, Y.-X.; Chen, H.-N.; Xiong, T.-Y.; Zheng, Y.-G. Effect of Ni interlayer on cavitation erosion resistance of NiTi cladding by Tungsten Inert Gas (TIG) surfacing process. Acta Metall. Sin. 2019, 33, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.T.; Lo, K.H.; Man, H.C. NiTi cladding on stainless steel by TIG surfacing process. Surf. Coat. Technol. 2003, 172, 308–315. [Google Scholar] [CrossRef]
- Han, T.; Xiao, M.; Zhang, Y.; Shen, Y. Laser cladding Ni-Ti-Cr alloy coatings with different process parameters. Mater. Manuf. Process. 2019, 34, 1710–1718. [Google Scholar] [CrossRef]
- Chai, L.; Wang, C.; Xiang, K.; Wang, Y.; Wang, T.; Ma, Y. Phase constitution, microstructure and properties of pulsed laser-clad ternary CrNiTi medium-entropy alloy coating on pure titanium. Surf. Coat. Technol. 2020, 402. [Google Scholar] [CrossRef]
- Gao, X.-L.; Wang, X.-Q.; Liu, J.; Li, L.-K. A novel laser welding method for the reliable joining of NiTi/301SS. Mater. Lett. 2020, 268, 127573. [Google Scholar] [CrossRef]
- Bao, S.; Zhang, L.; Peng, H.; Fan, Q.; Wen, Y. Effects of heat treatment on martensitic transformation and wear resistance of as-cast 60NiTi alloy. Mater. Res. Express 2019, 6, 086573. [Google Scholar] [CrossRef]
- Li, Y.; Liang, H.; Nie, Q.; Qi, Z.; Deng, D.; Jiang, H.; Cao, Z. Microstructures and wear resistance of CoCrFeNi2V0.5Tix high-entropy alloy coatings prepared by laser cladding. Crystals 2020, 10, 352. [Google Scholar] [CrossRef]
