Synthesis of Ag-La0.8Sr0.2MnO3 (LSM-Ag) Composite Powder and Its Application in Magnesium Air Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of LSM Powder
2.2. Preparation of LSM-Ag Composite Powder
2.3. Characterization of LSM-Ag Composite Powders
2.4. Electrocatalytic Performance Test of LSM-Ag Composite Powder
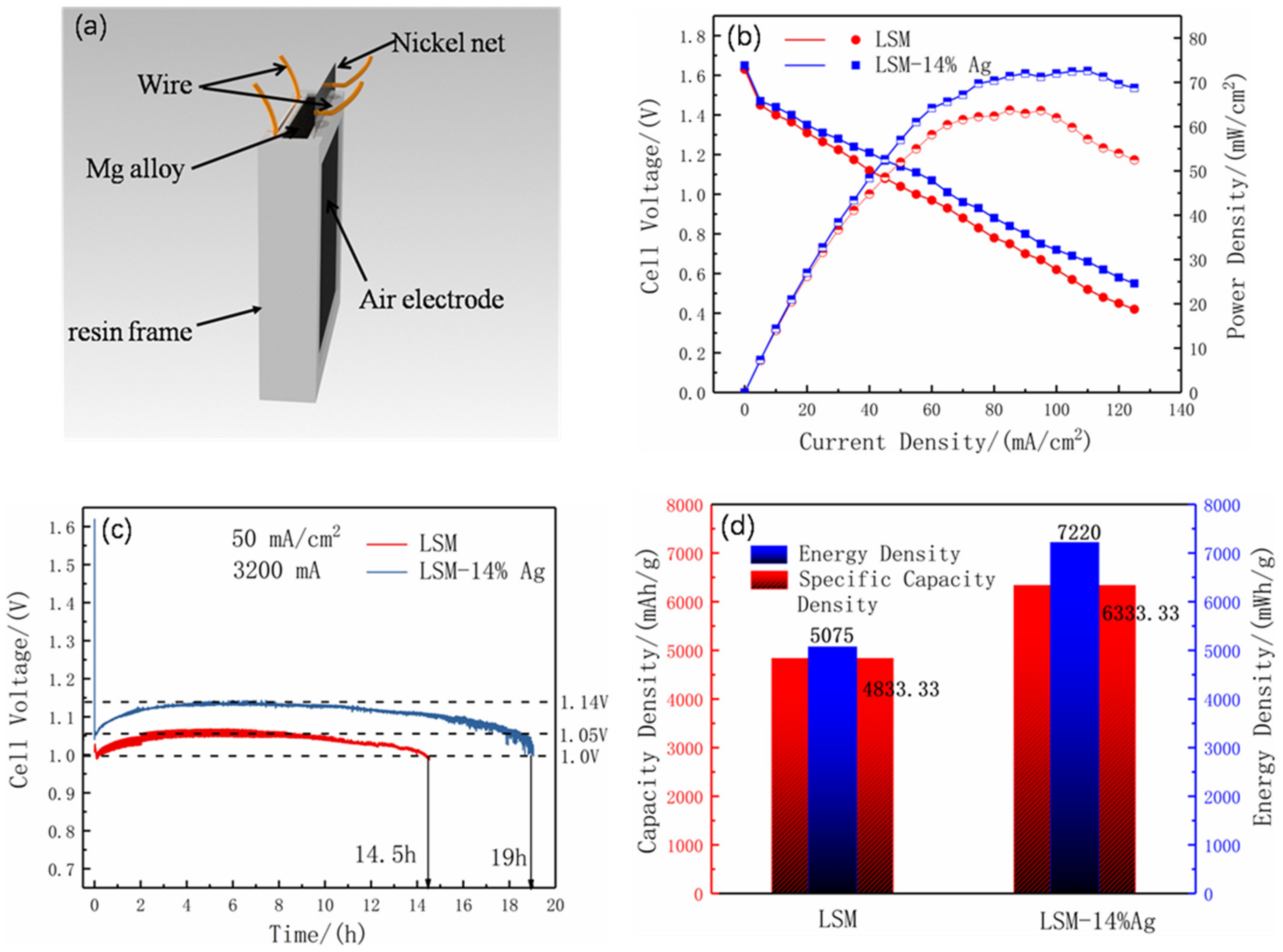
2.5. Assembly and Testing of Magnesium Air Battery
3. Results
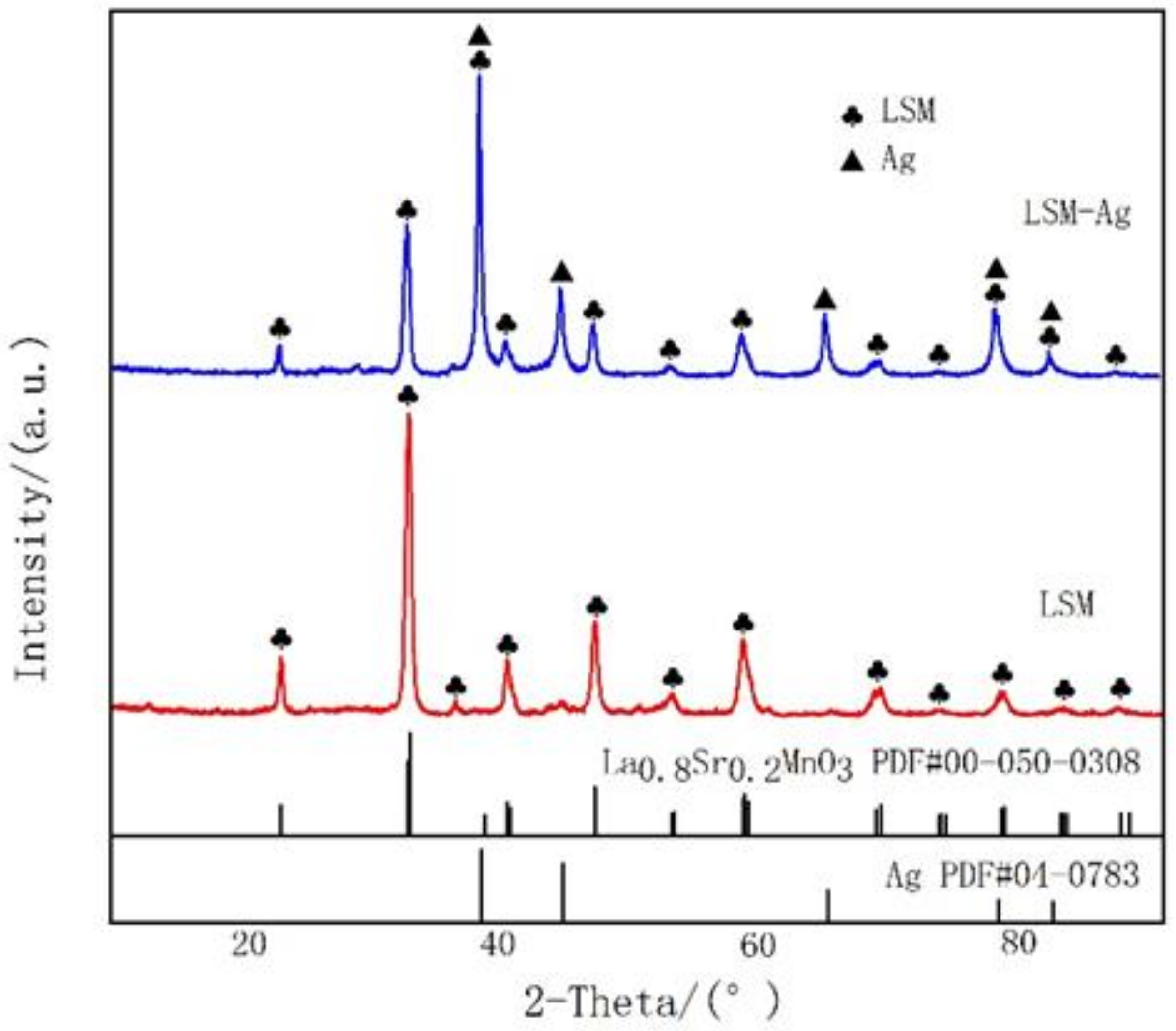
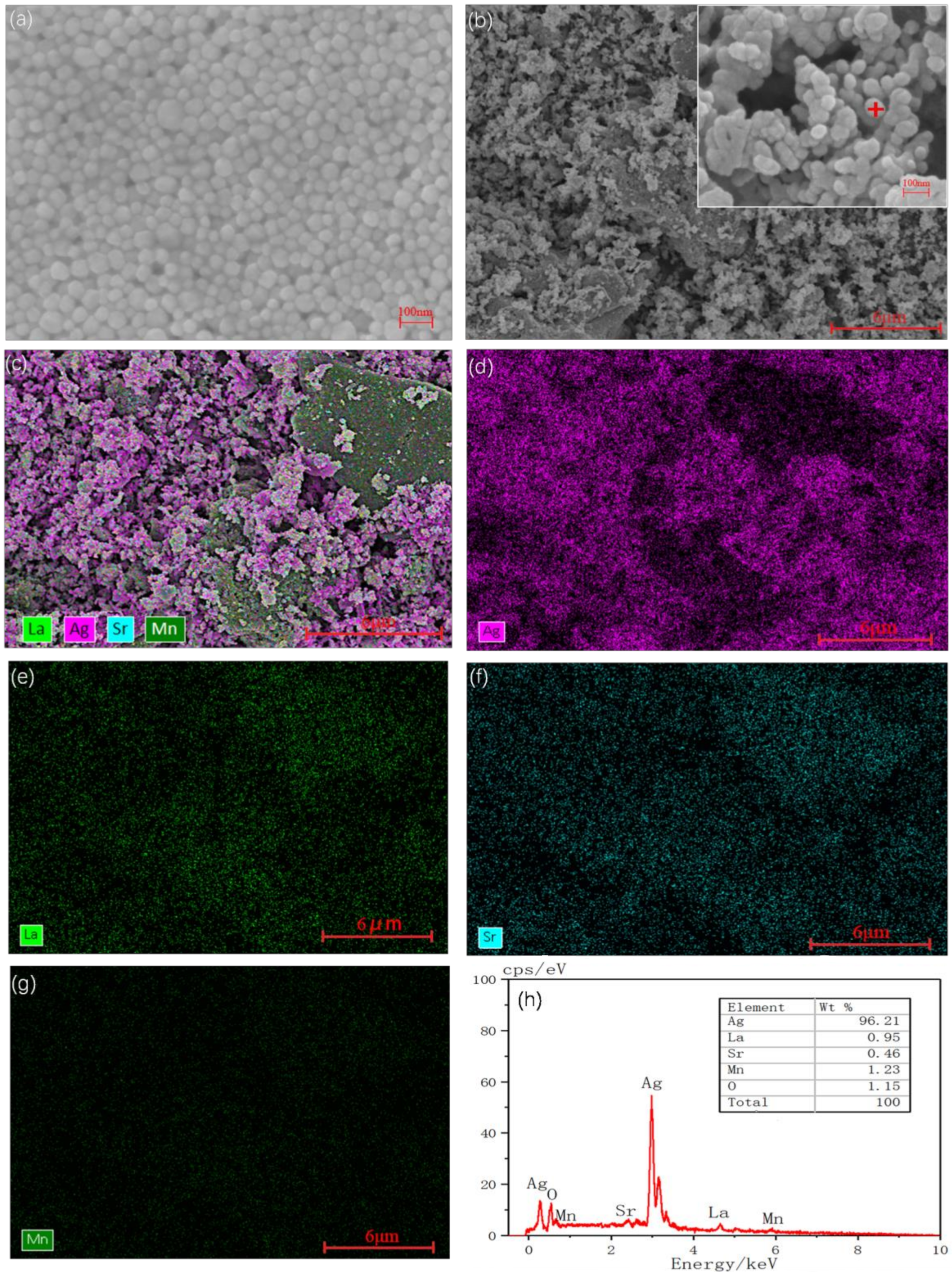
3.1. Microstructure Characterization
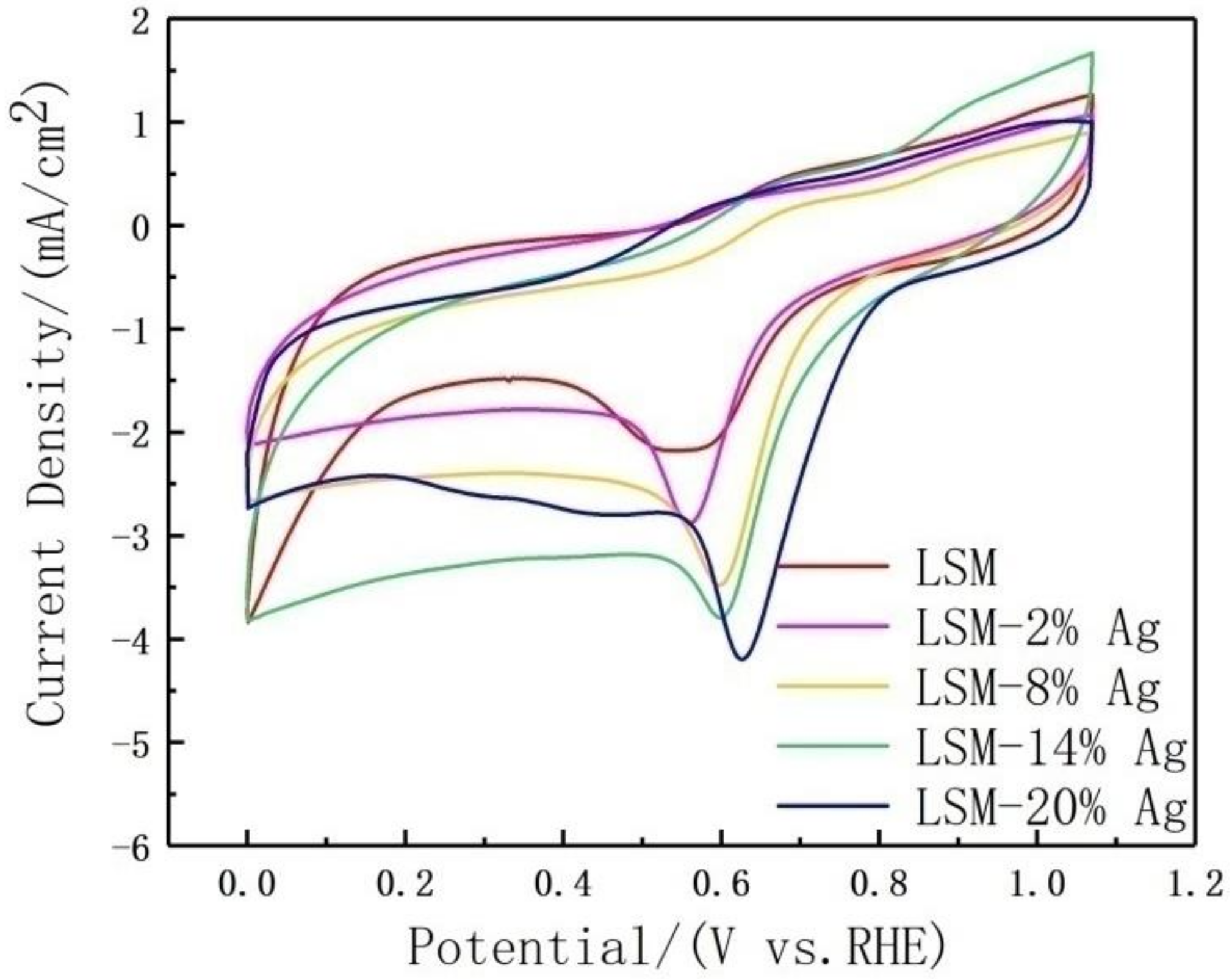
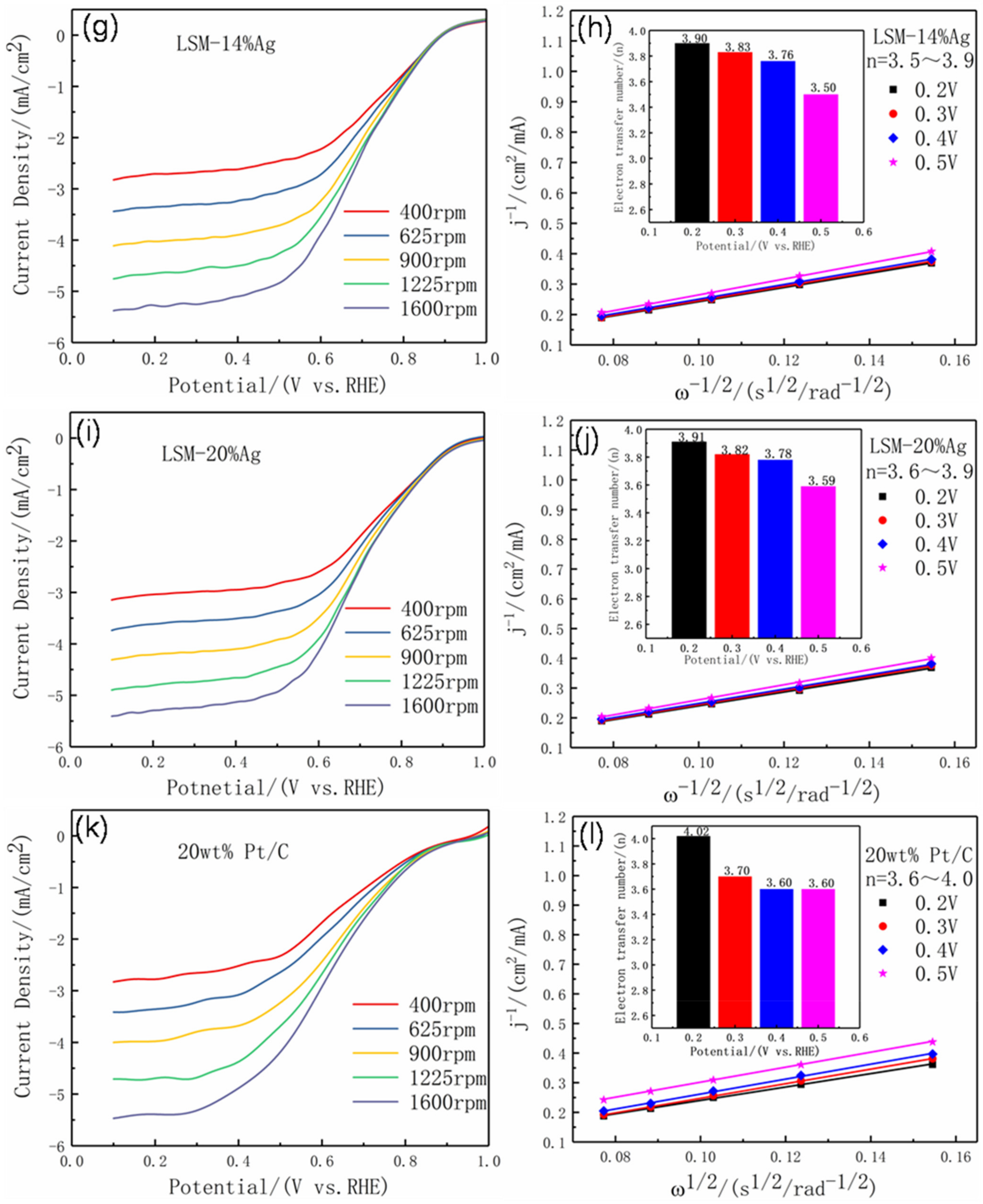
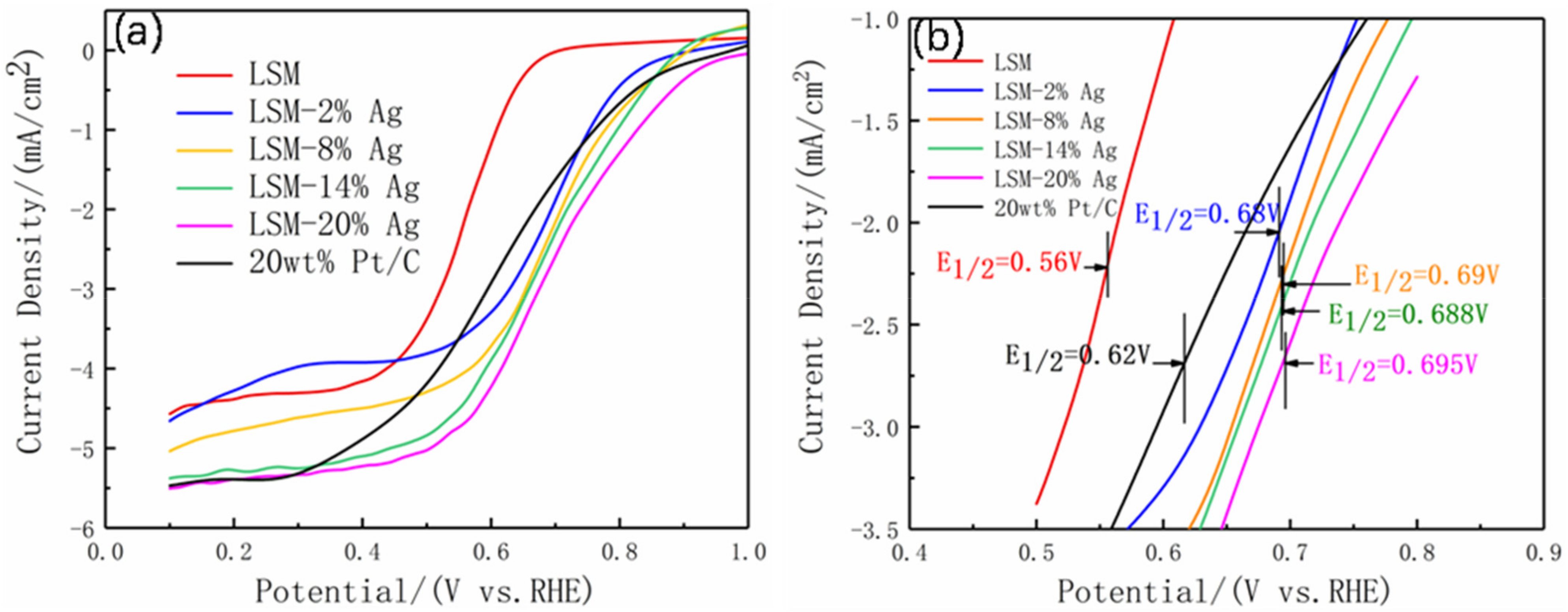
3.2. Electrochemical Performance
3.3. Magnesium-Air Battery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, F.W.; Chen, X.H.; Yu, J.; Ma, W.H.; Li, Y.P.; Ni, Y. Synthesis of three-dimensionally ordered porous perovskite type LaMnO3 for Al-air batter. J. Mater. Sci. Technol. 2018, 34, 1532–1537. [Google Scholar] [CrossRef]
