Recovery of Copper and Magnetite from Copper Slag Using Concentrated Solar Power (CSP)
Abstract
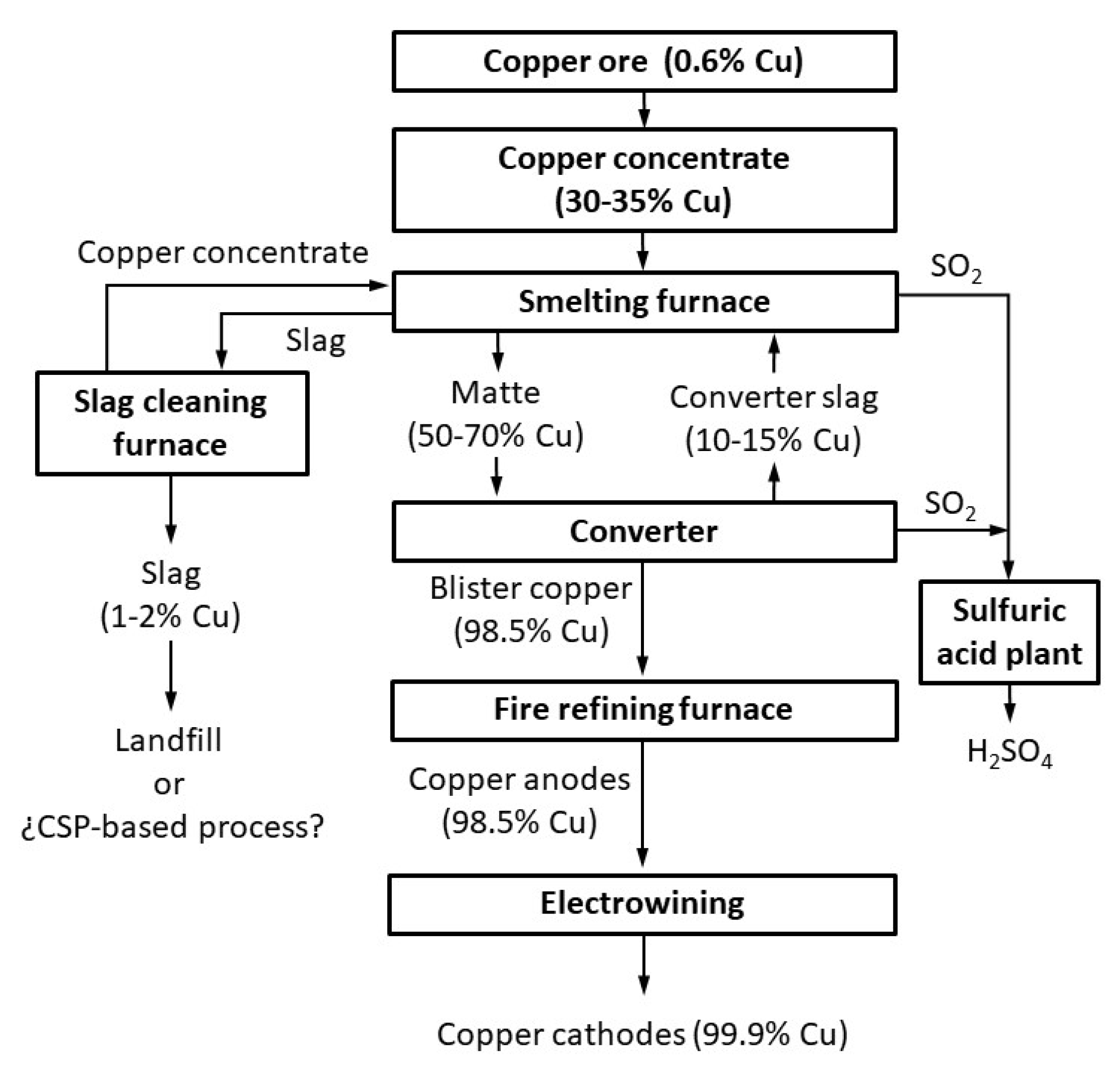
1. Introduction
2. Materials and Methods
2.1. Materials
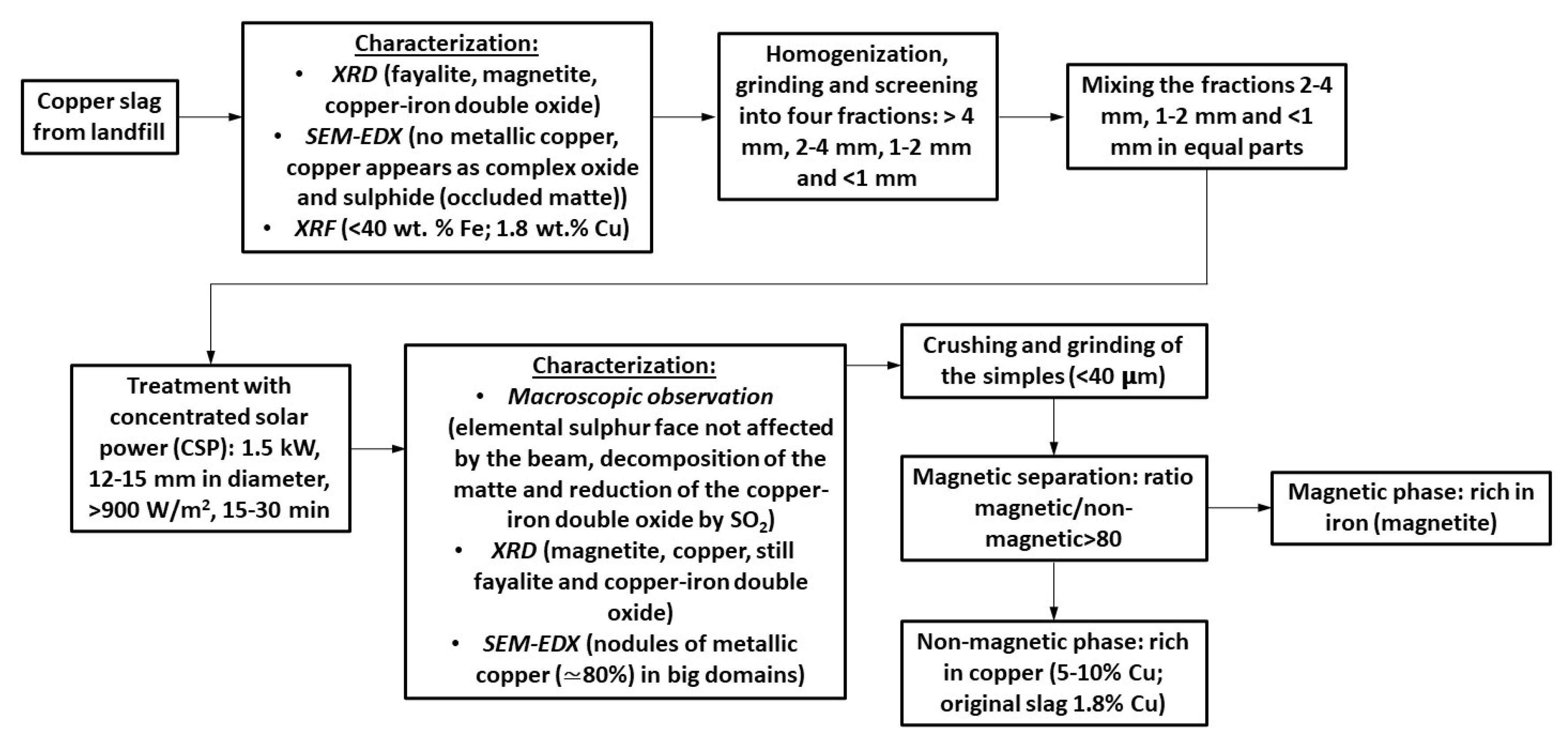
2.2. Experimental Procedure
3. Results
3.1. Macroscopic Analysis
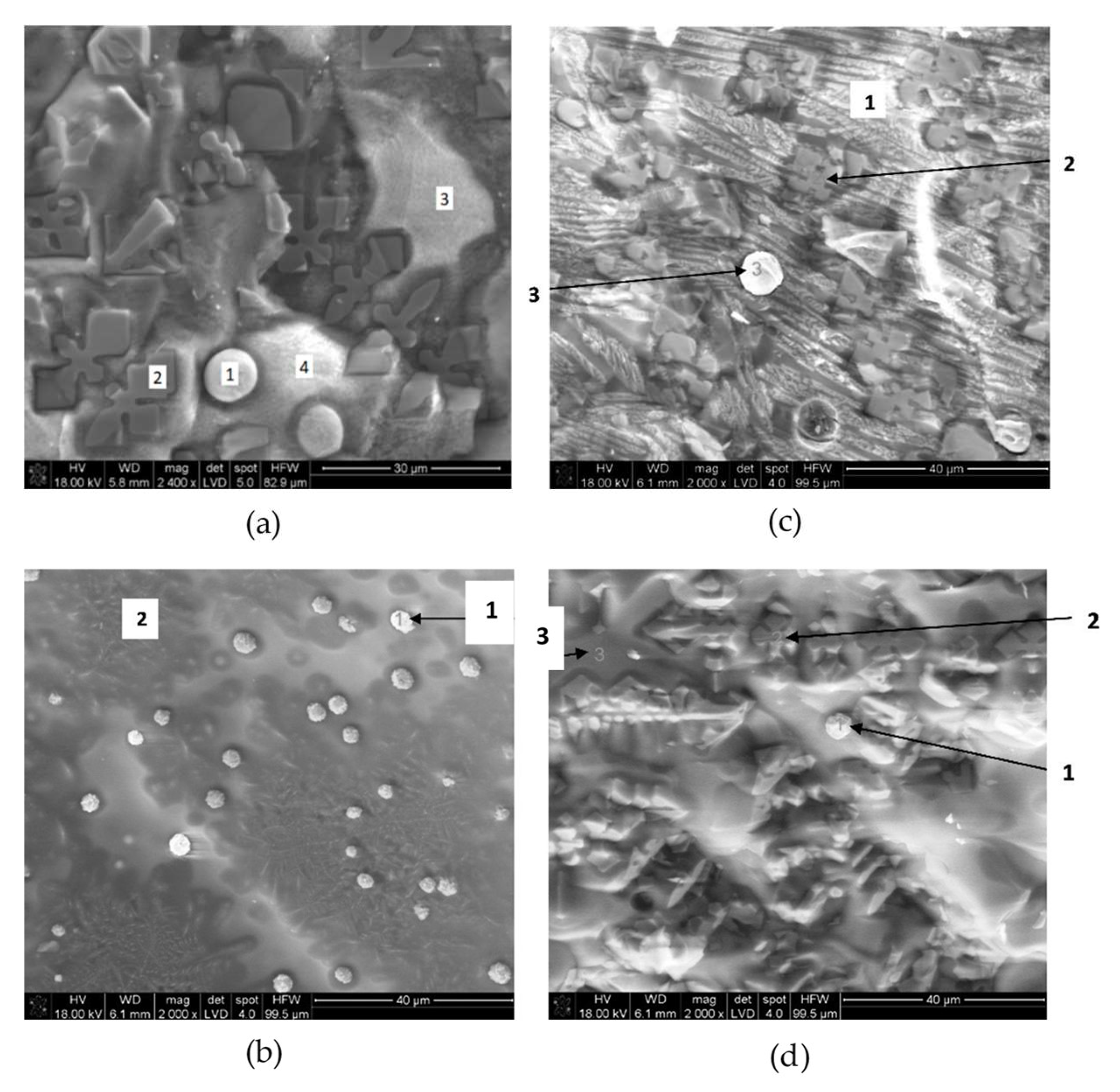
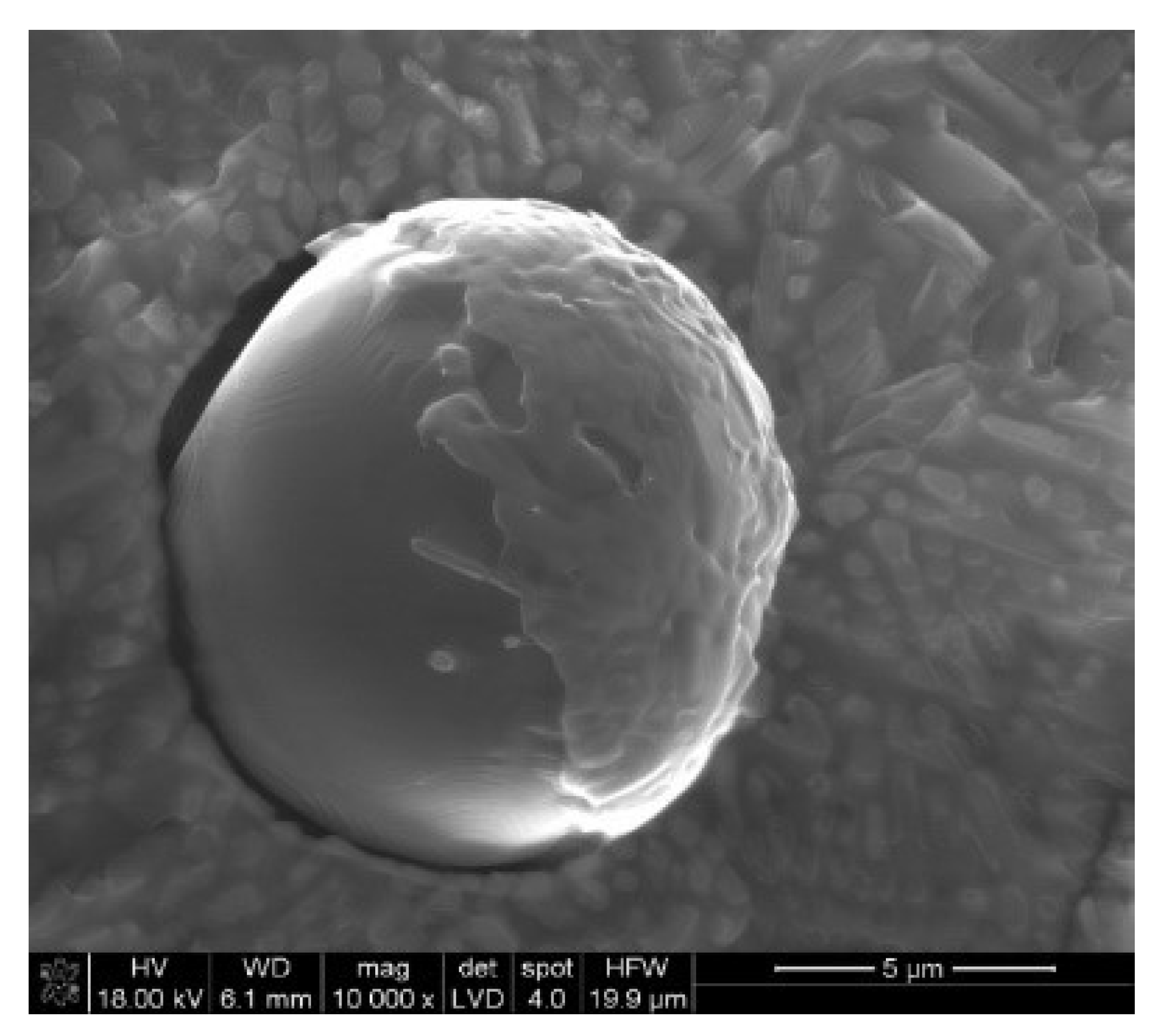
3.2. SEM-EDX
3.3. Size of the Nodules
3.4. Grinding and Magnetic Separation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sancho, J.P.; Verdeja, L.F.; Ballester, A. Metalurgia Extractiva. Volumen II. Procesos de Obtención, 1st ed.; Síntesis: Madrid, Spain, 2000. [Google Scholar]
- Fan, Y.; Shibata, E.; Iizuka, A.; Nakamura, T. Crystallization behaviors of copper smelter slag studied using time-temperature-transformation diagram. Mater. Trans. 2014, 55, 958–963. [Google Scholar] [CrossRef]
- Davenport, W.G.; King, M.; Schlesinger, M.; Biswas, A.K. Extractive Metallurgy of Copper, 4th ed.; Pergamon-Elsevier Science Ltd.: Oxford, UK, 2002. [Google Scholar]
- Nazer, A.; Pavez, O.; Rojas, F.; Aguilar, C. Una revisión de los usos de las escorias de cobre. In Proceedings of the IBEROMET XI. X CONAMET/SAM, Viña del Mar, Chile, 2–5 November 2010. [Google Scholar]
- Coursol, P.; Cardona, N.; Mackey, P.; Bell, S.; Davis, B. Minimization of copper losses in copper smelting slag during electric furnace treatment. JOM 2012, 64, 1305–1313. [Google Scholar] [CrossRef]
- Cardona, N.; Coursol, P.; Vargas, J.; Parra, R. Physical chemistry of copper smelting slags and copper losses at the Paipote smelter. Part 1-Thermodynamic modelling. Can. Metall. Quart. 2011, 50, 318–329. [Google Scholar] [CrossRef]
- Cardona, N.; Coursol, P.; Vargas, J.; Parra, R. Physical chemistry of copper smelting slags and copper losses at the Paipote smelter. Part 2-Characterization of industrial slags. Can. Metall. Quart. 2011, 50, 330–340. [Google Scholar] [CrossRef]
- Madheswaran, C.K.; Ambily, P.S.; Dattatreaya, J.K.; Rajamane, N.P. Studies on use of copper slag as replacement material for river sand in building constructions. J. Inst. Eng. Ser. A 2014, 95, 169–177. [Google Scholar] [CrossRef]
