Flotation Separation of Chalcopyrite and Talc Using Calcium Ions and Calcium Lignosulfonate as a Combined Depressant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flotation Experiment
- α—The primary ore grade, %.
- β—The concentrate grade, %.
- γ—The concentrate yield, %.
- ε—Recovery, %.
- βmax—Pure mineral grade, %.
2.3. Adsorption Capacity Experiment
- Γ—Adsorption density of calcium lignosulfonate on the mineral surface, mg/m2.
- Co—Original concentration of CLS, mg/L.
- Ce—Supernatant concentration of calcium lignosulfonate, mg/L.
- V—Volume of solution, ml.
- m—Quality of minerals, g.
- A—Specific surface area of minerals, m2/g.
2.4. Zeta Potential Measurements
2.5. Fourier Transform Infrared Spectrometry (FTIR) Analysis
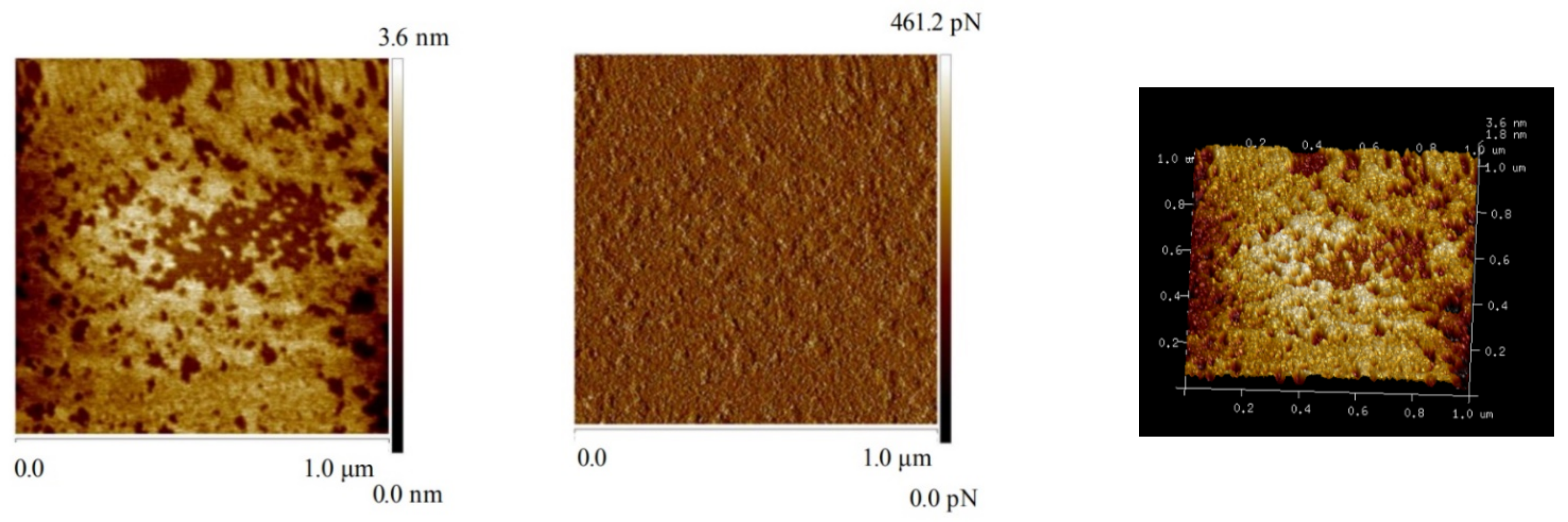
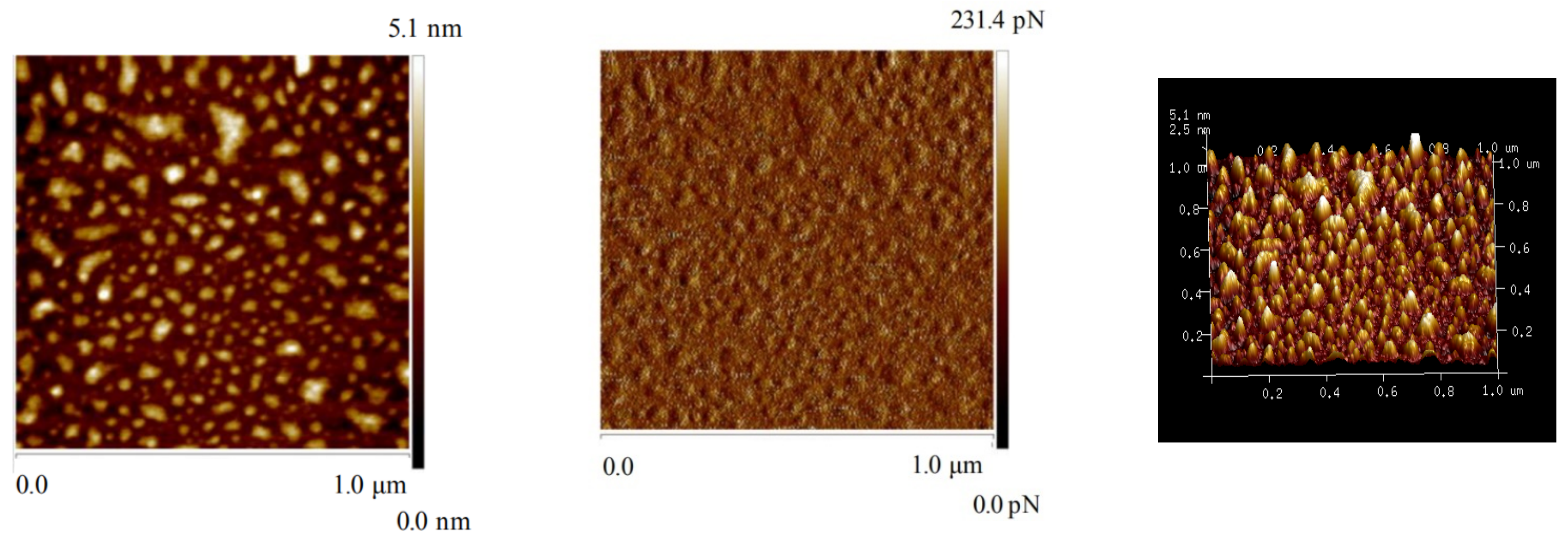
2.6. AFM Imaging
3. Results and Discussion
3.1. Flotation Test Results
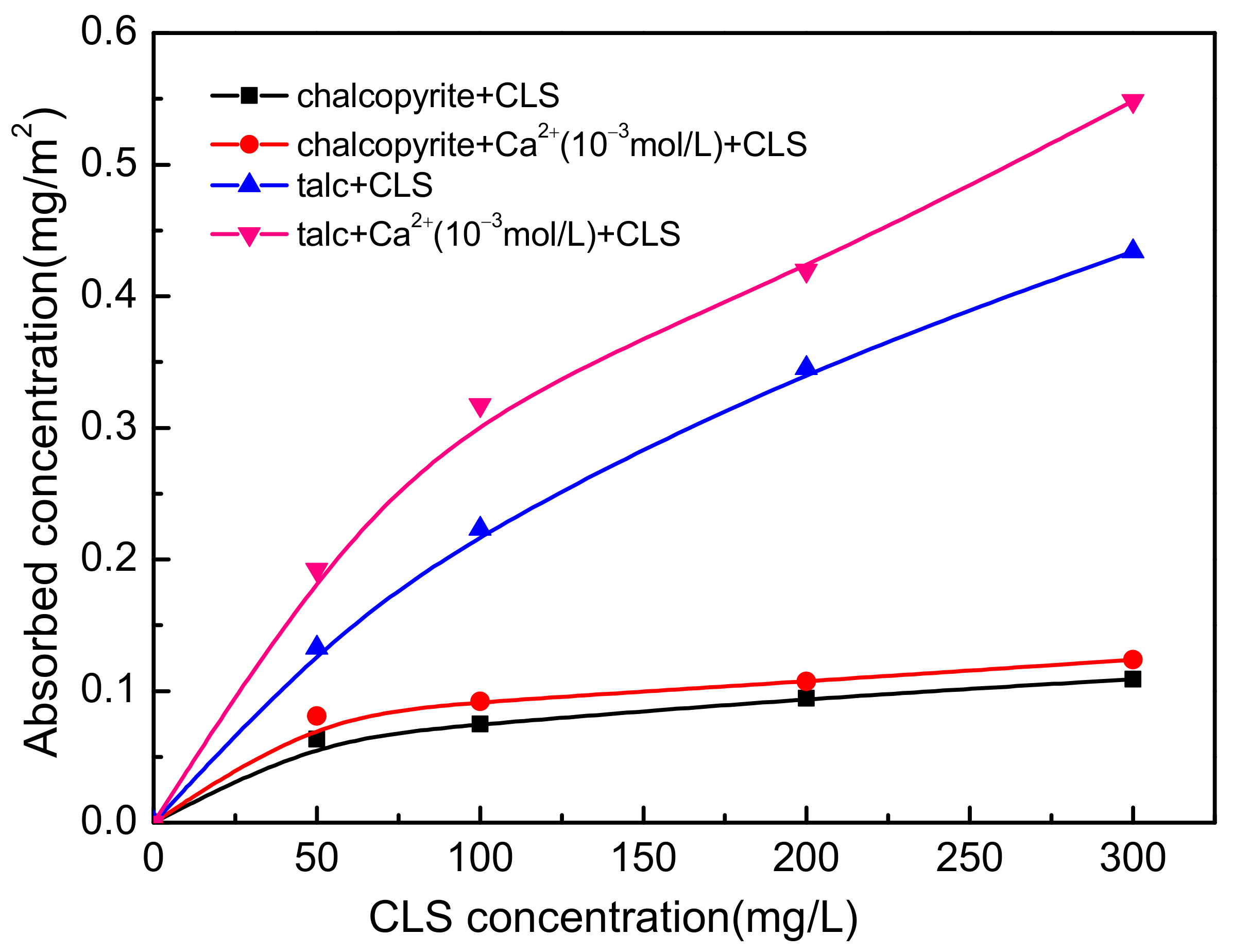
3.2. Adsorption Capacity Experimental Results
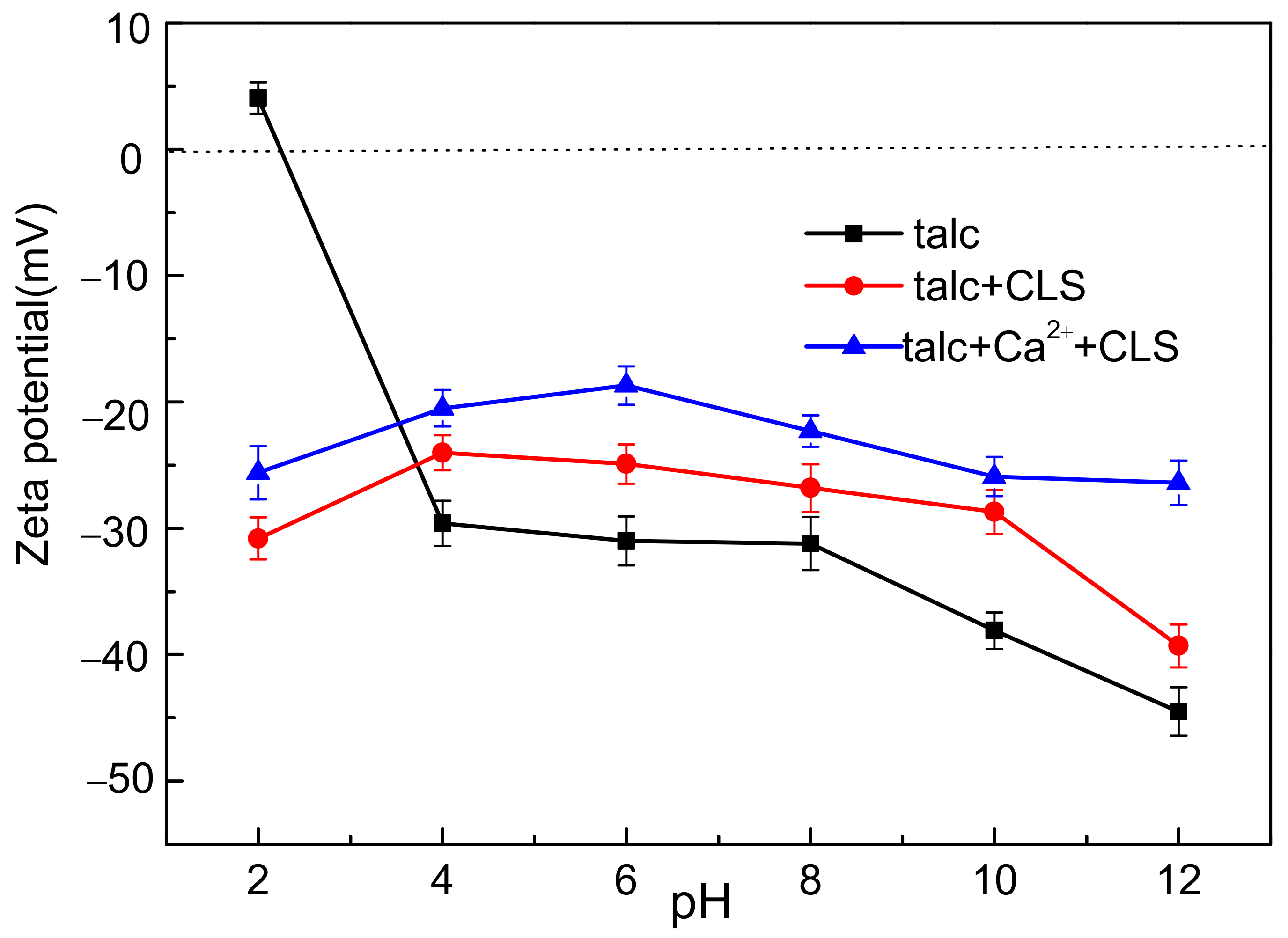
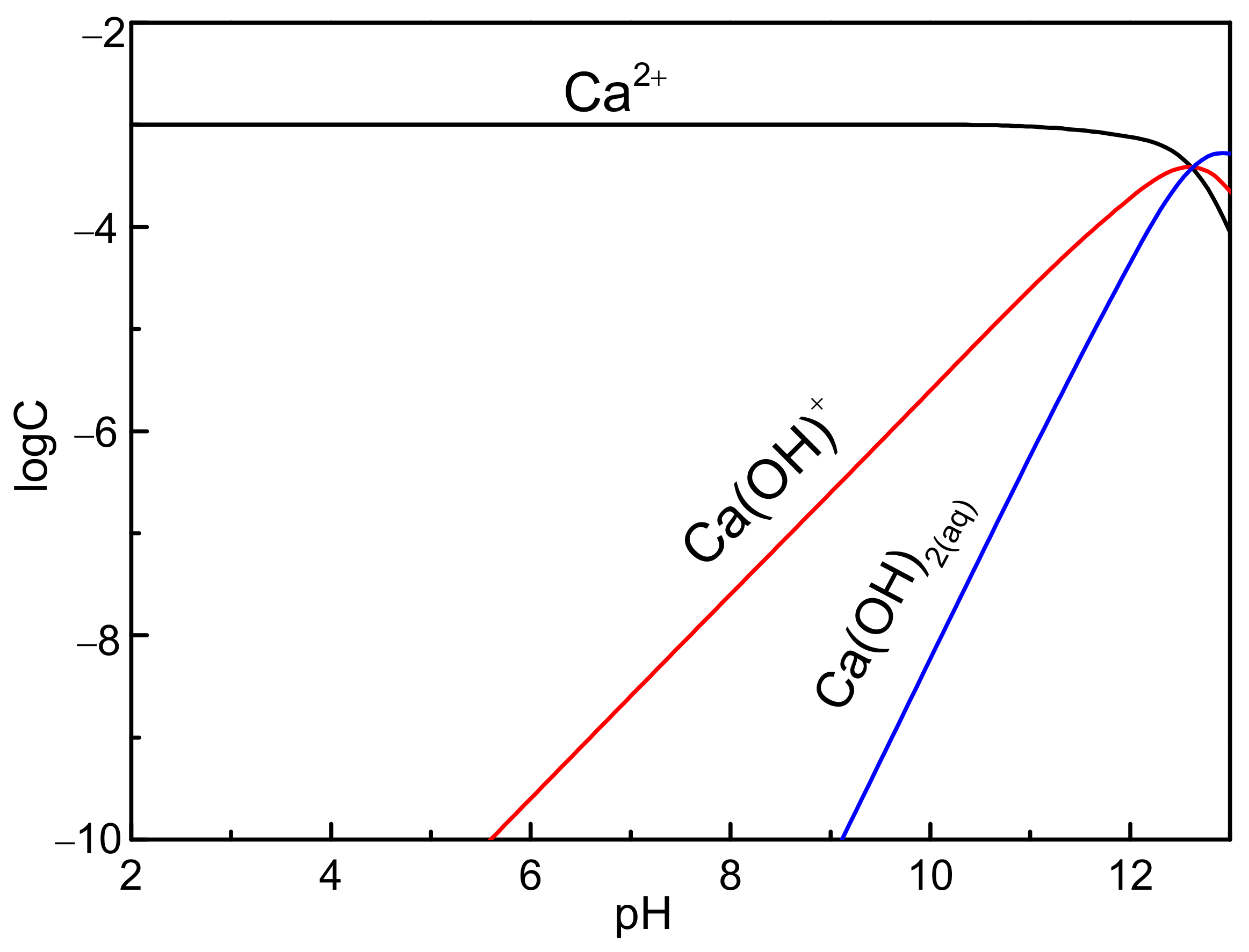
3.3. Zeta Potential Measurements
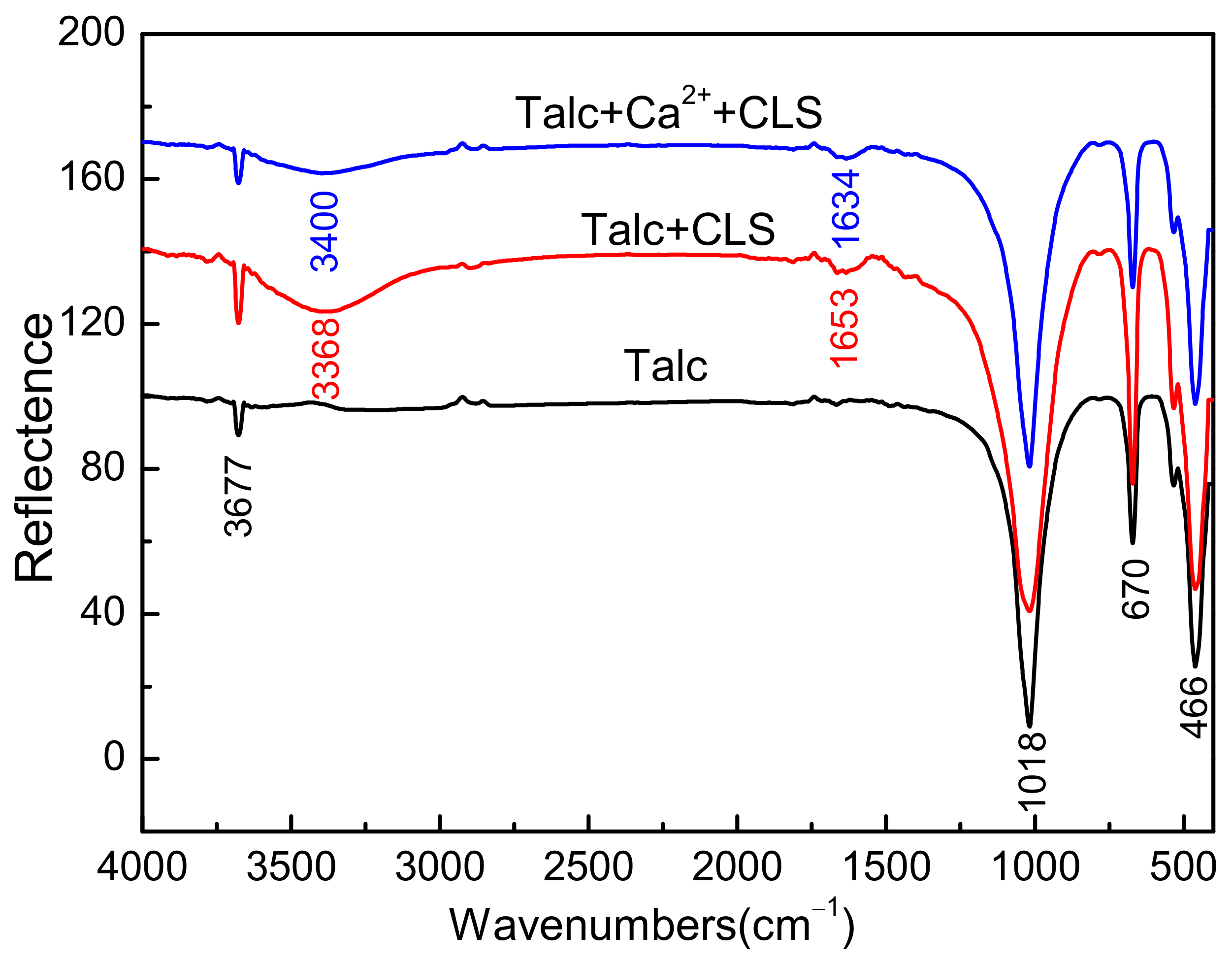
