Improving the Corrosion Behavior of Biodegradable AM60 Alloy through Plasma Electrolytic Oxidation
Abstract
1. Introduction
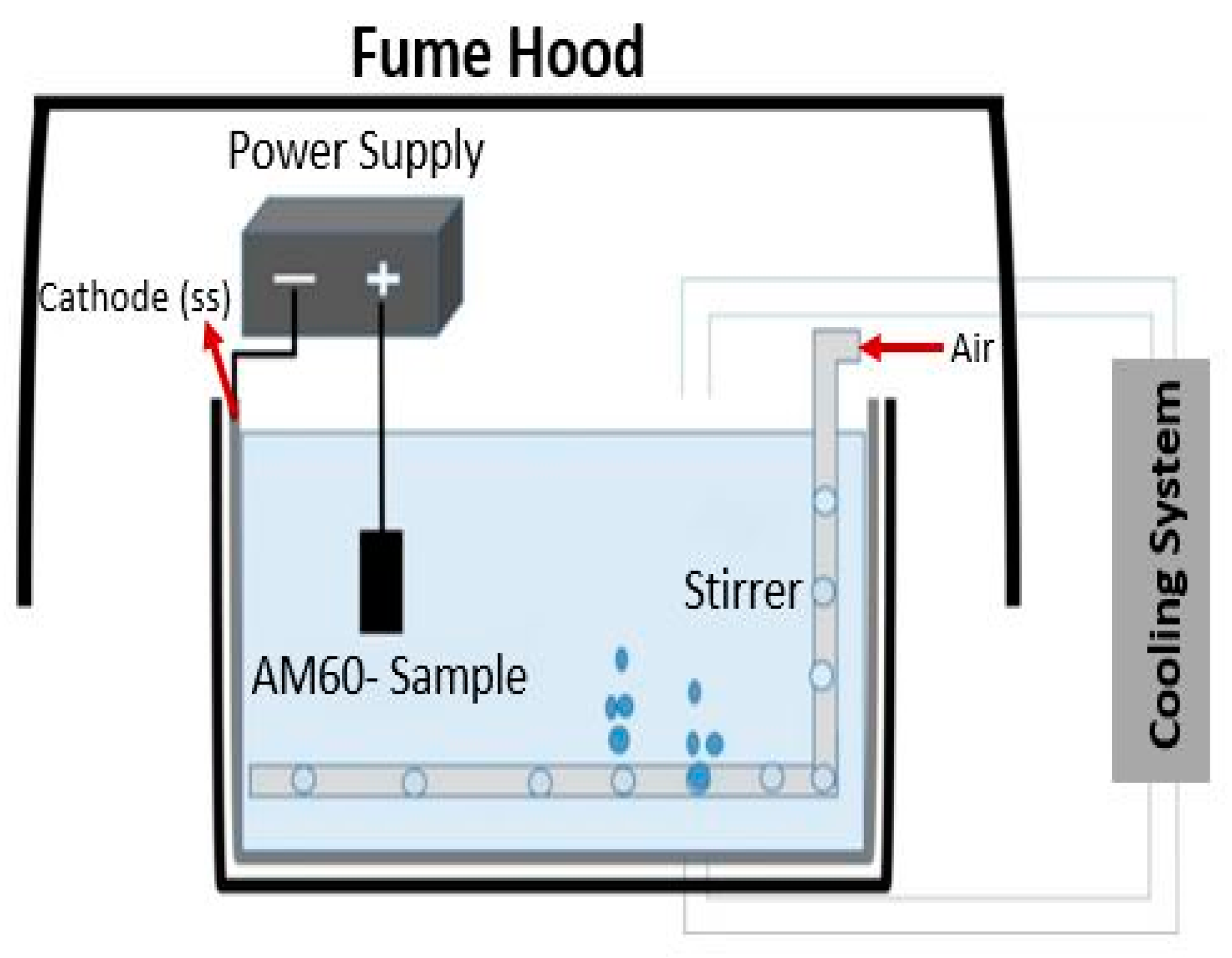
2. Materials and Methods
3. Results and Discussion
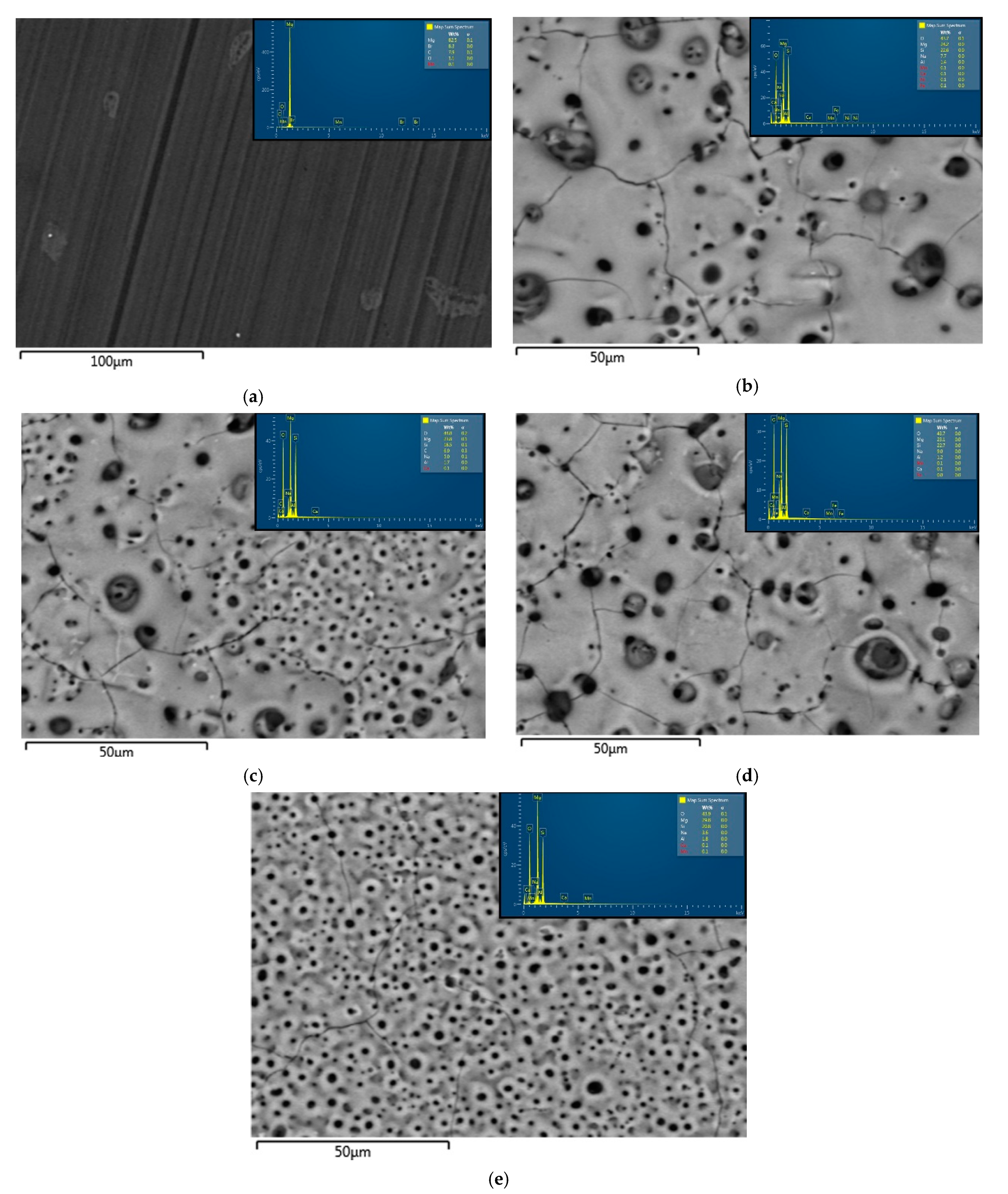
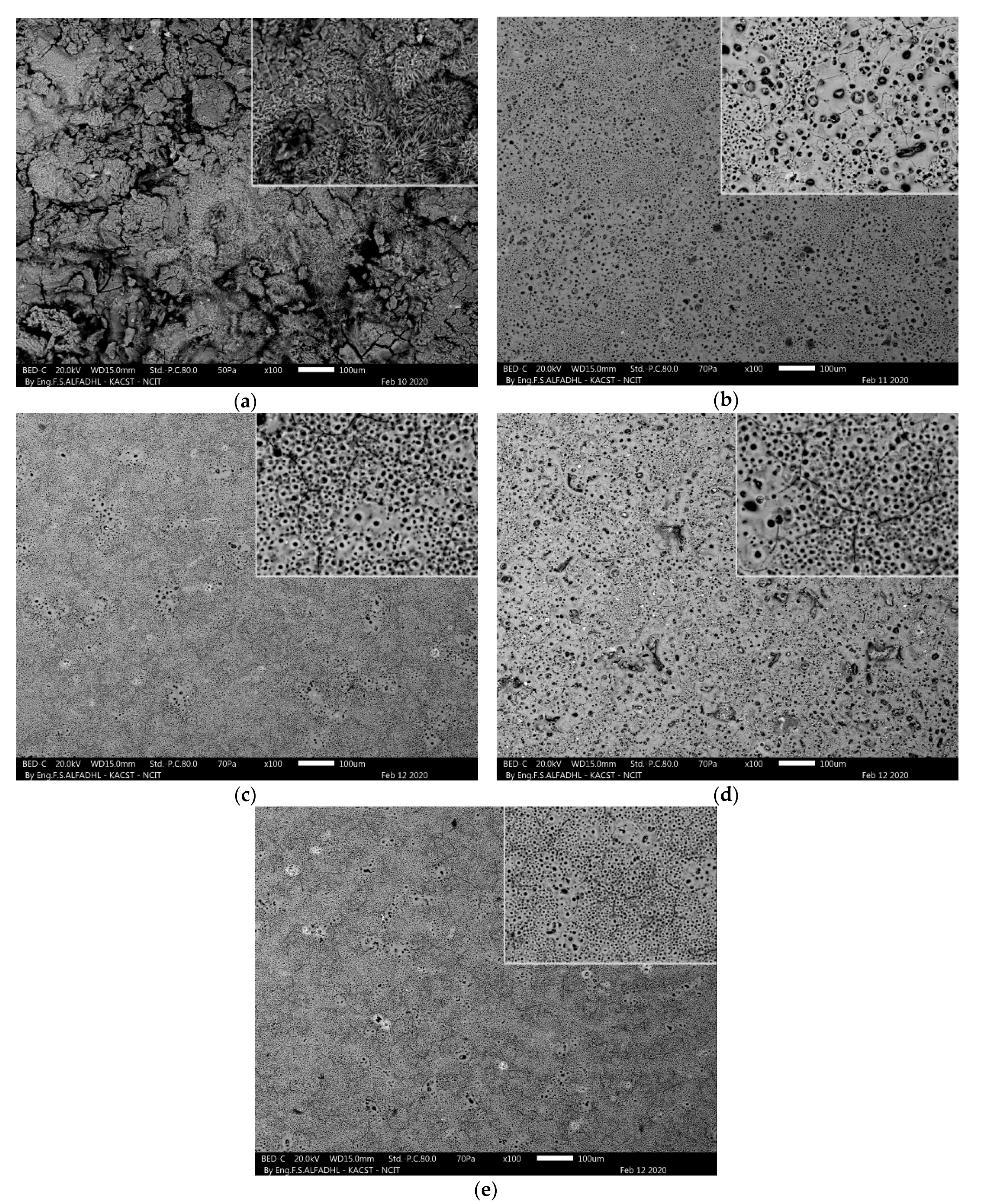
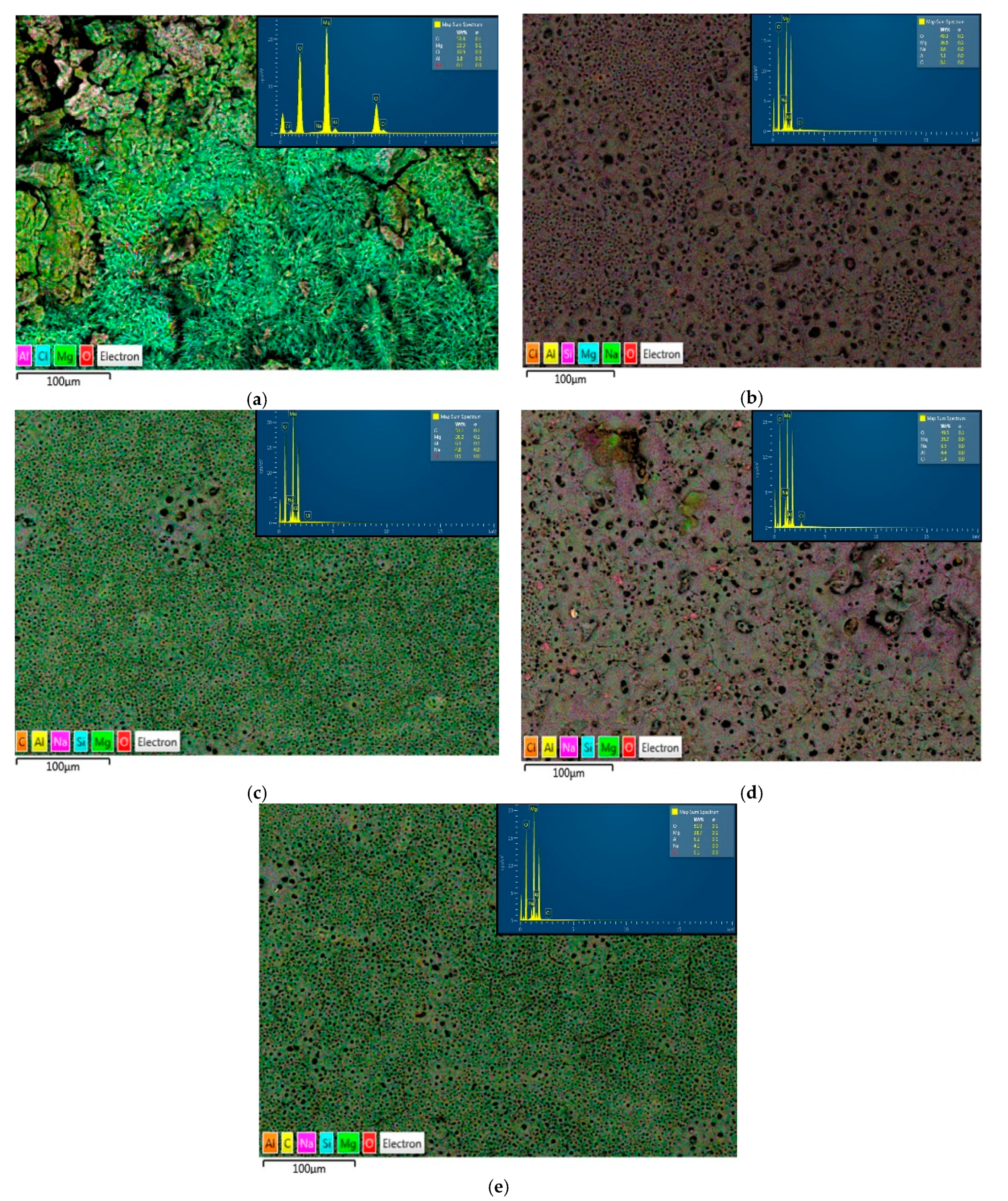
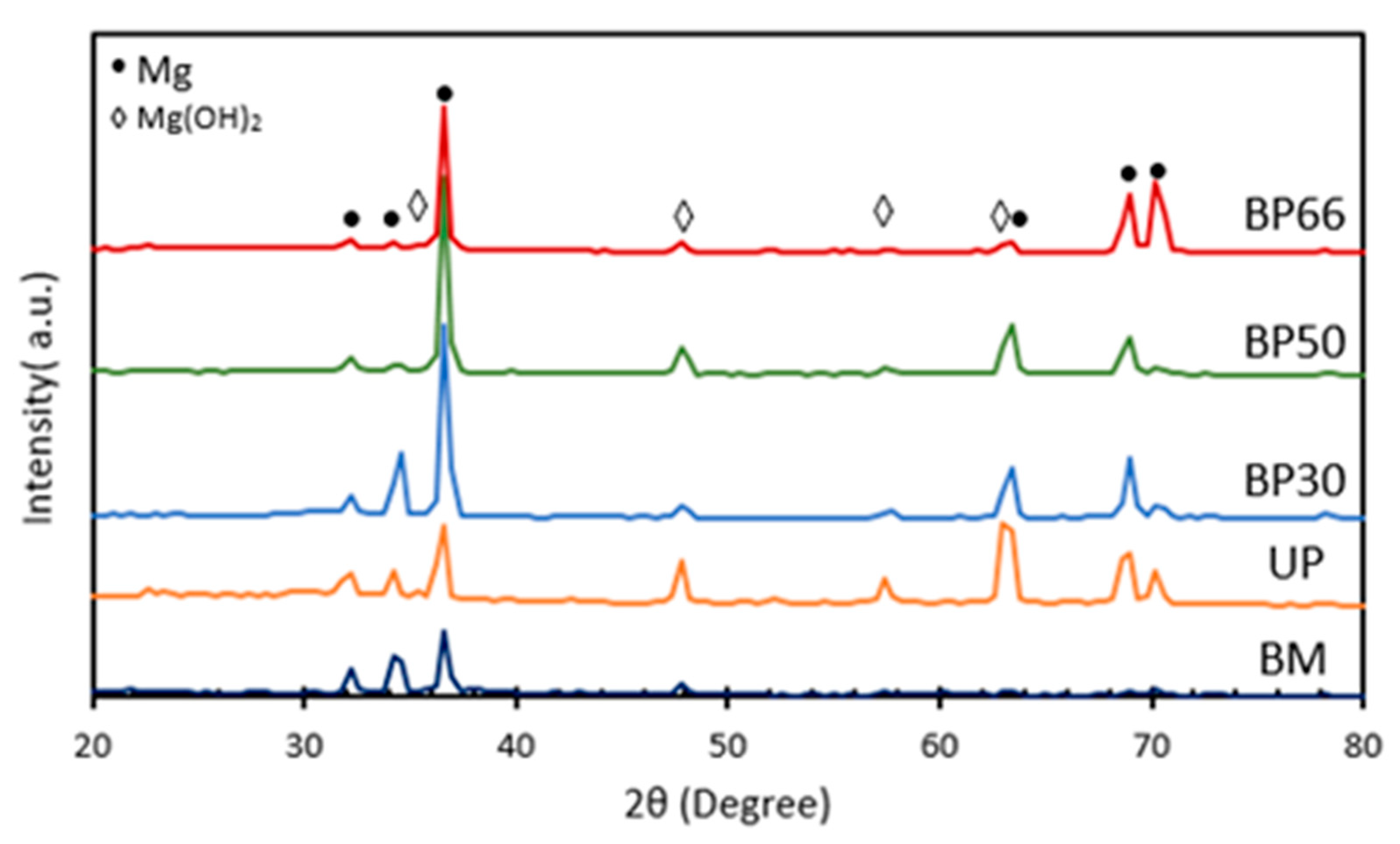
3.1. Phase Composition and Microstructure Morphology of the Samples
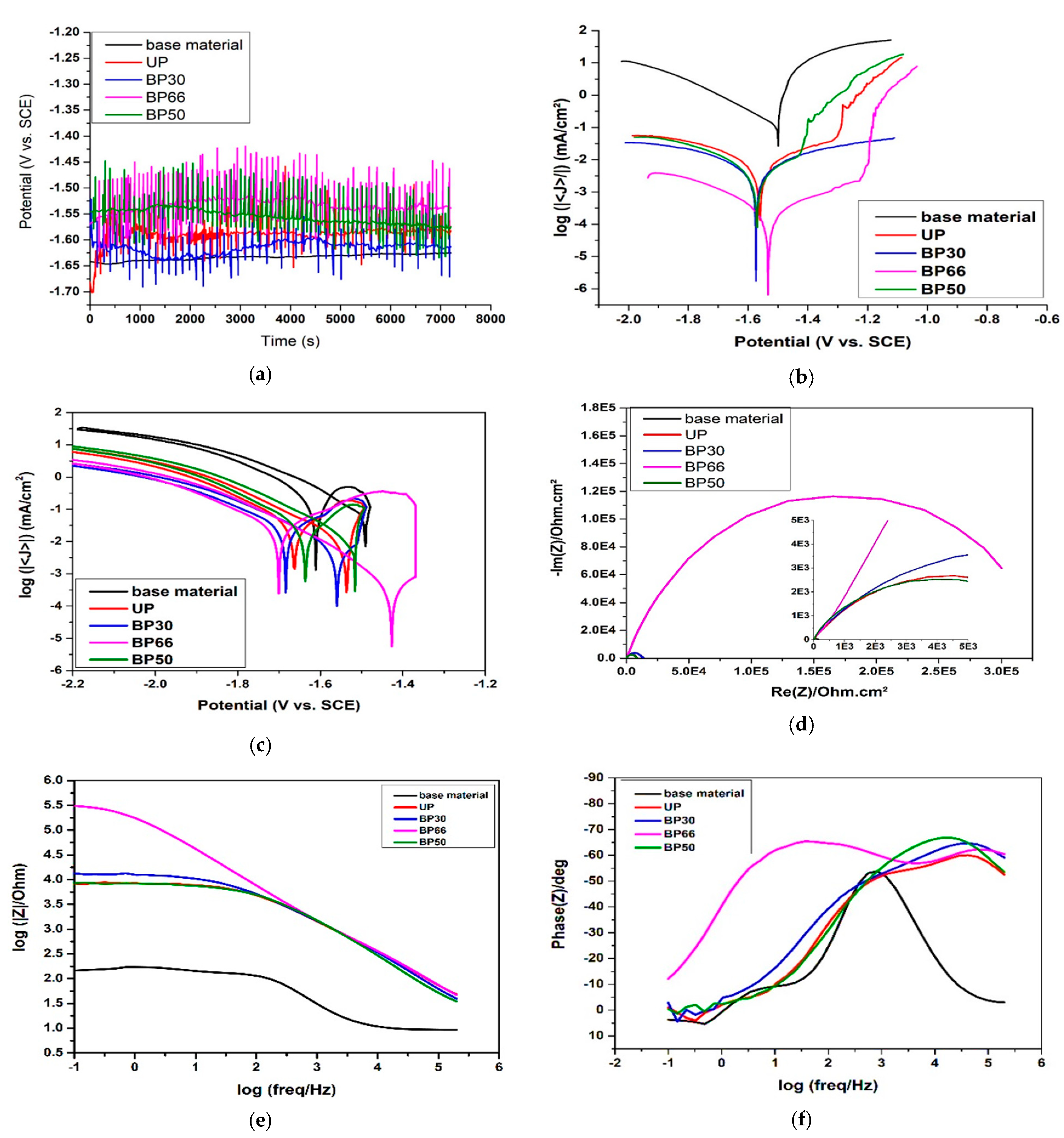
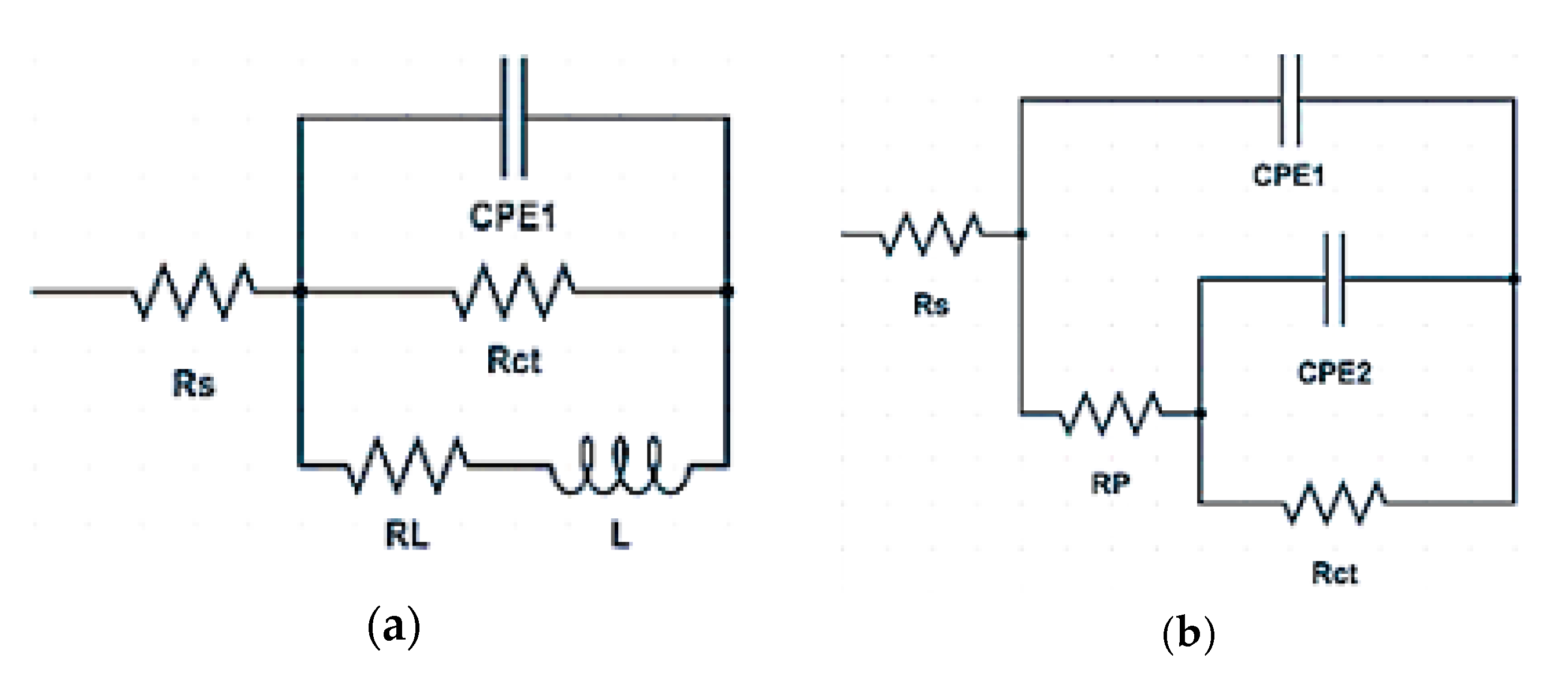
3.2. Electrochemical Measurements
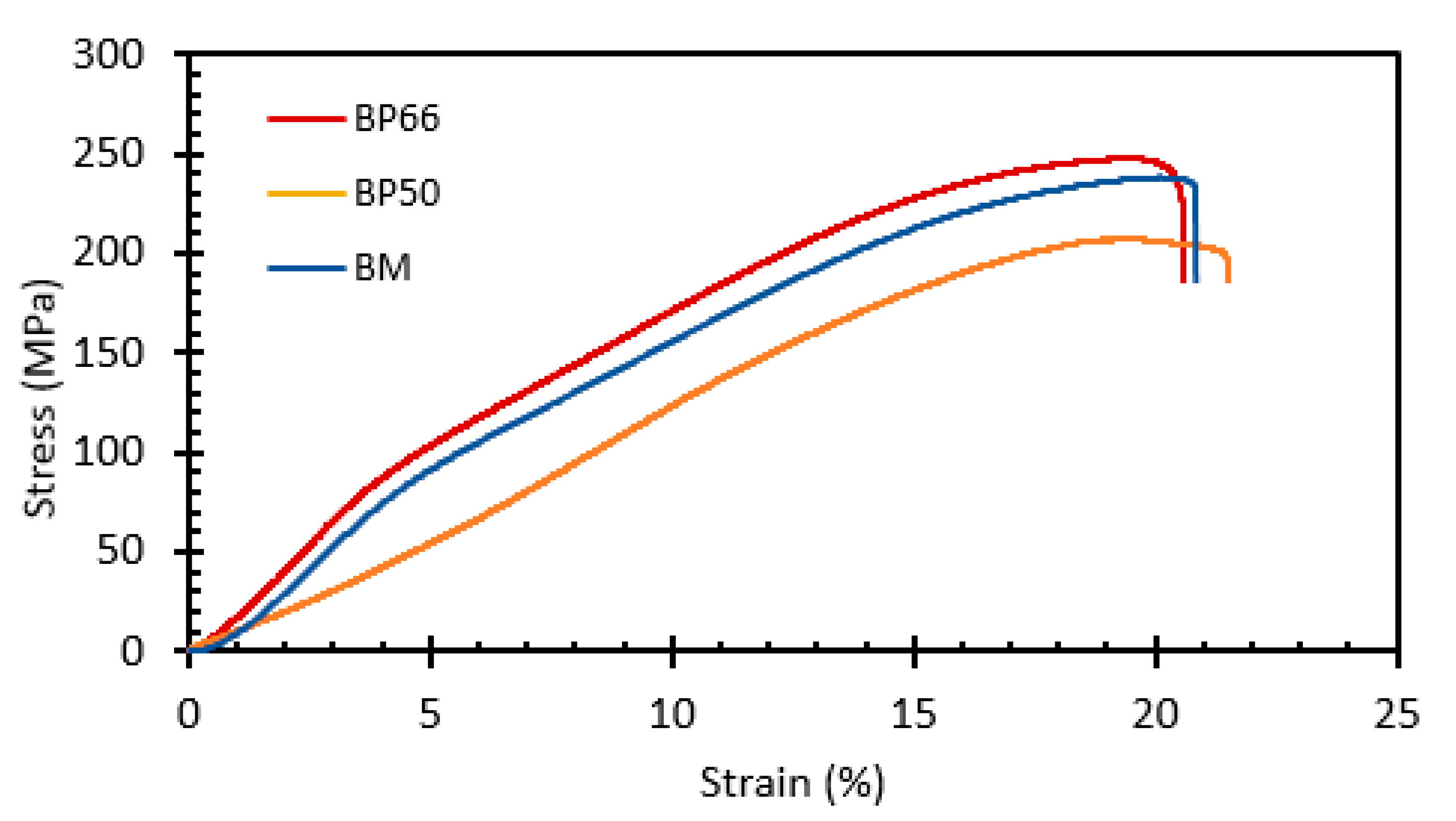
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witte, F. The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Amani, S.; Faraji, G. Recrystallization and Mechanical Properties of WE43 Magnesium Alloy Processed via Cyclic Expansion Extrusion. Int. J. Miner. Metall. Mater. 2018, 25, 672–681. [Google Scholar] [CrossRef]
- Mostaed, E.; Vedani, M.; Hashempour, M.; Bestetti, M. Influence of ECAP Process on Mechanical and Corrosion Properties of Pure Mg and ZK60 Magnesium Alloy for Biodegradable Stent Applications. Biomatter 2014, 4, 28283. [Google Scholar] [CrossRef] [PubMed]
- Mordike, B.L.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Mordike, B.L.; Hehmann, F. Magnesium Alloys and Their Applications. In Proceedings of the International Conference on Magnesium Alloys and Their Applications, Internationale Tagung Magnesium-Magnesiumverbindungen und ihre Anwendungen, Garmisch-Partenkirchen, Germany, 8–10 April 1992; Kainer, K.U., Barry, L.M., Eds.; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Aune, T.K.; Busk, R.S.; Carnahan, R.D.; Decker, R.F.; Hawke, D.; Hillis, J.E.; King, J.; Lowak, H.; Miller, W.K.; Mordike, B.L.; et al. ASM Specialty Handbook: Magnesium and Magnesium Alloys; ASM International Publishing: Almere, The Netherlands, 1999; pp. 106–118. [Google Scholar]