- Nazer, A.; Pavez, O.; Rojas, F. Use of copper slag in cement mortar. REM Rev. Esc. Minas 2012, 65, 87–91. [Google Scholar] [CrossRef][Green Version]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of copper slag in cement and concrete. Resour. Conserv. Recy. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- Potysz, A.; Van Hullebusch, E.D.; Kierczak, J.; Grybos, M.; Lens, P.N.L.; Guibaud, G. Copper metallurgy slags- Current knowledge and fate: A review. Crit. Rev. Env. Sci. Tec. 2015, 45, 2424–2488. [Google Scholar] [CrossRef]
- Palacios, J.; Sánchez, M. Wastes as resources: Update on recovery of valuable metals from copper slags. Miner. Process. Extr. Metall. 2011, 120, 218–223. [Google Scholar] [CrossRef]
- Cendoya, P. Efecto en la resistencia de las escorias de fundición de cobre como agregado fino en el comportamiento resistente del hormigón. Ingeniare Rev. Chil. Ing. 2009, 17, 85–94. [Google Scholar] [CrossRef]
- Murari, K.; Siddique, R.; Jain, K.K. Use of waste copper slag, a sustainable material. J. Mater. Cycles. Waste Manag. 2015, 17, 13–26. [Google Scholar] [CrossRef]
- Kambham, K.; Sangameswaran, S.; Datar, S.R.; Kura, B. Copper slag: Optimization of productivity and consumption for cleaner production in dry abrasive blasting. J. Clean Prod. 2007, 15, 465–473. [Google Scholar] [CrossRef]
- Jiménez-Padilla, B. Armado de Tuberías. FMEC0108, 1st ed.; IC Editorial: Antequera, Spain, 2014. [Google Scholar]
- Biswas, S.; Satapathy, A. Use of copper slag in glass-epoxy composites for improved wear resistance. Waste Manag. Res. 2010, 28, 615–625. [Google Scholar] [CrossRef]
- Pundhir, N.K.S.; Kamaraj, C.; Nanda, P.K. Use of copper slag as construction material in bituminous pavements. J. Sci. Ind. Res. India. 2005, 64, 997–1002. [Google Scholar]
- Busolic, D.; Parada, F.; Parra, R.; Sánchez, M.; Palacios, J.; Hino, M. Recovery of iron from copper flash smelting slags. Miner. Process. Extr. Metall. 2011, 120, 32–36. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, D.; Pan, J.; Wu, T.; Zhang, F. Improving beneficiation of copper and iron from copper slag by modifying the molten copper slag. Metals 2016, 6, 86. [Google Scholar] [CrossRef]
- Xian-Lin, Z.; De-Qing, Z.; Jian, P.; Teng-Jiao, W. Utilization of waste copper slag to produce directly reduced iron for weathering resistant steel. ISIJ Int. 2015, 55, 1347–1352. [Google Scholar] [CrossRef]