3.4. FTIR Measurements
3.5. AFM Imaging Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seck, G.S.; Hache, E.; Bonnet, C.; Simoën, M.; Carcanague, S. Copper at the crossroads: Assessment of the interactions between low-carbon energy transition and supply limitations. Resour. Conserv. Recycl. 2020, 163, 105072. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, G.; Chen, Y.; Huang, G.; Gao, Y. Investigations on the utilization of konjac glucomannan in the flotation separation of chalcopyrite from pyrite. Min. Eng. 2020, 145, 106089. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Khraisheh, M.; Holland, C.; Creany, C.; Harris, P.; Parolis, L. Effect of molecular weight and concentration on the adsorption of CMC onto talc at different ionic strengths. Int. J. Min. Process. 2005, 75, 197–206. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Beattie, D.A. The effect of impurities and cleavage character istics on talc hydrophobicity and polymer adsorption. J. Min. Process. 2013, 118, 34–42. [Google Scholar] [CrossRef]
- Morris, G.E.; Fornasiero, D.; Ralston, J. Polymer depressants at the talc-water interface: Adsorption isotherm, microflotation and electrokinetic studies. J. Min. Process. 2002, 67, 211–227. [Google Scholar] [CrossRef]
- Kursun, H.; Ulusoy, U. Influence of shape characteristics of talc mineral on the column flotation behavior. Int. J. Min. Process. 2005, 78, 262–268. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Beattie, D.A. Adsorption of tailored carboxymethyl cellulose polymers on talc and chalcopyrite: Correlation between coverage, wettability, and flotation. Min. Eng. 2010, 23, 985–993. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Feng, Q.; Zhang, G.; Chang, Z. The role of calcium and carbonate ions in the separation of pyrite and talc. Min. Eng. 2018, 119, 205–211. [Google Scholar] [CrossRef]
- Lotter, N.O.; Bradshaw, D.J.; Becker, M.; Parolis, L.A.S.; Kormos, L.J. A discussion of the occurrence and undesirable flotation behaviour of orthopyroxene and talc in the processing of mafic deposits. Min. Eng. 2008, 21, 905–912. [Google Scholar] [CrossRef]
- Zhao, K.; Gu, G.; Wang, C.; Rao, X.; Wang, X.; Xiong, X. The effect of a new polysaccha ride on the depression of talc and the flotation of a nickel-copper sulfide ore. Min. Eng. 2015, 77, 99–106. [Google Scholar] [CrossRef]