- Gupta, M.; Ling, S.N.M. Magnesium, Magnesium Alloys, and Magnesium Composites; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2011; pp. 39–85. [Google Scholar]
- Kuwahara, H.; Al-Abdullat, Y.; Ohta, M.; Tsutsumi, S.; Ikeuchi, K.; Mazaki, N.; Aizawa, T. Surface Reaction of Magnesium in Hank’s Solutions. Mater. Sci. Forum 2000, 350–351, 349–358. [Google Scholar] [CrossRef]
- Kojima, Y. Platform Science and Technology for Advanced Magnesium Alloys. Mater. Sci. Forum 2000, 350–351, 3–18. [Google Scholar] [CrossRef]
- Kim, J.J.; Han, D.S. Recent Development and Applications of Magnesium Alloys in the Hyundai and Kia Motors Corporation. Mater. Trans. 2008, 49, 894–897. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Yang, M. Research on Microstructure and Alloy Phases of AM50 Magnesium Alloy. J. Alloys Compd. 2009, 470, 515–521. [Google Scholar] [CrossRef]
- Wang, R.M.; Eliezer, A.; Gutman, E.M. An Investigation on the Microstructure of an AM50 Magnesium Alloy. Mater. Sci. Eng. A 2003, 355, 201–207. [Google Scholar] [CrossRef]
- Hu, H.; Zhou, M.; Sun, Z.; Li, N. Tensile Behaviour and Fracture Characteristics of Die Cast Magnesium Alloy AM50. J. Mater. Process. Technol. 2008, 201, 364–368. [Google Scholar] [CrossRef]
- Khaleghi, A.A.; Akbaripanah, F.; Sabbaghian, M.; Máthis, K.; Minárik, P.; Veselý, J.; El-Tahawy, M.; Gubicza, J. Influence of High-Pressure Torsion on Microstructure, Hardness and Shear Strength of AM60 Magnesium Alloy. Mater. Sci. Eng. 2021, 799, 140158. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Chang, L.; Zhang, Z.; Zhang, J. Effect of Rare Earth Element Ce and La on Corrosion Behavior of AM60 Magnesium Alloy. Corros. Sci. 2009, 51, 1334–1343. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef]
- Bettles, C.; Barnett, M. Advances in Wrought Magnesium Alloys: Fundamentals of Processing, Properties and Applications; ASM International, Woodhead Publishing: Sawston, UK, 2012. [Google Scholar]