- Heo, J.H.; Chung, Y.; Park, J.H. Recovery of iron and removal of hazardous elements from waste copper slag via a novel aluminothermic smelting reduction (ASR) process. J. Clean. Prod. 2016, 137, 777–787. [Google Scholar] [CrossRef]
- Siwiec, G.; Sozanska, M.; Blacha, L.; Smalcerz, A. Behaviour of iron during reduction of slag obtained from copper flash smelting. Metabk 2015, 54, 113–115. [Google Scholar]
- Sarfo, P.; Wyss, G.; Ma, G.; Das, A.; Young, C. Carbothermal reduction of copper smelter slag for recycling into pig iron and glass. Miner. Eng. 2017, 107, 8–19. [Google Scholar] [CrossRef]
- Liao, Y.; Zhou, J.; Huang, F. Separating and recycling of Fe, Cu, Zn from dumped copper slag by microwave irradiation assisted carbothermic method. J. Residuals Sci. Technool. 2016, 13, S155–S160. [Google Scholar] [CrossRef]
- Li, K.; Ping, S.; Wang, H.; Ni, W. Recovery of iron from copper slag by deep reduction and magnetic beneficiation. Int. J. Min. Met. Mater. 2013, 20, 1035–1041. [Google Scholar] [CrossRef]
- Fernández-González, D.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Mochón-Castaños, J.; Sancho-Gorostiaga, J.; Verdeja, L.F. Concentrated solar energy applications in materials science and metallurgy. Sol. Energy 2018, 170, 520–540. [Google Scholar] [CrossRef]
- Murray, J.P.; Flamant, G.; Roos, C.J. Silicon and solar-grade silicon production by solar dissociation of Si3N4. Sol. Energy 2006, 80, 1349–1354. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Tuerk, O.; Steinfeld, A. Production of Si by vacuum carbothermal reduction of SiO2 using concentrated solar energy. JOM 2010, 62, 49–54. [Google Scholar] [CrossRef]
- Murray, J.P. Aluminum production using high-temperature solar process heat. Sol. Energy 1999, 66, 133–142. [Google Scholar] [CrossRef]
- Murray, J.P. Aluminum-silicon carbothermal reduction using high-temperature solar process heat. In Proceedings of the 128th TMS Annual Meeting, San Diego, CA, USA, 28 February–4 March 1999. [Google Scholar]
- Murray, J.P. Solar production of aluminum by direct reduction: Preliminary results for two processes. J. Sol. Energy Eng. 2001, 123, 125–132. [Google Scholar] [CrossRef]
- Lytvynenko, Y.M. Obtaining aluminum by the electrolysis with the solar radiation using. Appl. Sol. Energy 2013, 49, 4–6. [Google Scholar] [CrossRef]