- Ilhwan, P.; Seunggwan, H.; Sanghee, J.; Mayumi, I.; Naoki, H. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation. Metals 2020, 10, 1667. [Google Scholar]
- Johnston, H.E. Reverse Flotation of a Chalcopyrite Concentrate. Bachelor’s Thesis, University of Queensland, St Lucia, Australia, 2005. [Google Scholar]
- Ahmed, M.M.; Ibrahim, G.A.; Hassan, M. Improvement of Egyptian talc quality for industrial uses by flotation process and leaching. Int. J. Min. Process. 2007, 83, 132–145. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Ndlovu, B.; Bradshaw, D. Behavior of talc and mica in copper ore flotation. Appl. Clay Sci. 2018, 160, 270–275. [Google Scholar] [CrossRef]
- Parolis, L.A.; van der Merwe, R.; Groenmeyer, G.V.; Harris, P.J. The influence of metal cations on the behaviour of carboxymethyl celluloses as talc depressants. Colloids Surf. A Phys. Eng. Asp. 2007, 317, 109–115. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, W.; Guo, Y.; Peng, J.; Ning, X.; Wang, H. Synergistic effect of acidified water glass and locust bean gum in the flotation of a refractory copper sulfide ore. J. Clean. Prod. 2018, 202, 1077–1084. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Shi, Q. Utilization of N-carboxymethyl Chitosan as a Selective Depressant for Talc In Flotation of Chalcopyrite. Min. Min. 2019, 55, 108–115. [Google Scholar]
- Pan, G.; Shi, Q.; Zhang, G. Selective depression of talc in chalcopyrite flotation by xanthan gum: Flotation response and adsorption mechanism. Colloids Surf. A Phys. Eng. Asp. 2020, 600, 124902. [Google Scholar] [CrossRef]
- Liu, C.; Ai, G.; Song, S. The effect of amino trimethylene phosphonic acid on the flotation separation of pentlandite from lizardite. Powder Technol. 2018, 336, 527–532. [Google Scholar] [CrossRef]
- Ma, X.; Pawlik, M. The effect of lignosulfonates on the floatability of talc. Int. J. Min. Process. 2007, 83, 1–2. [Google Scholar] [CrossRef]
- Lim, Z.Q.; Aziz, N.A.A.; Idris, A.K.; Akhir, N.A.M. Green Lignosulphonate as co-surfactant for wettability alteration. Pet. Res. 2020, 5, 154–163. [Google Scholar] [CrossRef]
- Liu, R.; Sun, W.; Hu, Y.; Wang, D. Effect of organic depressant lignosulfonate calcium on separation of chalcopyrite from pyrite. J. Cent. South Univ. Technol. 2012, 16, 753–757. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, G.; Song, S.; Li, H. Flotation separation of smithsonite from quartz using calcium lignosulphonate as a depressant and sodium oleate as a collector. Miner. Eng. 2019, 131, 385–391. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Ou, L.; Mai, Q. Flotation separation of chalcopyrite from talc using a new depressant carrageenan. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125274. [Google Scholar] [CrossRef]
- Paredes, Á.; Acuña, S.M.; Toledo, P.G. AFM Study of Pyrite Oxidation and Xanthate Adsorption in the Presence of Seawater Salts. Metals 2019, 9, 1177. [Google Scholar] [CrossRef]
- Le Guern, C.; Conil, P.; Houot, R. Role of calcium ions in the mechanism of action of a lignosulphonate used to modify the wettability of plastics for their separation by flotation. Miner. Eng. 2000, 13, 53–63. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Song, S.; Gao, H.; Niu, F.; Zhang, J.; Ai, G. Selective separation of hematite from quartz with sodium oleate collector and calcium lignosulphonate depressant. J. Mol. Liq. 2020, 322, 114502. [Google Scholar] [CrossRef]