- Azumi, K.; Elsentriecy, H.H.; Tang, J. Corrosion Prevention of Magnesium Alloys: 12. Plating Techniques to Protect Magnesium (Mg) Alloys from Corrosion; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Strategies to Improve the Corrosion Resistance of Microarc Oxidation (MAO) Coated Magnesium Alloys for Degradable Implants: Prospects and Challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Khaselev, O.; Weiss, D.; Yahalom, J. Structure and Composition of Anodic Films Formed on Binary Mg–Al Alloys in KOH–Aluminate Solutions under Continuous Sparking. Corros. Sci. 2001, 43, 1295–1307. [Google Scholar] [CrossRef]
- Birss, V.; Xia, S.; Yue, R.; Richard, G.R., Jr. Characterization of oxide films formed on Mg-based WE43 alloy using AC/DC anodization in silicate solutions. J. Electrochem. Soc. 2003, 151, B1–B10. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An Investigation of Ceramic Coating Growth Mechanisms in Plasma Electrolytic Oxidation (PEO) Processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Duan, H.; Du, K.; Yan, C.; Wang, F. Electrochemical Corrosion Behavior of Composite Coatings of Sealed MAO Film on Magnesium Alloy AZ91D. Electrochim. Acta 2006, 51, 2898–2908. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Tsai, W.-T. Effect of Heat Treatment on Anodization and Electrochemical Behavior of AZ91D Magnesium Alloy. J. Mater. Res. 2005, 20, 2763–2771. [Google Scholar] [CrossRef]
- Guo, H.-X.; Liu, S.-X. Hydrothermal Synthesis and Crystal Structure of a Novel 1D Molybdenum(V) Phosphate with Mixed-Valence Cobalt Coordination Cations. J. Mol. Struct. 2005, 741, 229–234. [Google Scholar] [CrossRef]
- Cai, Q.; Wang, L.; Wei, B.; Liu, Q. Electrochemical Performance of Microarc Oxidation Films Formed on AZ91D Magnesium Alloy in Silicate and Phosphate Electrolytes. Surf. Coat. Technol. 2006, 200, 3727–3733. [Google Scholar] [CrossRef]