- Epstein, M.; Olalde, G.; Santén, S.; Steinfeld, A.; Wieckert, C. Towards the industrial solar carbothermal production of zinc. J. Sol. Energ. 2008, 130, 014501–014504. [Google Scholar] [CrossRef]
- Fletcher, E.A.; Noring, J.E. High temperature solar electrothermal processing—Zinc from zinc oxide. Energy 1983, 8, 247–254. [Google Scholar] [CrossRef]
- Fletcher, E.A.; Macdonald, F.J.; Kunnerth, D. High temperature solar electrothermal processing—II. Zinc from zinc oxide. Energy 1985, 10, 1255–1272. [Google Scholar] [CrossRef]
- Palumbo, R.D.; Fletcher, E.A. High temperature solar electrothermal processing—III. Zinc from zinc oxide at 1200–1675K using a non-consumable anode. Energy 1988, 13, 319–332. [Google Scholar] [CrossRef]
- Osinga, T.; Frommherz, U.; Steinfeld, A.; Wieckert, C. Experimental investigation of the solar carbothermic reduction of ZnO using a two-cavity solar reactor. J. Sol. Energ. 2004, 126, 633–637. [Google Scholar] [CrossRef]
- Epstein, M.; Ehrensberger, K.; Yogev, A. Ferro-reduction of ZnO using concentrated solar energy. Energy 2004, 29, 745–756. [Google Scholar] [CrossRef]
- Steinfeld, A.; Brack, M.; Meier, A.; Weidenkaff, A.; Wuillemin, D. A solar chemical reactor for co-production of zinc and synthesis gas. Energy 1998, 23, 803–814. [Google Scholar] [CrossRef]
- Wieckert, C.; Palumbo, R.; Frommherz, U. A two-cavity reactor for solar chemical processes: Heat transfer model and application to carbothermic reduction of ZnO. Energy 2004, 29, 771–787. [Google Scholar] [CrossRef]
- Wieckert, C.; Frommherz, U.; Kräupl, S.; Guillot, E.; Olalde, G.; Epstein, M.; Santén, S.; Osinga, T.; Steinfeld, A. A 300 kW solar chemical pilot plant for the carbothermic production of zinc. J. Sol. Energy 2006, 129, 190–196. [Google Scholar] [CrossRef]
- Schunk, L.O.; Lipinski, W.; Steinfeld, A. Heat transfer model of a solar receiver-reactor for the thermal dissociation of ZnO-Experimental validation at 10 kW and scale-up to 1 MW. Chem. Eng. J. 2009, 150, 502–508. [Google Scholar] [CrossRef]
- Villasmil, W.; Brkic, M.; Wuillemin, D.; Meier, A.; Steinfeld, A. Pilot scale demonstration of a 100-kWth solar thermochemical plant for the thermal dissociation of ZnO. J. Sol. Energy 2013, 136, 011016–0111027. [Google Scholar] [CrossRef]