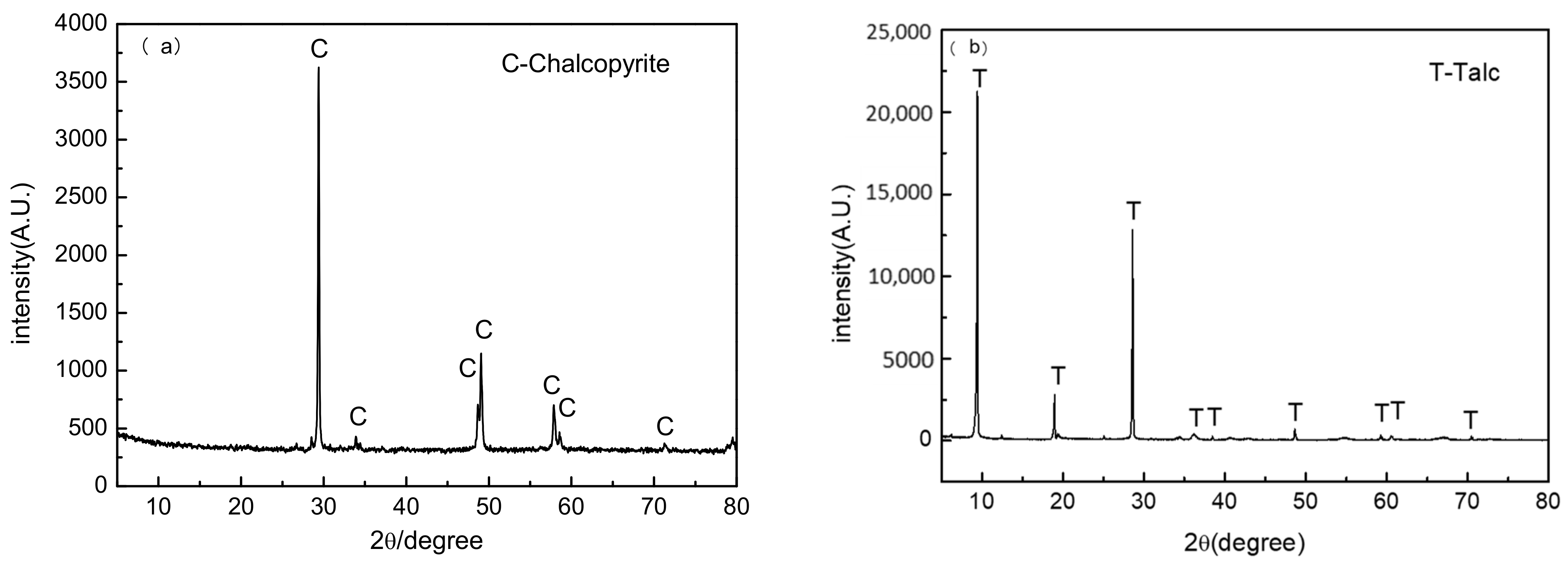
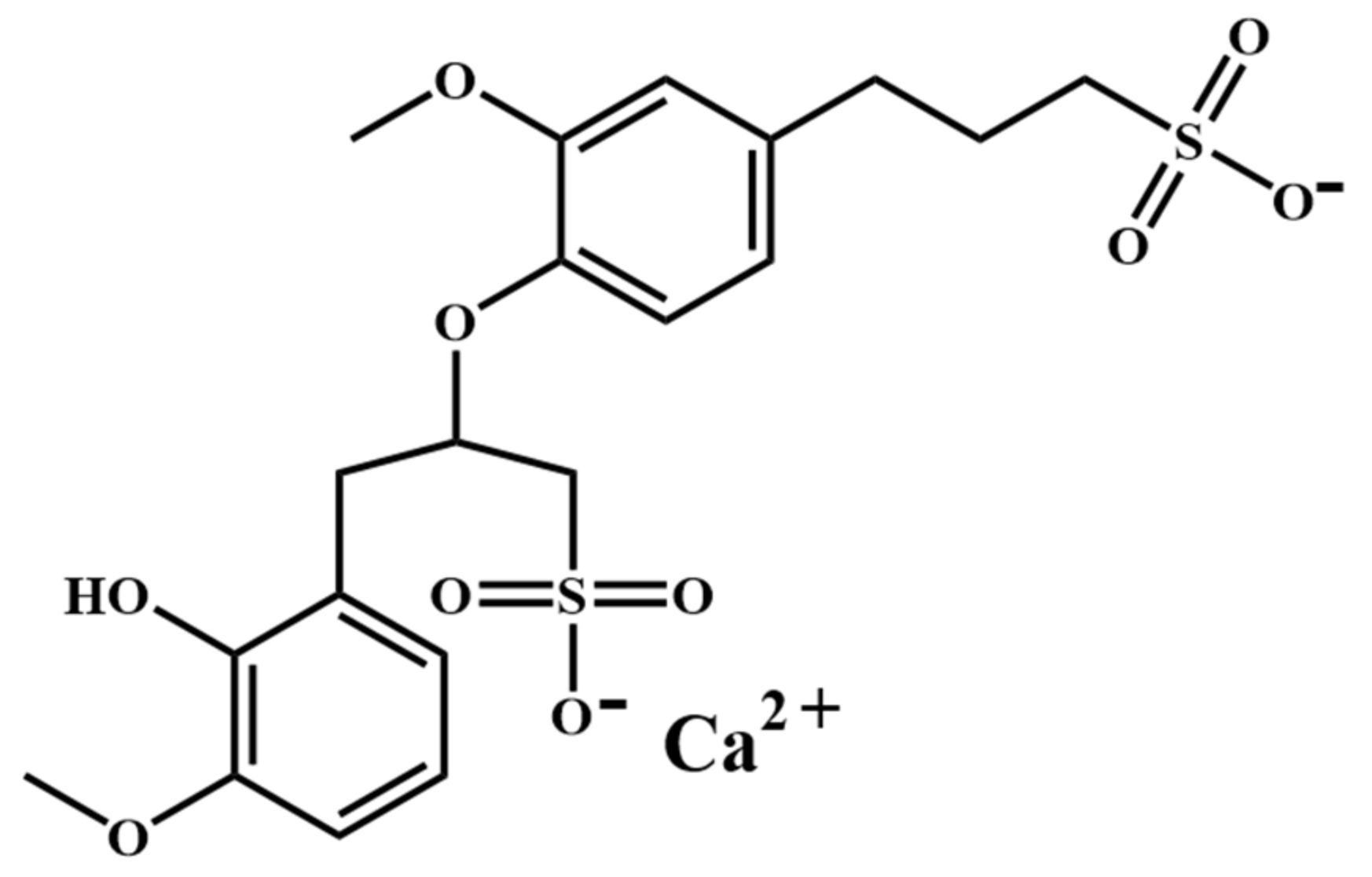


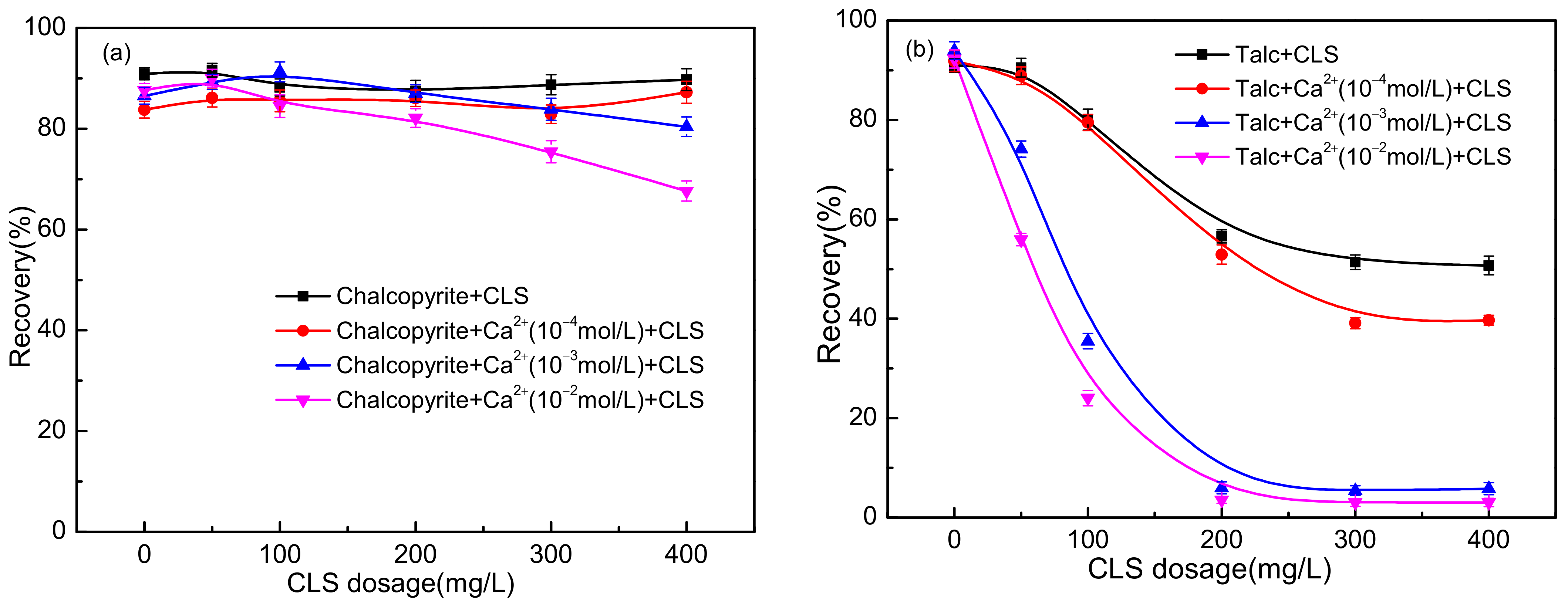
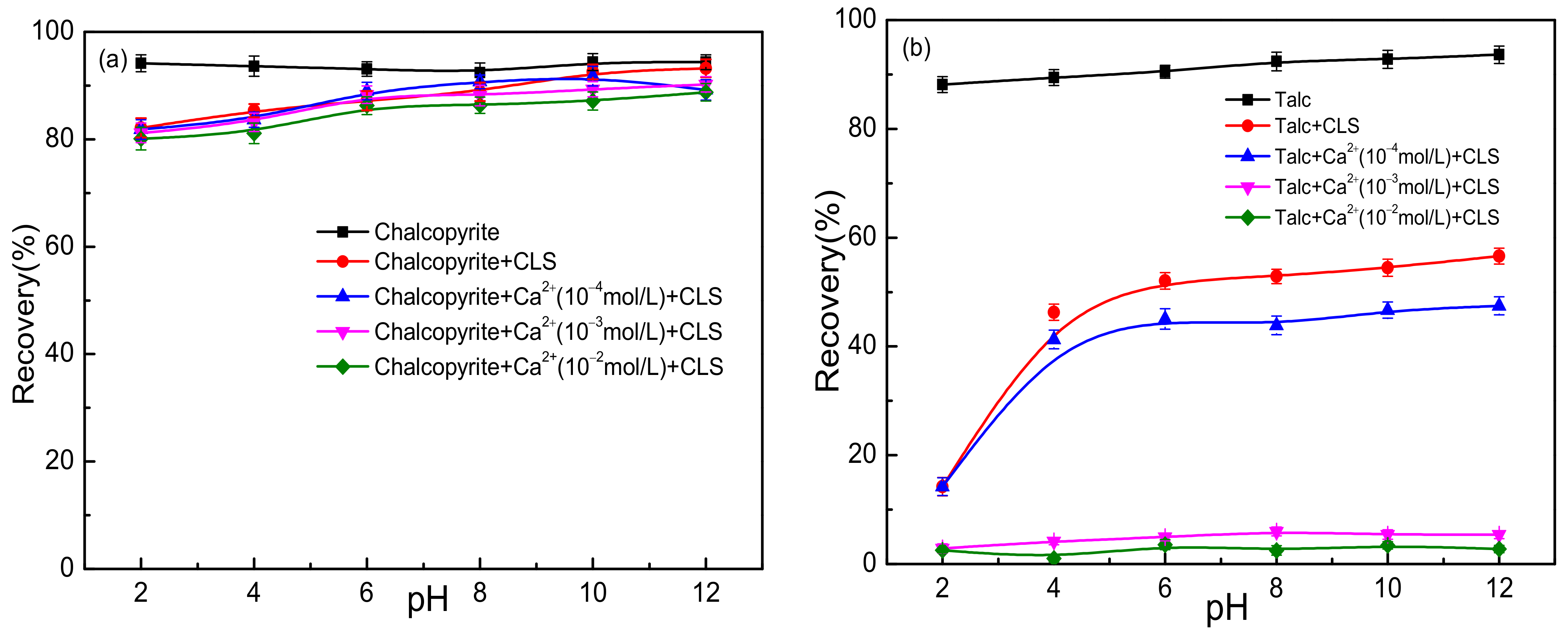


| Sample | Mass Fraction/% | |||||
|---|---|---|---|---|---|---|
| Cu | TFe | S | MgO | SiO2 | Al2O3 | |
| Chalcopyrite | 33.635 | 29.764 | 33.584 | - | 2.782 | - |
| Talc | - | 0.774 | - | 29.561 | 64.830 | 0.366 |
| Conditions | Product | Yield/% | Grade/% | Recovery/% | Separation Efficiency/% | ||
|---|---|---|---|---|---|---|---|
| Cu | MgO | Cu | MgO | ||||
| - | Concentrate | 81.5 | 15.3 | 17.8 | 88.7 | 79.2 | 2.3 |
| Tailings | 18.5 | 8.6 | 20.5 | 11.3 | 20.8 | ||
| Feed | 100.0 | 14.0 | 18.3 | 100.0 | 100.0 | ||
| CLS | Concentrate | 27.9 | 23.8 | 6.8 | 46.2 | 11.0 | 11.7 |
| Tailings | 72.1 | 10.8 | 21.1 | 53.8 | 89.0 | ||
| Feed | 100.0 | 14.4 | 17.1 | 100.0 | 100.0 | ||
| Ca2+ + CLS | Concentrate | 23.4 | 29.7 | 3.0 | 50.0 | 4.1 | 26.3 |
| Tailings | 76.6 | 9.0 | 21.7 | 50.0 | 95.9 | ||
| Feed | 100.0 | 13.9 | 17.3 | 100.0 | 100.0 | ||