- Tjiang, F.; Ye, L.-W.; Huang, Y.-J.; Chou, C.-C.; Tsai, D.-S. Effect of Processing Parameters on Soft Regime Behavior of Plasma Electrolytic Oxidation of Magnesium. Ceram. Int. 2017, 43, S567–S572. [Google Scholar] [CrossRef]
- Hussein, R.O.; Northwood, D.O.; Nie, X. The Effect of Processing Parameters and Substrate Composition on the Corrosion Resistance of Plasma Electrolytic Oxidation (PEO) Coated Magnesium Alloys. Surf. Coat. Technol. 2013, 237, 357–368. [Google Scholar] [CrossRef]
- Arrabal, R.; Pardo, A.; Merino, M.C.; Mohedano, M.; Casajús, P.; Matykina, E.; Skeldon, P.; Thompson, G.E. Corrosion Behaviour of a Magnesium Matrix Composite with a Silicate Plasma Electrolytic Oxidation Coating. Corros. Sci. 2010, 52, 3738–3749. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion Resistance of WE43 and AZ91D Magnesium Alloys with Phosphate PEO Coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Martin, J.; Nominé, A.V.; Stef, J.; Nominé, A.; Zou, J.X.; Henrion, G.; Grosdidier, T. The Influence of Metallurgical State of Substrate on the Efficiency of Plasma Electrolytic Oxidation (PEO) Process on Magnesium Alloy. Mater. Des. 2019, 178, 107859. [Google Scholar] [CrossRef]
- Gunduz, K.O.; Oter, Z.C.; Tarakci, M.; Gencer, Y. Plasma Electrolytic Oxidation of Binary Mg-Al and Mg-Zn Alloys. Surf. Coat. Technol. 2017, 323, 72–81. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Yan, Z.; Wang, H.; Peng, J. Effect of Potassium Fluoride on Structure and Corrosion Resistance of Plasma Electrolytic Oxidation Films Formed on AZ31 Magnesium Alloy. J. Alloys Compd. 2009, 480, 469–474. [Google Scholar] [CrossRef]
- Bala Srinivasan, P.; Liang, J.; Balajeee, R.G.; Blawert, C.; Störmer, M.; Dietzel, W. Effect of Pulse Frequency on the Microstructure, Phase Composition and Corrosion Performance of a Phosphate-Based Plasma Electrolytic Oxidation Coated AM50 Magnesium Alloy. Appl. Surf. Sci. 2010, 256, 3928–3935. [Google Scholar] [CrossRef]