- Bozzolo, G.; Noebe, R.D.; Mosca, H.O. Site preference of ternary alloying additions to NiTi: Fe, Pt, Pd, Au, Al, Cu, Zr and Hf. J. Alloy. Compd. 2005, 389, 80–94. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.X.; Zheng, L.J.; Zhang, F.X.; Zhang, H. Influence of solution heat treatment on the microstructural evolution and mechanical behavior of 60NiTi. J. Alloy. Compd. 2019, 775, 698–706. [Google Scholar] [CrossRef]
- Cacciamani, G.; De Keyzer, J.; Ferro, R.; Klotz, U.E.; Lacaze, J.; Wollants, P. Critical evaluation of the Fe–Ni, Fe–Ti and Fe–Ni–Ti alloy systems. Intermetallics 2006, 14, 1312–1325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Cheng, F.T.; Man, H.C. Laser cladding of austenitic stainless steel using NiTi strips for resisting cavitation erosion. Mater. Sci. Eng. A 2005, 402, 126–134. [Google Scholar] [CrossRef]
- Ghosh, M.; Chatterjee, S. Diffusion bonded transition joints of titanium to stainless steel with improved properties. Materials Sci. Eng. Struct. Mater. Prop. Microstruct. Process. 2003, 358, 152–158. [Google Scholar] [CrossRef]
- Cheng, F.T.; Lo, K.H.; Man, H.C. NiTi cladding on stainless steel by TIG surfacing process Part II. Corrosion behavior. Surf. Coat. Technol. 2003, 172, 316–321. [Google Scholar] [CrossRef]
- Robin, A.; Meirelis, J.P. Influence of fluoride concentration and pH on corrosion behavior of titanium in artificial saliva. J. Appl. Electrochem. 2007, 37, 511–517. [Google Scholar] [CrossRef]