| Samples | RMS (nm) ± 0.005 | PTV (nm) ± 0.005 | ΔPTV (nm) ± 0.005 | Coverage (%) |
|---|---|---|---|---|
| Bare talc | 0.275 | 0.176 | - | - |
| Talc + CLS | 1.17 | 1.007 | 0.831 | 76.75 |
| Talc + Ca2++ CLS | 1.27 | 1.091 | 0.915 | 91.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, Q.; Zhou, H.; Ou, L. Flotation Separation of Chalcopyrite and Talc Using Calcium Ions and Calcium Lignosulfonate as a Combined Depressant. Metals 2021, 11, 651. https://doi.org/10.3390/met11040651
Mai Q, Zhou H, Ou L. Flotation Separation of Chalcopyrite and Talc Using Calcium Ions and Calcium Lignosulfonate as a Combined Depressant. Metals. 2021; 11(4):651. https://doi.org/10.3390/met11040651
Chicago/Turabian StyleMai, Qiongyin, Hanyu Zhou, and Leming Ou. 2021. "Flotation Separation of Chalcopyrite and Talc Using Calcium Ions and Calcium Lignosulfonate as a Combined Depressant" Metals 11, no. 4: 651. https://doi.org/10.3390/met11040651
APA StyleMai, Q., Zhou, H., & Ou, L. (2021). Flotation Separation of Chalcopyrite and Talc Using Calcium Ions and Calcium Lignosulfonate as a Combined Depressant. Metals, 11(4), 651. https://doi.org/10.3390/met11040651