- Dehnavi, V.; Luan, B.L.; Shoesmith, D.W.; Liu, X.Y.; Rohani, S. Effect of Duty Cycle and Applied Current Frequency on Plasma Electrolytic Oxidation (PEO) Coating Growth Behavior. Surf. Coat. Technol. 2013, 226, 100–107. [Google Scholar] [CrossRef]
- Martin, J.; Nominé, A.; Brochard, F.; Briançon, J.-L.; Noël, C.; Belmonte, T.; Czerwiec, T.; Henrion, G. Delay in Micro-Discharges Appearance during PEO of Al: Evidence of a Mechanism of Charge Accumulation at the Electrolyte/Oxide Interface. Appl. Surf. Sci. 2017, 410, 29–41. [Google Scholar] [CrossRef]
- Muhaffel, F.; Cimenoglu, H. Development of Corrosion and Wear Resistant Micro-Arc Oxidation Coating on a Magnesium Alloy. Surf. Coat. Technol. 2019, 357, 822–832. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Song, R.G.; Xiang, N.; Xiong, Y.; Hu, Q. Effect of Current Density on Microstructure and Properties of PEO Ceramic Coatings on Magnesium Alloy. Surf. Eng. 2017, 33, 744–752. [Google Scholar] [CrossRef]
- Bala Srinivasan, P.; Liang, J.; Blawert, C.; Störmer, M.; Dietzel, W. Effect of Current Density on the Microstructure and Corrosion Behaviour of Plasma Electrolytic Oxidation Treated AM50 Magnesium Alloy. Appl. Surf. Sci. 2009, 255, 4212–4218. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. Thermo-Physical Properties of Plasma Electrolytic Oxide Coatings on Aluminium. Surf. Coat. Technol. 2005, 199, 168–176. [Google Scholar] [CrossRef]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The Effect of Current Mode and Discharge Type on the Corrosion Resistance of Plasma Electrolytic Oxidation (PEO) Coated Magnesium Alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Zavidnaya, A.G.; Sinebryukhov, S.L.; Egorkin, V.S.; Nistratova, M.V.; Yerokhin, A.; Matthews, A. PEO Coatings Obtained on an Mg–Mn Type Alloy under Unipolar and Bipolar Modes in Silicate-Containing Electrolytes. Surf. Coat. Technol. 2010, 204, 2316–2322. [Google Scholar] [CrossRef]