- Figueira, N.; Silva, T.M.; Carmezim, M.J.; Fernandes, J.C.S. Corrosion behaviour of NiTi alloy. Electrochim. Acta 2009, 54, 921–926. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Y.F. The electrochemical behavior of a Ti50Ni47Fe3 shape memory alloy. Mater. Lett. 2006, 60, 1646–1650. [Google Scholar] [CrossRef]
- Wever, D.J.; Veldhuizen, A.G.; de Vries, J.; Busscher, H.J.; Uges, D.R.A.; van Horn, J.R. Electrochemical and surface characterization of a nickel-titanium alloy. Biomaterials 1998, 19, 761–769. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Pei, R.; Liu, S.; Wang, S.-L.; Dong, L.-J.; Zhou, L.-J.; Xi, Y.-C.; Bai, S.-L. Microstructure and corrosion behavior of different clad zones in multi-track Ni-based laser-clad coating. Surf. Coat. Technol. 2020, 402, 126310. [Google Scholar] [CrossRef]
- Shen, F.; Tao, W.; Li, L.; Zhou, Y.; Wang, W.; Wang, S. Effect of microstructure on the corrosion resistance of coatings by extreme high speed laser cladding. Appl. Surf. Sci. 2020, 517, 146085. [Google Scholar] [CrossRef]
- Marattukalam, J.J.; Singh, A.K.; Datta, S.; Das, M.; Balla, V.K.; Bontha, S.; Kalpathy, S.K. Microstructure and corrosion behavior of laser processed NiTi alloy. Mater. Sci Eng. C Mater. Biol. Appl. 2015, 57, 309–313. [Google Scholar] [CrossRef]
- Marattukalam, J.J.; Balla, V.K.; Das, M.; Bontha, S.; Kalpathy, S.K. Effect of heat treatment on microstructure, corrosion, and shape memory characteristics of laser deposited NiTi alloy. J. Alloy. Compd. 2018, 744, 337–346. [Google Scholar] [CrossRef]