- Jayasayee, K.; Veen, R.V.; Manivasagam, T.G.; Celebi, S.; Hensen, E.J.M.; Bruijn, F.A.D. Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: Influence of particle size and non-noble metals. Appl. Catal. B-Environ. 2012, 111, 515–526. [Google Scholar] [CrossRef]
- Tang, Q.W.; Wang, L.M.; Wu, M.J.; Xu, N.N.; Jiang, L.; Qiao, J.L. Achieving high-powered Zn/air fuel cell through N and S co-doped hierarchically porous carbons with tunable active-sites as oxygen electrocatalysts. J. Power Sources 2017, 365, 348–353. [Google Scholar] [CrossRef]
- Li, X.M.; Dong, F.; Xu, N.N.; Zhang, T.; Li, K.X.; Qiao, J.L. Co3O4/MnO2/hierarchically porous carbon as superior bifunctional electrodes for liquid and all-solid-state rechargeable zinc-air batteries. ACS Appl. Mater. Int. 2018, 10, 15591–15601. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, K.X.; Qiu, Y.F.; Liu, X.Z.; Cao, C.Y.; Feng, Y.J.; Hu, P.A. Nitrogen and sulfur co-doped porous carbon derived from bio-waste as a promising electrocatalyst for zinc-air battery. Energy 2018, 143, 43–55. [Google Scholar] [CrossRef]
- Li, L.Q.; Yang, J.; Yang, H.B.; Zhang, L.P.; Shao, J.J.; Huang, W.; Liu, B.; Dong, X.C. Anchoring Mn3O4 nanoparticles on oxygen functionalized carbon nanotubes as bifunctional catalyst for rechargeable zinc-air battery. ACS Appl. Energy Mater. 2018, 1, 963–969. [Google Scholar] [CrossRef]
- Li, G.; Mezaal, M.A.; Zhang, R.; Zhang, K.; Lei, L. Electrochemical Performance of MnO2-based Air Cathodes for Zinc-air Batteries. Fuel Cells 2016, 16, 395–400. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, A.P.; Ahmed, R.; Wang, H.J.; Li, H.; Chen, Z.W. Manganese dioxide nanotube and nitrogen-doped carbon nanotube based composite bifunctional catalyst for rechargeable zinc-air battery. Electrochim. Acta 2012, 69, 295–300. [Google Scholar] [CrossRef]
- Wu, X.; Scott, K. A non-precious metal bifunctional oxygen electrode for alkaline anion exchange membrane cells. J. Power Sources 2012, 206, 14–19. [Google Scholar] [CrossRef]
- De Koninck, M.; Marsan, B. MnxCu1-xCo2O4 used as bifunctional electrocatalyst in alkaline medium. Electrochim. Acta 2008, 53, 7012–7021. [Google Scholar] [CrossRef]
- Chen, D.J.; Chen, C.; Baiyee Zarah, M.; Shao, Z.P.; Ciucci, F. Nonstoichiometric oxides as low-cost and highly-efficient oxygen reduction/evolution catalysts for low-temperature electrochemical devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef]
- Hardin, W.G.; Mefford, J.T.; Slanac, D.A.; Patel, B.B.; Wang, X.Q.; Dai, S.; Zhao, X.; Ruoff, R.S.; Johnston, K.P.; Stevenson, K.J. Tuning the electrocatalytic activity of perovskites through active site variation and support interactions. Chem. Mater. 2014, 26, 3368–3376. [Google Scholar] [CrossRef]
- Lopez, K.; Park, G.; Sun, H.-J.; An, J.-C.; Eom, S.; Shim, J. Electrochemical characterizations of LaMO3 (M = Co, Mn, Fe, and Ni) and partially substituted LaNixM1−xO3 (x = 0.25 or 0.5) for oxygen reduction and evolution in alkaline solution. J. Appl. Electrochem. 2015, 45, 313–323. [Google Scholar] [CrossRef]
- Seo, M.H.; Park, H.W.; Lee, D.U.; Park, M.G.; Chen, Z.W. Design of Highly Active Perovskite Oxides for Oxygen Evolution Reaction by Combining Experimental and ab Initio Studies. ACS Catal. 2015, 5, 4337–4344. [Google Scholar] [CrossRef]