- Cao, F.; Cao, J.; Zhang, Z.; Zhang, J.; Cao, C. Plasma Electrolytic Oxidation of AZ91D Magnesium Alloy with Different Additives and Its Corrosion Behavior. Mater. Corros. 2007, 58, 696–703. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabal, R.; Mohedano, M.; Llamazares, Y.; Matykina, E.; Yerokhin, A.; Pardo, A. Influence of Sealing Post-Treatments on the Corrosion Resistance of PEO Coated AZ91 Magnesium Alloy. Appl. Surf. Sci. 2018, 433, 653–667. [Google Scholar] [CrossRef]
- Liu, F.; Shan, D.; Song, Y.; Han, E. Formation Process of Composite Plasma Electrolytic Oxidation Coating Containing Zirconium Oxides on AM50 Magnesium Alloy. Trans. Nonferrous Met. Soc. China 2011, 21, 943–948. [Google Scholar] [CrossRef]
- Rapheal, G.; Kumar, S.; Scharnagl, N.; Blawert, C. Effect of Current Density on the Microstructure and Corrosion Properties of Plasma Electrolytic Oxidation (PEO) Coatings on AM50 Mg Alloy Produced in an Electrolyte Containing Clay Additives. Surf. Coat. Technol. 2016, 289, 150–164. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, X.; Jiang, K.; Chen, J.; Zuo, Y. The Influences of Duty Cycle on the Bonding Strength of AZ31B Magnesium Alloy by Microarc Oxidation Treatment. Surf. Coat. Technol. 2010, 205, 1789–1792. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Němcová, A.; Gholinia, A.; Mohedano, M.; Sun, M.; Matthews, A.; Yerokhin, A. Incorporation of Halloysite Nanotubes into Forsterite Surface Layer during Plasma Electrolytic Oxidation of AM50 Mg Alloy. Electrochim. Acta 2019, 299, 772–788. [Google Scholar] [CrossRef]
- Zhou, X.; Thompson, G.E.; Skeldon, P.; Wood, G.C.; Shimizu, K.; Habazaki, H. Film Formation and Detachment during Anodizing of Al–Mg Alloys. Corros. Sci. 1999, 41, 1599–1613. [Google Scholar] [CrossRef]
- Sarbishei, S.; Sani, M.A.F.; Mohammadi, M.R. Study plasma electrolytic oxidation process and characterization of coatings formed in an alumina nanoparticle suspension. Vacuum 2014, 108, 12–19. [Google Scholar] [CrossRef]