- Koepft, E.; Villasmil, W.; Meier, A. Pilot-scale solar reactor operation and characterization for fuel production via the Zn/ZnO thermochemical cycle. Appl. Energy 2016, 165, 1004–1023. [Google Scholar] [CrossRef]
- Ruiz-Bustinza, I.; Cañadas, I.; Rodríguez, J.; Mochón, J.; Verdeja, L.F.; García-Carcedo, F.; Vázquez, A. Magnetite production from steel wastes with concentrated solar energy. Steel Res. Int. 2013, 84, 207–217. [Google Scholar] [CrossRef]
- Sibieude, F.; Ducarroir, M.; Tofighi, A.; Ambriz, J. High temperature experiments with a solar furnace: The decomposition of Fe3O4, Mn3O4, CdO. Int. J. Hydrogen Energy 1982, 7, 79–88. [Google Scholar] [CrossRef]
- Steinfeld, A.; Fletcher, E.A. Theoretical and experimental investigation of the carbothermic reduction of Fe2O3 using solar energy. Energy 1991, 16, 1011–1019. [Google Scholar] [CrossRef]
- Steinfeld, A.; Kuhn, P.; Karni, J. High-temperature solar thermochemistry: Production of iron and synthesis gas by Fe3O4-reduction with methane. Energy 1993, 18, 239–249. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. Solar synthesis of calcium aluminates. Sol. Energy 2018, 171, 658–666. [Google Scholar] [CrossRef]
- Mochón, J.; Ruiz-Bustinza, Í.; Vázquez, A.; Fernández, D.; Ayala, J.M.; Barbés, M.F.; Verdeja, L.F. Transformations in the iron-manganese-oxygen-carbon system resulted from treatment of solar energy with high concentration. Steel Res. Int. 2014, 85, 1469–1476. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. Iron Metallurgy via Concentrated Solar Energy. Metals-Basel 2018, 8, 873. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. The treatment of Basic Oxygen Furnace (BOF) slag with concentrated solar energy. Sol. Energy 2019, 180, 372–382. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. Transformations in the Mn-O-Si system using concentrated solar energy. Sol. Energy 2019, 184, 148–152. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. Transformations in the Si-O-Ca system: Silicon-calcium via solar energy. Sol. Energy 2019, 181, 414–423. [Google Scholar] [CrossRef]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Gómez-Rodríguez, C.; Verdeja, L.F. Treatment of copper slag with concentrated solar energy. In Proceedings of the Environmental Safety-Non-Energy and Raw Materials, I International Conference on Engineering Materials, Safety, Environment and Technology, Zielona Gora, Poland, 3–4 June 2020; Gabryelewicz, I., Wedrychowicz, M., Eds.; University of Zielona Gora: Zielona Gora, Poland, 2021; pp. 39–59. [Google Scholar]