- Neelakantan, L.; Monchev, B.; Frotscher, M.; Eggeler, G. The influence of secondary phase carbide particles on the passivity behaviour of NiTi shape memory alloys. Mater. Corros. Werkst. Korros. 2012, 63, 979–984. [Google Scholar] [CrossRef]
- Vandenkerckhove, R.; Chandrasekaran, M.; Vermaut, P.; Portier, R.; Delaey, L. Corrosion behaviour of a superelastic Ni-Ti alloy. Mater. Sci. Eng. Struct. Mater. Prop. Microstruct. Process. 2004, 378, 532–536. [Google Scholar] [CrossRef]
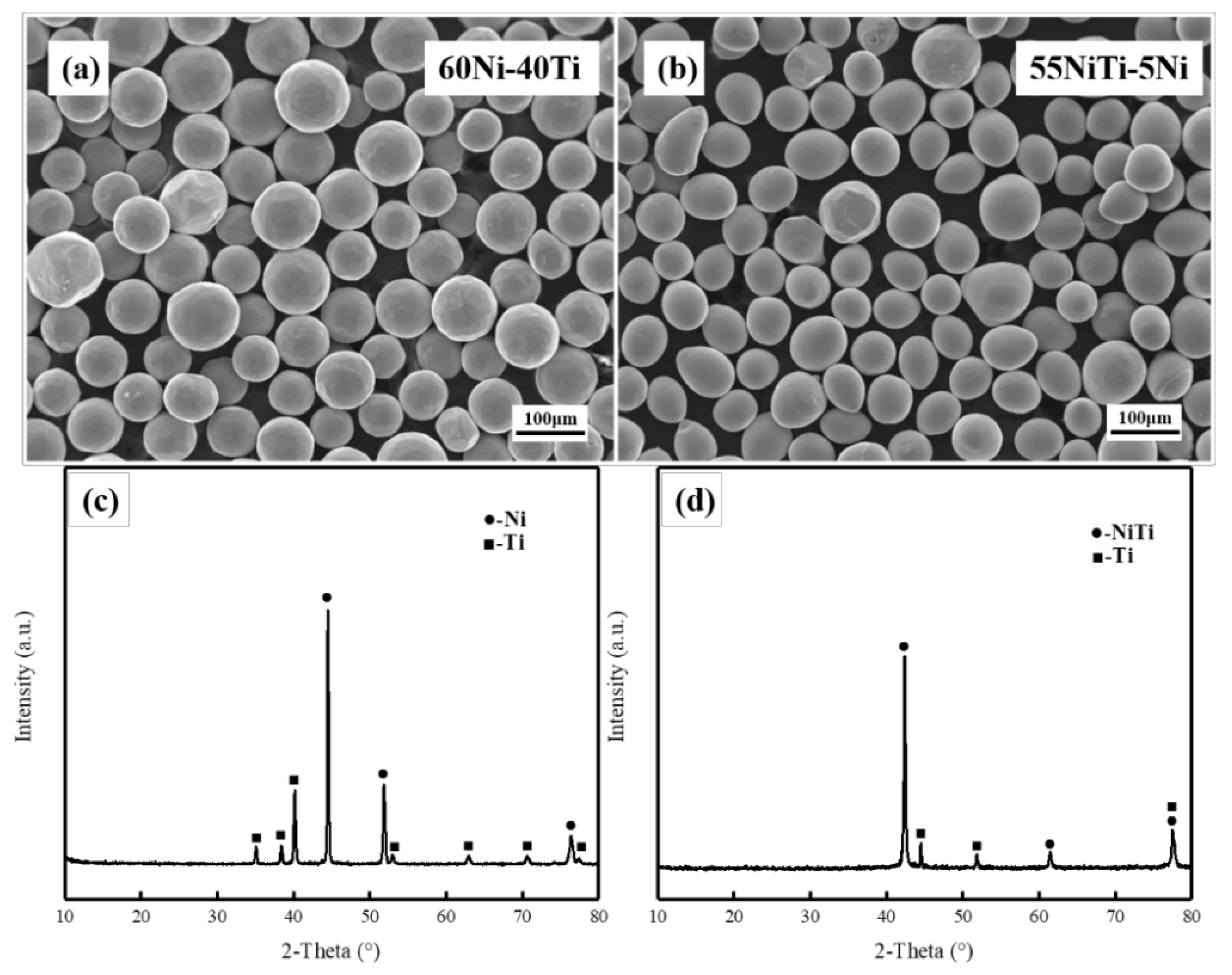
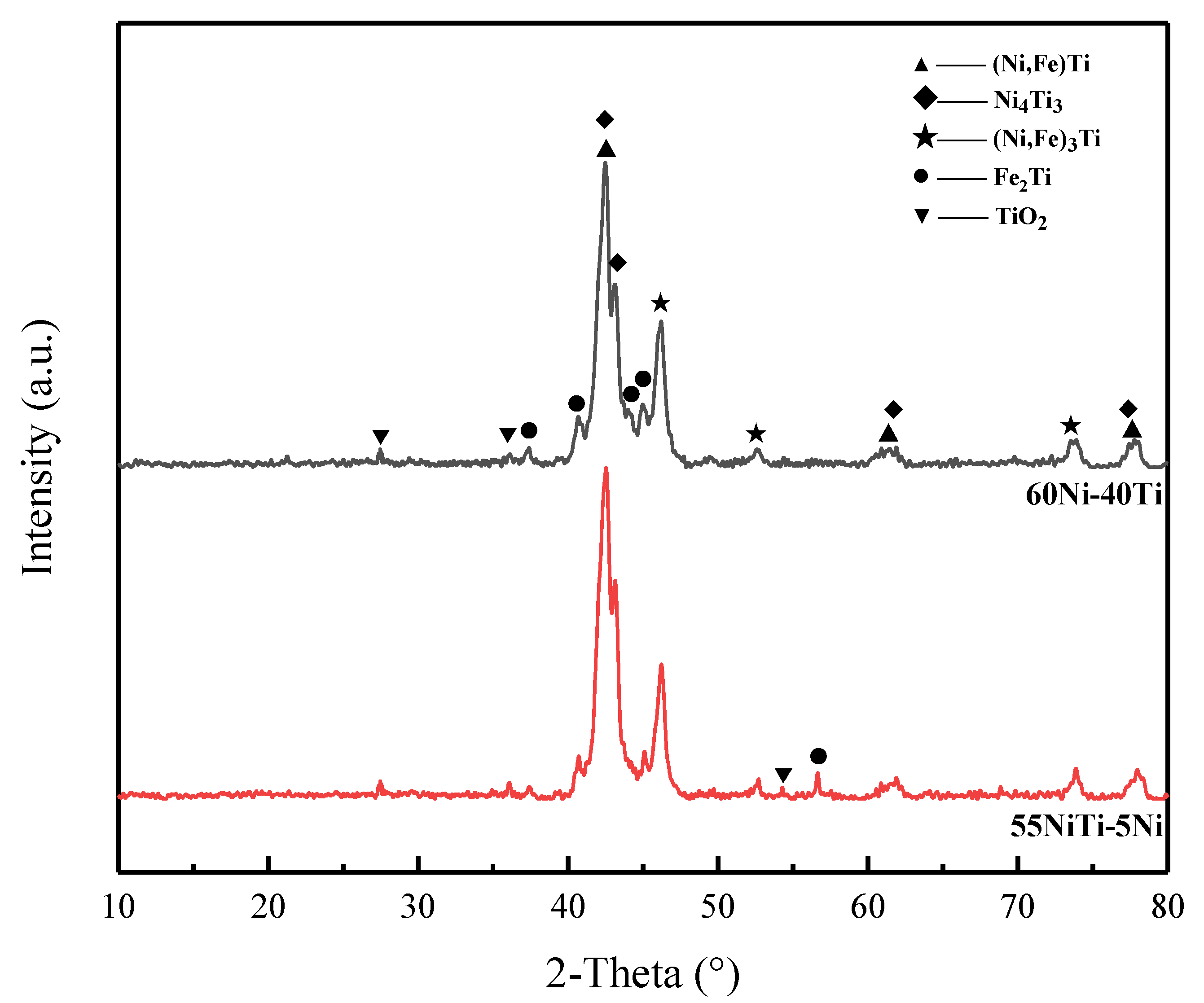
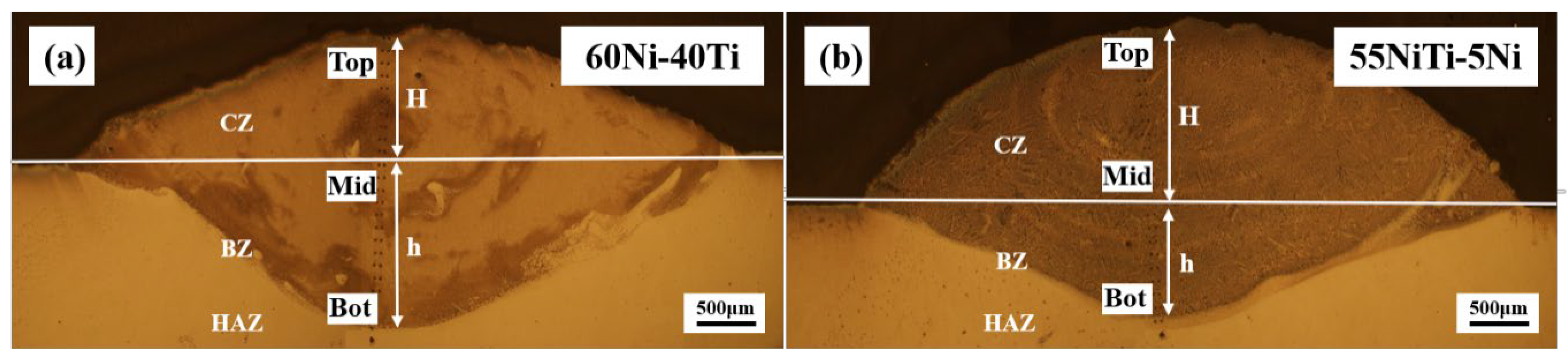
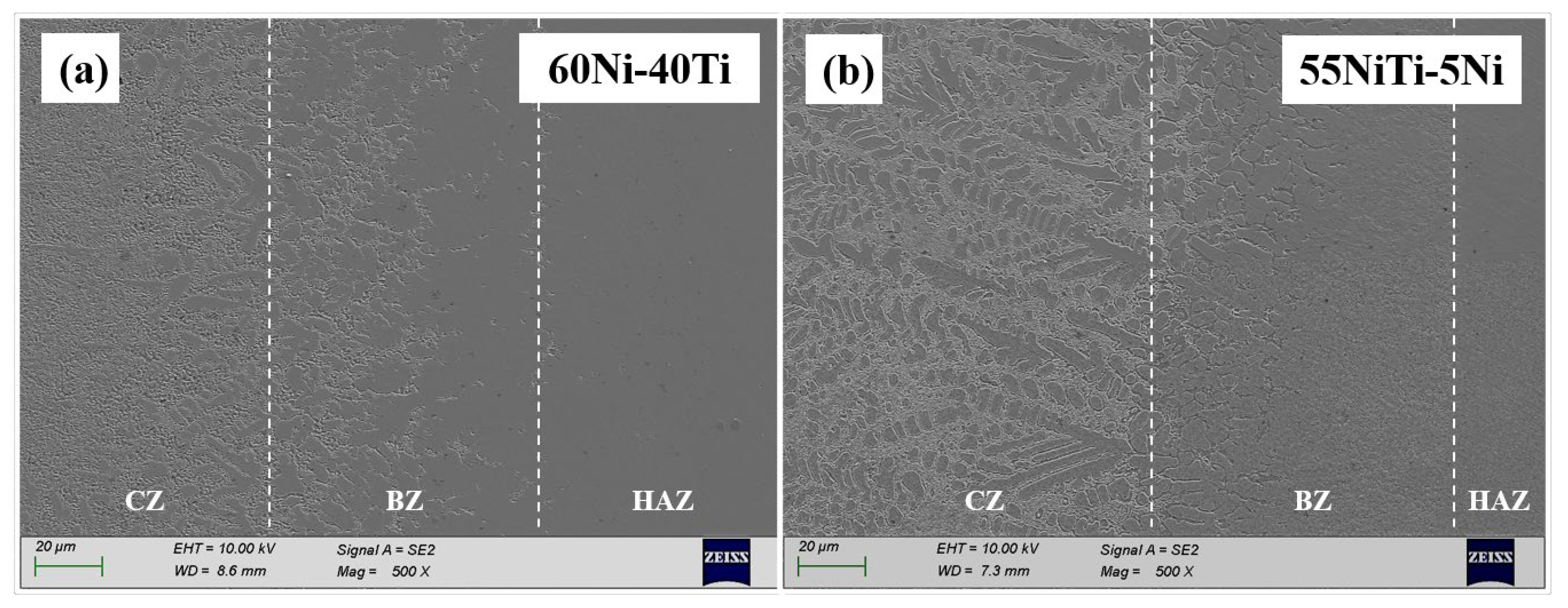



| Powder | Ni | Ti | Co | Na | Cu | Si | Fe | C | O |
|---|---|---|---|---|---|---|---|---|---|
| Pure Ni | Bal. | - | 0.020 | <0.005 | 0.001 | 0.003 | 0.003 | 0.002 | 0.006 |
| Pure Ti | - | Bal. | - | - | - | - | 0.016 | 0.006 | 0.058 |
| Alloy 55NiTi | 56.46 | Bal. | - | <0.005 | <0.005 | - | <0.005 | 0.003 | 0.037 |
| Sample | H (mm) | H (mm) | η |
|---|---|---|---|
| 60Ni-40Ti | 1.645 | 1.393 | 0.54 |
| 55NiTi-5Ni | 0.99 | 1.745 | 0.36 |
| Element | 60Ni-40Ti (Coarse) | 60Ni-40Ti (Fine) | 55NiTi-5Ni | ||||
|---|---|---|---|---|---|---|---|
| A | B | A | B | C | D | E | |
| Ni (At%) | 44.7 | 30.2 | 40.12 | 28.03 | 35.31 | 38.96 | 64.89 |
| Ti (At%) | 14 | 27.13 | 13.76 | 26.31 | 31.62 | 29.37 | 22.8 |
| Fe (At%) | 32.85 | 35.69 | 37.35 | 36.39 | 26.16 | 27.16 | 9.62 |
| Cr (At%) | 8.45 | 7.99 | 8.77 | 9.29 | 6.91 | 8.51 | 2.69 |
| Sample | 60Ni-40Ti | 55NiTi-5Ni | 316L |
|---|---|---|---|
| OCP (mV) | −526.1 | −432.6 | 208.1 |