- Ezhilselvi, V.; Nithin, J.; Balaraju, J.N.; Subramanian, S. The influence of current density on the morphology and corrosion properties of MAO coatings on AZ31B magnesium alloy. Surf. Coat. Technol. 2016, 288, 221–229. [Google Scholar] [CrossRef]
- Lu, X.; Sah, S.P.; Scharnagl, N.; Störmer, M.; Starykevich, M.; Mohedano, M.; Kainer, K.U. Degradation behavior of PEO coating on AM50 magnesium alloy produced from electrolytes with clay particle addition. Surf. Coat. Technol. 2015, 269, 155–169. [Google Scholar] [CrossRef]
- Alateyah, A.; Aljohani, T.A.; Alawad, M.O.; El-Hafez, H.A.; Almutairi, A.N.; Alharbi, E.S.; Alhamada, R.; El-Garaihy, B.W.; El-Garaihy, W.H. Improved Corrosion Behavior of AZ31 Alloy through ECAP Processing. Metals 2021, 11, 363. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Leyland, A.; Matthews, A. Kinetic Aspects of Aluminium Titanate Layer Formation on Titanium Alloys by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2002, 200, 172–184. [Google Scholar] [CrossRef]



| Sample Designation | Polarity | Frequency (Hz) | Off/On (ms) | Duty Cycle (%) | Anode (mA/cm2) | Cathode (mA/cm2) |
|---|---|---|---|---|---|---|
| AM60-UP | Unipolar | 1000 | 0.7/0.3 | 30 | 30 | 0 |
| AM60-BP30 | Bipolar | 1000 | 0.7/0.3 | 30 | 30 | 10 |
| AM60-BP50 | Bipolar | 1000 | 0.5/0.5 | 50 | 30 | 10 |
| AM60-BP66 | Bipolar | 1666 | 0.2/0.4 | 66.6 | 30 | 10 |
| Sample Condition | Before/After Corrosion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mg | Si | O | Na | Al | Mn | Ca | Br | C | Cl | |
| AM60-BM | 82.5/33.3 | 0/0 | 1.1/53.5 | 0/0.1 | 0/1.8 | 0.1/0 | 0/0 | 8.3 | 7.9/0 | 0/10.9 |
| AM60-UP | 24.2/36.5 | 22.6/0 | 43.7/49.3 | 7.7/8.6 | 1.4/5.1 | 0.1/0 | 0.1/0 | 0/0 | 0/0 | 0/0.4 |
| AM60-BP30 | 23.8/38.0 | 18.5/0 | 44.0/51.1 | 5.0/4.8 | 1.7/6.1 | 0.1/0 | 0.1/0 | 0/0 | 6.9/0 | 0/0.1 |
| AM60-BP50 | 23.1/35.2 | 22.7/0 | 43.749.5 | 9.0/9.5 | 1.2/4.4 | 0.1/0 | 0.1/0 | 0/0 | 0/0 | 0/1.4 |
| AM60-BP66 | 29.8/38.7 | 20.8/0 | 43.9/51.0 | 3.6/4.1 | 1.8/6.2 | 0.1/0 | 0.1/0 | 0/0 | 0/0 | 0/0.1 |