- Winkel, H.E. Thermal Decomposition of Copper Sulfides under Concentrated Irradiation. Ph.D. Thesis, Swiss Federal Institute of Technology Zurich, Zurich, Switzerland, 2006. [Google Scholar]
- Alcock, C.B. Principles of Pyrometallurgy; Academic Press: London, UK, 1976; pp. 15–16. [Google Scholar]
- Ballester, A.; Verdeja, L.F.; Sancho, J.P. Metalurgia Extractiva. Volumen I. Fundamentos, 1st ed.; Síntesis: Madrid, Spain, 2000. [Google Scholar]
- Richardson, F.D.; Jeffers, J.H.E. The Ellingham diagram for metal oxides. In Introduction to Metallurgical Thermodynamics; Hemisphere Publishing Corporation: San Francisco, CA, USA, 1981. [Google Scholar]
- Verdeja, L.F.; Sancho, J.P.; Ballester, A. Refractory and Ceramic Materials, 1st ed.; Síntesis: Madrid, Spain, 2014. [Google Scholar]
- Pero-Sanz, J.A.; Quintana, M.J.; Verdeja, L.F. Solidification and Solid-state Transformations of Metals and Alloys, 1st ed.; Elsevier: Boston, MA, USA, 2017. [Google Scholar]
- Flamant, G.; Ferriere, A.; Laplaze, D.; Monty, D. Solar processing of materials: Opportunities and new frontiers. Sol. Energy 1999, 66, 117–132. [Google Scholar] [CrossRef]
- Alvarez, M.A. Environmental Impact of Plastics. J. Mater. Ed. 2018, 40, 119–124. [Google Scholar]
- Dobiszewska, M. Waste Materials Used in Making Mortar and Concrete. J. Mater. Ed. 2017, 39, 133–156. [Google Scholar]
- Fernández-González, D.; Sancho-Gorostiaga, J.; Piñuela-Noval, J.; Verdeja, L.F. Anodic Lodes and Scrapings as a Source of Electrolytic Manganese. Metals 2018, 8, 162. [Google Scholar] [CrossRef]
- Ordiales, M.; Iglesias, J.; Fernández-González, D.; Sancho-Gorostiaga, J.; Fuentes, A.; Verdeja, L.F. Cold agglomeration of Ultrafine Oxidized Dust (UOD) from ferromanganese and silicomanganese industrial process. Metals 2016, 6, 203. [Google Scholar] [CrossRef]
- Fernández-González, D.; Piñuela-Noval, J.; Verdeja, L.F. Silicomanganese and ferromanganese slags treated with concentrated solar energy. Proceedings 2018, 2, 1450. [Google Scholar] [CrossRef]
- Fernández-González, D. Aplicaciones de la Energía Solar Concentrada en Metalurgia y Ciencia de los Materiales. Ph.D. Thesis, Department of Materials Science and Metallurgical Engineering, University of Oviedo, Oviedo/Uviéu, Asturias, Spain, September 2019. [Google Scholar]