| Sample | E0 (mV) | I0 (μA) | Corrosion Rate (mpy) |
|---|---|---|---|
| 60Ni-40Ti | −570.0 | 7.050 | 1.930 |
| 55NiTi-5Ni | −404.0 | 5.540 | 1.495 |
| SS 316L | 260.0 | 79.90 | 33.01 |
| Sample | Rs (Ω·cm2) | Rt (Ω·cm2) | Rct (Ω·cm2) | CPE1 (Ω−1·m2·sn) | n1 | CPE2 (Ω−1·m2·sn) | n2 | X2 |
|---|---|---|---|---|---|---|---|---|
| 60Ni-40Ti | 16.02 | 9.49 × 10−5 | 1.12 × 105 | 1.98 × 10−5 | 0.327 | 4.30 × 10−5 | 0.559 | 9.99 × 10−5 |
| 55NiTi-5Ni | 16.7 | 2.216 × 105 | 1.84 × 105 | 2.751 × 10−5 | 0.927 | 7.96 × 10−6 | 0.891 | 3.56 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Hu, Z.; Feng, Y.; Mo, F. The Effect of Powder Composition on the Microstructure and Corrosion Resistance of Laser Cladding 60NiTi Alloy Coatings on SS 316L. Metals 2021, 11, 1104. https://doi.org/10.3390/met11071104
Du Z, Hu Z, Feng Y, Mo F. The Effect of Powder Composition on the Microstructure and Corrosion Resistance of Laser Cladding 60NiTi Alloy Coatings on SS 316L. Metals. 2021; 11(7):1104. https://doi.org/10.3390/met11071104
Chicago/Turabian StyleDu, Zexu, Zhengfei Hu, Yuqiang Feng, and Fan Mo. 2021. "The Effect of Powder Composition on the Microstructure and Corrosion Resistance of Laser Cladding 60NiTi Alloy Coatings on SS 316L" Metals 11, no. 7: 1104. https://doi.org/10.3390/met11071104
APA StyleDu, Z., Hu, Z., Feng, Y., & Mo, F. (2021). The Effect of Powder Composition on the Microstructure and Corrosion Resistance of Laser Cladding 60NiTi Alloy Coatings on SS 316L. Metals, 11(7), 1104. https://doi.org/10.3390/met11071104