| Condition | βa (mV·dec−1) | −βc (mV·dec−1) | Ecorr (V/SCE) | Icorr (µAcm−2) | Corrosion Rate mpy |
|---|---|---|---|---|---|
| BM | 51.5 | 198.5 | −1.527 | 192.08 | 5.315 |
| UP | 79.2 | 76.2 | −1.562 | 2.227 | 0.061 |
| BP 30 | 95.4 | 92.7 | −1.574 | 1.789 | 0.049 |
| BP 50 | 97.2 | 83.2 | −1.572 | 1.305 | 0.036 |
| BP 66 | 107.8 | 94.3 | −1.532 | 0.111 | 0.003 |
| Condition | Rs (Ω. cm2) | CPE1 (Ω-1. sn. cm−2) | n1 | Rp (Ω. cm2) | CPE2 (Ω-1. sn. cm−2) | n2 | Rct (Ω. cm2) |
|---|---|---|---|---|---|---|---|
| BM | 7.28 | 1.7 × 10−5 | 0.9 | 100.5 | 1.3 × 10−6 | 1 | 25.3 |
| UP | 5.40 | 6.6 × 10−7 | 0.78 | 1570 | 2.5 × 10−6 | 0.62 | 8695 |
| BP 30 | 4.59 | 9.3 × 10−7 | 0.75 | 2725 | 2.0 × 10−6 | 0.68 | 7427 |
| BP 50 | 9.8 | 1.5 × 10−7 | 0.89 | 2922 | 2.3 × 10−6 | 0.60 | 6634 |
| BP 66 | 4.99 | 6.5 × 10−7 | 0.76 | 1079 | 6.1 × 10−7 | 0.75 | 271,209 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alateyah, A.I.; Aljohani, T.A.; Alawad, M.O.; Elkatatny, S.; El-Garaihy, W.H. Improving the Corrosion Behavior of Biodegradable AM60 Alloy through Plasma Electrolytic Oxidation. Metals 2021, 11, 953. https://doi.org/10.3390/met11060953
Alateyah AI, Aljohani TA, Alawad MO, Elkatatny S, El-Garaihy WH. Improving the Corrosion Behavior of Biodegradable AM60 Alloy through Plasma Electrolytic Oxidation. Metals. 2021; 11(6):953. https://doi.org/10.3390/met11060953
Chicago/Turabian StyleAlateyah, Abdulrahman I., Talal A. Aljohani, Majed O. Alawad, Sally Elkatatny, and Waleed H. El-Garaihy. 2021. "Improving the Corrosion Behavior of Biodegradable AM60 Alloy through Plasma Electrolytic Oxidation" Metals 11, no. 6: 953. https://doi.org/10.3390/met11060953
APA StyleAlateyah, A. I., Aljohani, T. A., Alawad, M. O., Elkatatny, S., & El-Garaihy, W. H. (2021). Improving the Corrosion Behavior of Biodegradable AM60 Alloy through Plasma Electrolytic Oxidation. Metals, 11(6), 953. https://doi.org/10.3390/met11060953