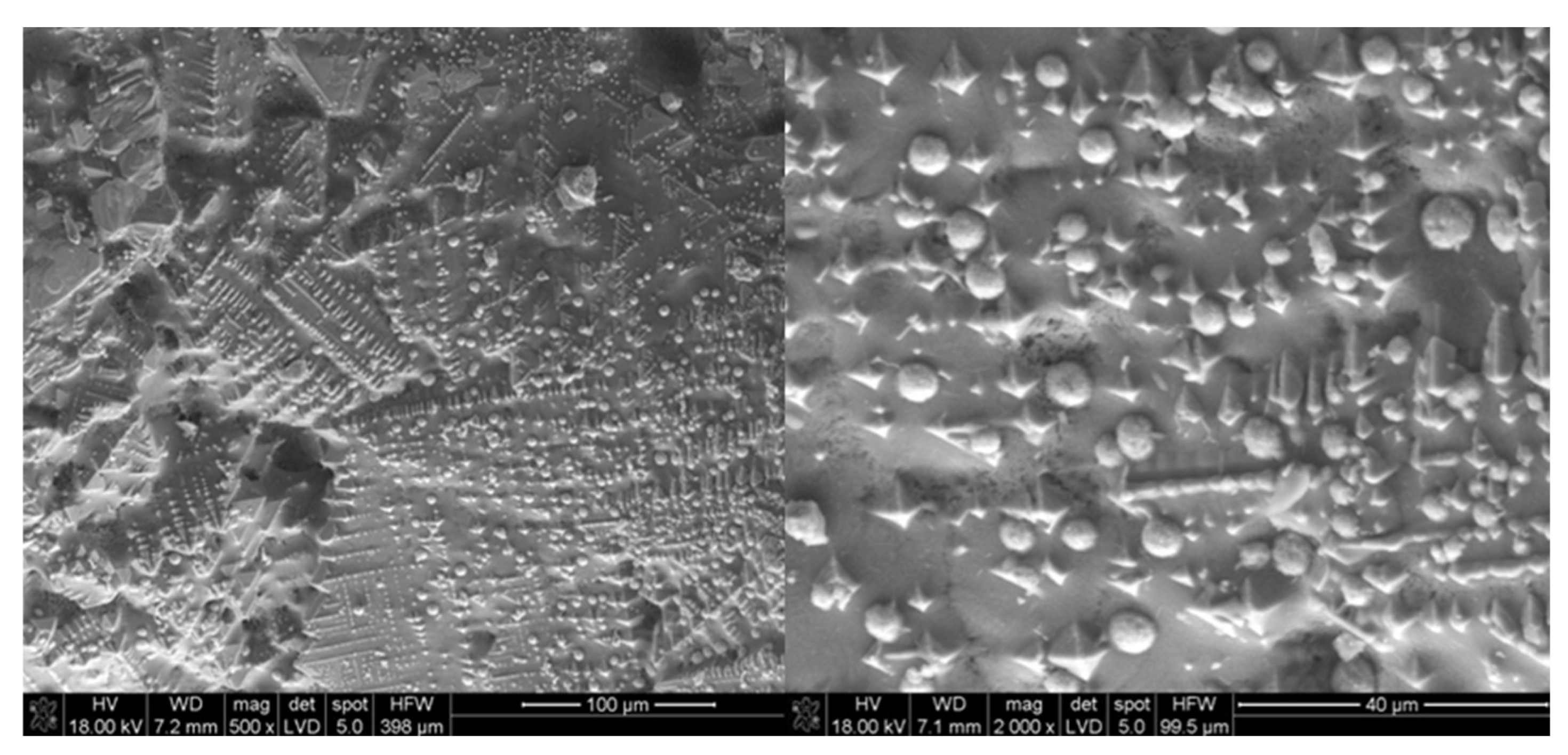

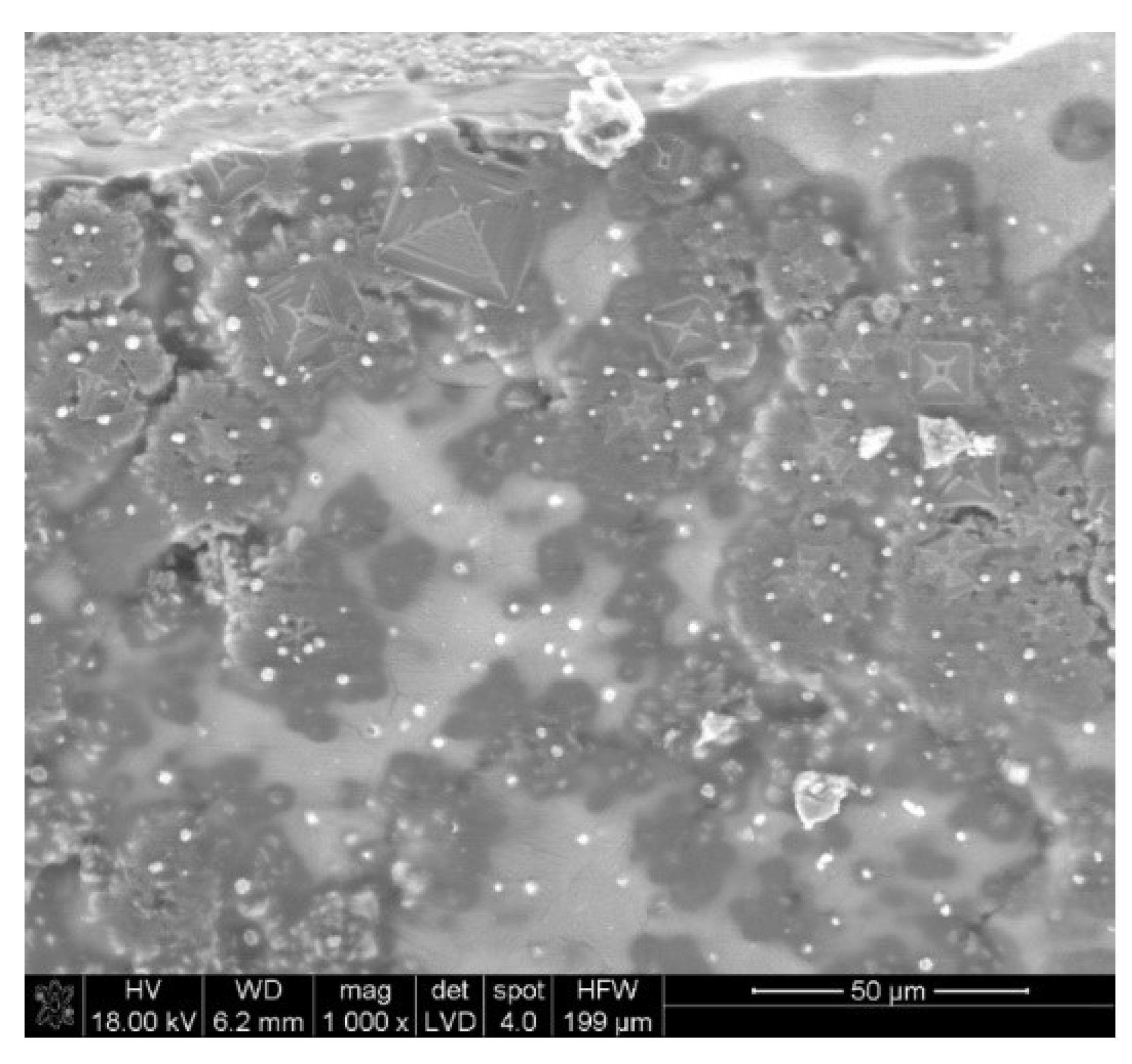
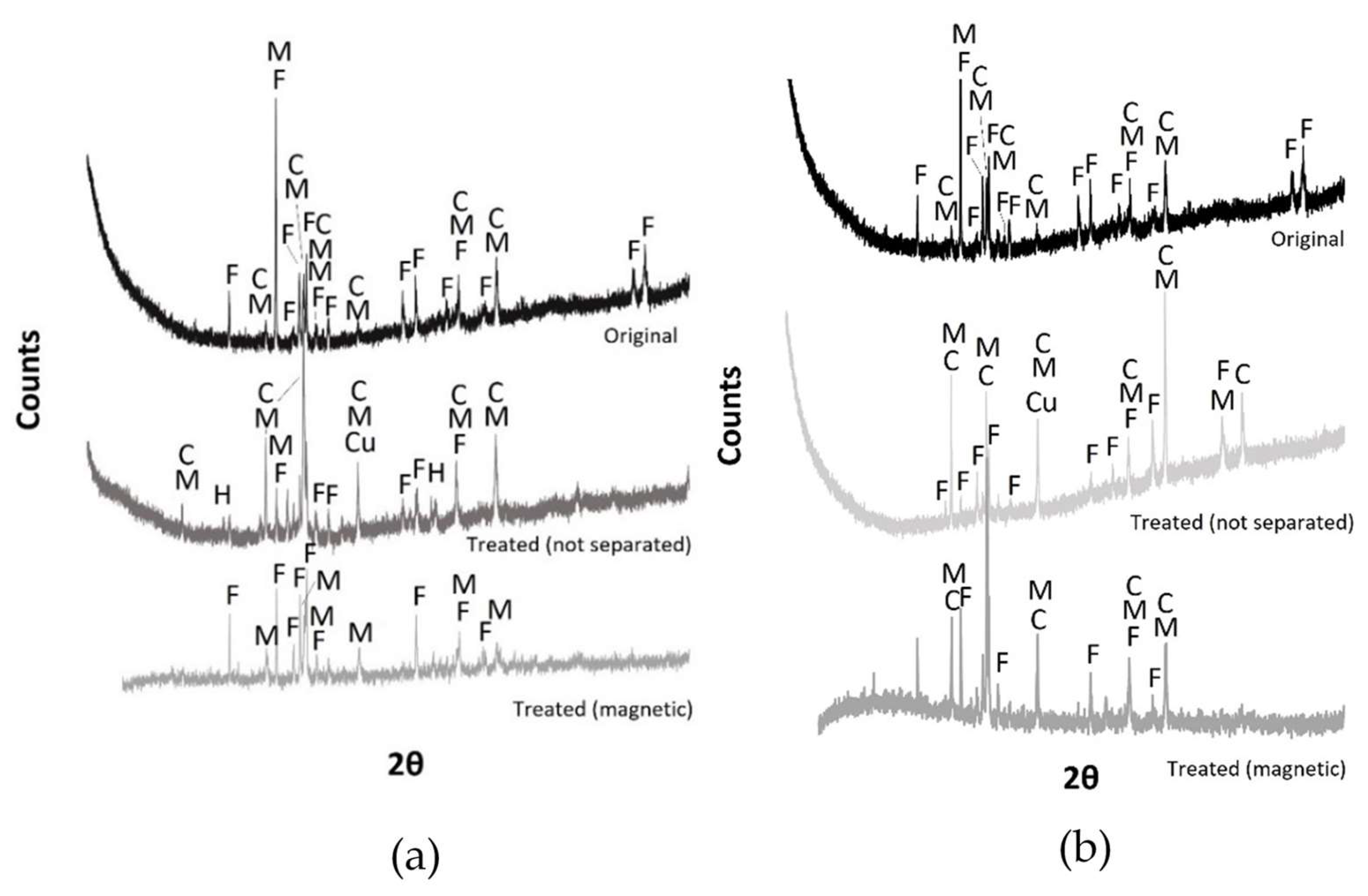
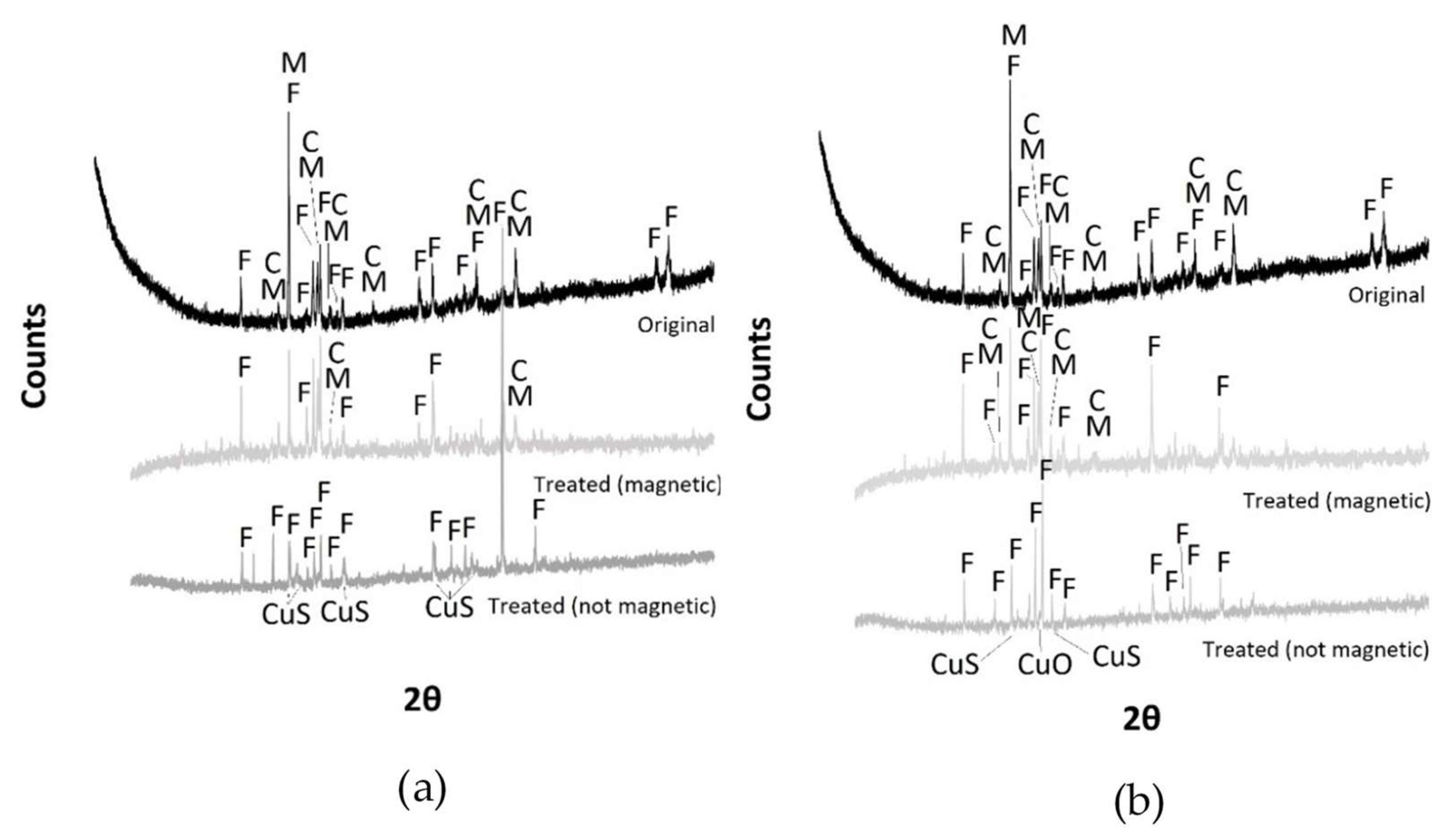
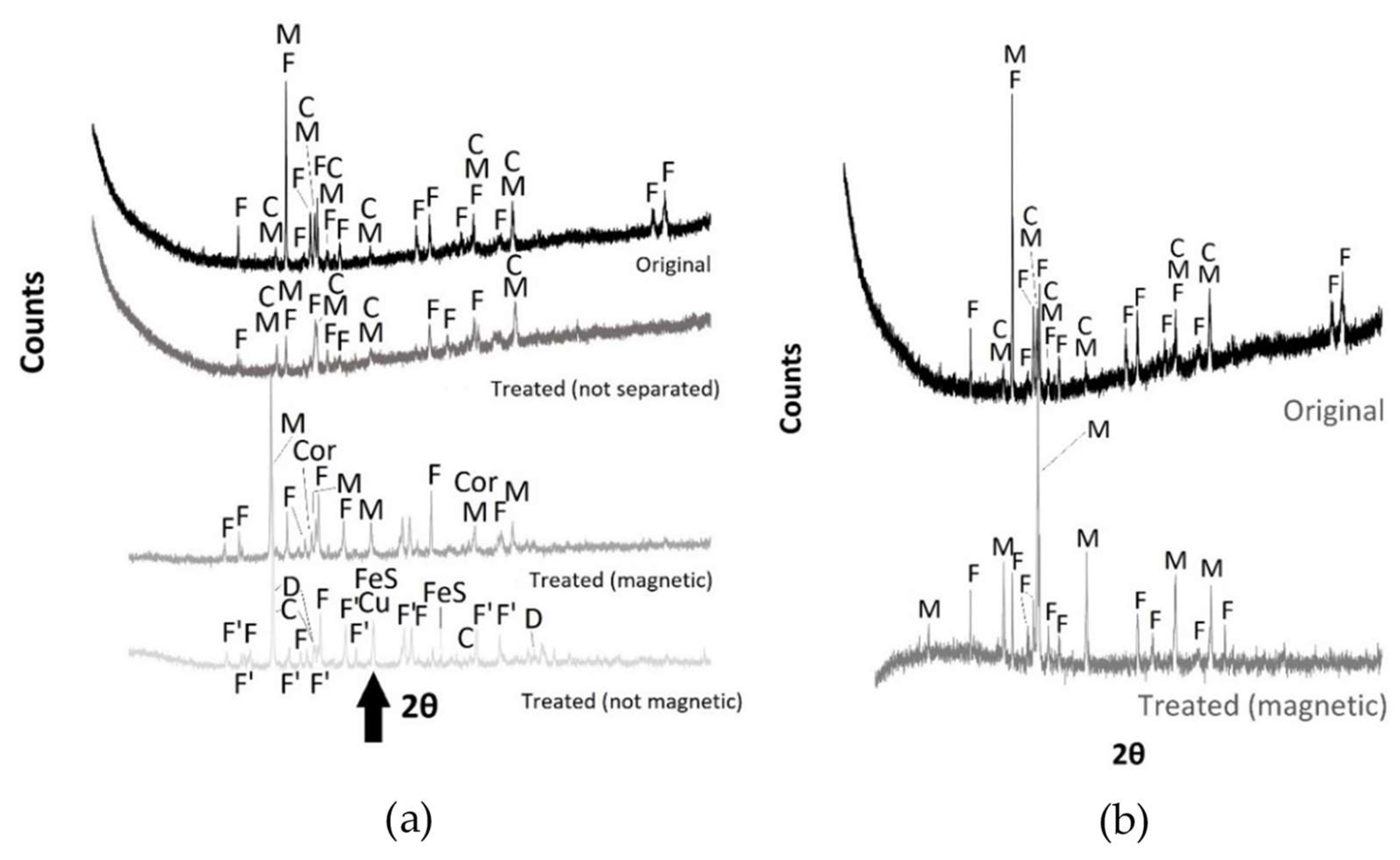
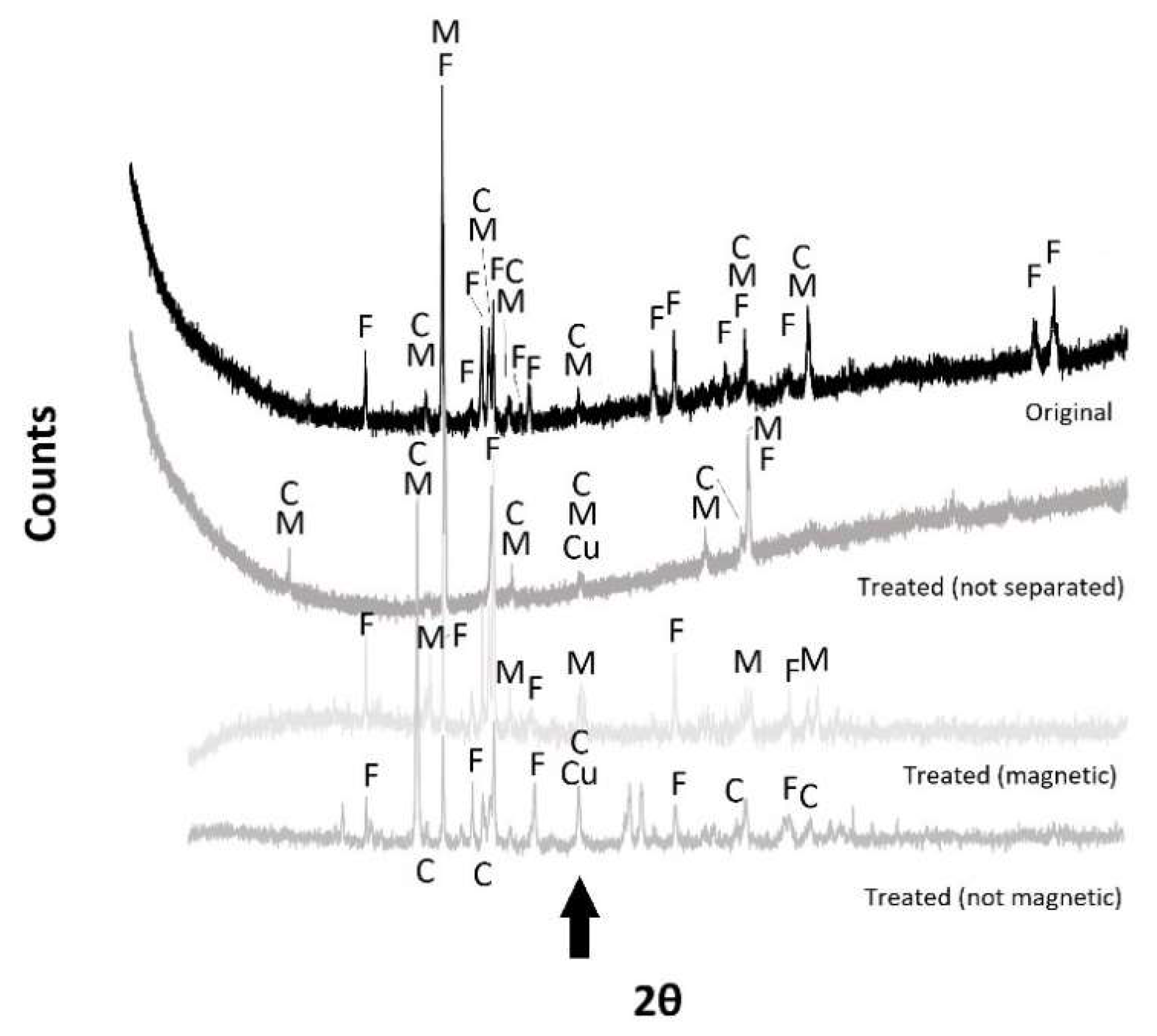
| Material | Process | Temperature | Installation | Researcher |
|---|---|---|---|---|
| Si | Dissociation of Si3N4 by carbothermal reduction of SiO2 under an N2 atmosphere | >1400 °C | OSF | Jean P. Murray, Gilles Flamant, Carolyn J. Roos [28] |
| Si | Carbothermic reduction of SiO2 under vacuum conditions at a high temperature | 1725–2000 °C | PSI | Peter G. Loutzenhiser, Ozan Tuerk, Aldo Steinfeld [29] |
| Al | Production of aluminum via carbothermal reduction | >2000 °C | PSI and OSF | Jean P. Murray [30,31,32] |
| Al | Production of aluminum using both electricity and heat generated using solar energy | ≃1000 °C | UKR | Y. M. Lytvynenko [33] |
| Zn | Production of zinc via CSP to be used in water and carbon dioxide splitting (scaled up to demonstration plant scale [34]) | >1750 °C | Mainly at the PSI, OSF, and WIS | E. A. Fletcher, R. D. Palumbo, T. Osinga, M. Epstein A. Steinfeld, C. Wieckert, L. Schunck, W. Villasmil, E. Koepft, and others [35,36,37,38,39,40,41,42,43,44,45] |
| Fe, Mn, Cd | Treatment of different materials containing iron | >1100 °C | Mainly at the PSI, PSA, OSF, and WIS | F. Sibieude, A. Steinfeld, E. A. Fletcher, I. Ruiz-Bustinza, J. Mochón and others [46,47,48,49] |
| Fe | O | Si | Al | Cu | Ca | Na | S | Others |
|---|---|---|---|---|---|---|---|---|
| 42.82 | 36.15 | 10.36 | 3.24 | 1.84 | 1.76 | 0.99 | 0.52 | 2.32 |
| Sample | Lime | Shutter Opening | Time (min) | Average Incident Radiation (W/m2) | Power (W) |
|---|---|---|---|---|---|
| CuSin1 | No | 80 | 20 | 941.2 | 1129 |
| CuSin2 | No | 88 | 25 | 955.8 | 1262 |
| CuSin3 | No | 41 | 30 | 915.5 | 563 |
| Cusinbonus | No | 60 | 23 | 867.8 | 781 |
| CuCon1 (stop in the middle of the experiment) | Yes | 92 | - | 938 | 1294 |
| CuCon2 | Yes | 100 | 15 | 939.5 | 1409 |
| CuCon3 | Yes | 84 | 20 | 973 | 1226 |
| Element | Original Copper Slag | CuSin1 | CuSin2 | CuSin3 | ||
|---|---|---|---|---|---|---|
| Magnetic | Magnetic | Non-Magnetic | Magnetic | Non-Magnetic | ||
| Cu | 1.84 | 1.34 | 1.29 | 11.72 | 1.27 | 7.09 |
| Fe | 42.82 | 38.53 | 44.75 | 43.51 | 45.45 | 45.34 |
| O | 36.15 | 37.98 | 36.17 | 32.50 | 35.94 | 33.43 |
| Si | 10.36 | 12.28 | 10.39 | 6.20 | 9.85 | 6.90 |
| Al | 3.24 | 5.94 | 2.47 | 1.16 | 2.67 | 1.82 |
| Ca | 1.76 | 0.88 | 1.06 | 1.64 | 1.10 | 2.08 |
| Na | 0.99 | 0.71 | 0.87 | 0.30 | 0.78 | 0.43 |
| K | 0.69 | 0.64 | 0.75 | 0.56 | 0.76 | 0.62 |
| S | 0.52 | 0.25 | 0.63 | 0.88 | 0.60 | 0.58 |
| Others | 1.63 | 1.46 | 1.62 | 1.52 | 1.58 | 1.70 |
| Element | Cubonus | CuCon1 | CuCon2 | CuCon3 | ||
|---|---|---|---|---|---|---|
| Magnetic | Magnetic | Non-Magnetic | Magnetic | Non-Magnetic | Magnetic | |
| Cu | 0.88 | 1.28 | 7.25 | 1.01 | 5.34 | 1.52 |
| Fe | 39.05 | 42.21 | 23.24 | 38.02 | 32.27 | 38.26 |
| O | 37.63 | 34.64 | 35.68 | 37.83 | 34.78 | 37.55 |
| Si | 11.63 | 7.22 | 6.42 | 11.48 | 7.32 | 11.31 |
| Al | 5.80 | 2.85 | 10.02 | 6.50 | 5.24 | 6.31 |
| Ca | 1.93 | 8.52 | 14.29 | 2.05 | 12.24 | 2.22 |
| Na | 0.62 | 0.47 | 0.27 | 0.69 | 0.33 | 0.53 |
| K | 0.67 | 0.62 | 0.46 | 0.67 | 0.46 | 0.65 |
| S | 0.25 | 0.71 | 0.76 | 0.30 | 0.58 | 0.24 |
| Others | 1.53 | 1.47 | 1.61 | 1.45 | 1.44 | 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, Í.; González-Gasca, C.; Gómez-Rodríguez, C.; Verdeja, L.F. Recovery of Copper and Magnetite from Copper Slag Using Concentrated Solar Power (CSP). Metals 2021, 11, 1032. https://doi.org/10.3390/met11071032
Fernández-González D, Prazuch J, Ruiz-Bustinza Í, González-Gasca C, Gómez-Rodríguez C, Verdeja LF. Recovery of Copper and Magnetite from Copper Slag Using Concentrated Solar Power (CSP). Metals. 2021; 11(7):1032. https://doi.org/10.3390/met11071032
Chicago/Turabian StyleFernández-González, Daniel, Janusz Prazuch, Íñigo Ruiz-Bustinza, Carmen González-Gasca, Cristian Gómez-Rodríguez, and Luis Felipe Verdeja. 2021. "Recovery of Copper and Magnetite from Copper Slag Using Concentrated Solar Power (CSP)" Metals 11, no. 7: 1032. https://doi.org/10.3390/met11071032
APA StyleFernández-González, D., Prazuch, J., Ruiz-Bustinza, Í., González-Gasca, C., Gómez-Rodríguez, C., & Verdeja, L. F. (2021). Recovery of Copper and Magnetite from Copper Slag Using Concentrated Solar Power (CSP). Metals, 11(7), 1032. https://doi.org/10.3390/met11071032