- Zhou, W.; Sunarso, J. Enhancing Bi-functional Electrocatalytic Activity of Perovskite by Temperature Shock: A Case Study of LaNiO3−δ. J. Phys. Chem. Lett. 2013, 4, 2982–2988. [Google Scholar] [CrossRef]
- Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature Solid Oxide Fuel Cells: A brief review. J. Power Sources 2015, 298, 46–67. [Google Scholar] [CrossRef]
- Uchida, H.; Arisaka, S.; Watanabe, M. High performance electrodes for mediumtemperature solid oxide fuel cells: Activation of La(Sr)CoO cathode with highly dispersed-Pt metal electrocatalysts. Solid State Ion. 2000, 135, 347–351. [Google Scholar] [CrossRef]
- Uchida, H.; Watanabe, M. High-Performance Electrodes for Medium-Temperature Solid Oxide Fuel Cells. Mod. Asp. Electrochem. 2008, 149, 53–87. [Google Scholar] [CrossRef]
- Chen, K.; Ai, N.; Jiang, S.P. Enhanced electrochemical performance and stability of (La,Sr)MnO3-(Gd,Ce)O2 oxygen electrodes of solid oxide electrolysis cells by palladium infiltration. Int. J. Hydrog. Energy 2012, 37, 1301–1310. [Google Scholar] [CrossRef]
- Wang, L.S.; Barnett, S.A. Ag-perovskite cermets for thin film solid oxide fuel cell air-electrode applications. Solid State Ion. 1995, 76, 103–113. [Google Scholar] [CrossRef]
- Sholklapper, T.Z.; Radmilovic, V.; Jacobson, C.P.; Visco, S.J.; Jonghe, L.D. Nanocomposite Ag-LSM solid oxide fuel cell electrodes. J. Power Sources 2008, 175, 206–210. [Google Scholar] [CrossRef]
- Lu, F.; Wang, Y.; Jin, C.; Li, F.; Yang, R.; Chen, F. Microporous La0.8Sr0.2MnO3 perovskite nanorods as efficient electrocatalysts for lithium–air battery. J. Power Sources 2015, 293, 726–733. [Google Scholar] [CrossRef]
- Chen, B.; Cheng, D.; Zhu, J. Synthesis of PtCu nanowires in nonaqueous solvent with enhanced activity and stability for oxygen reduction reaction. J. Power Sources 2014, 267, 380–387. [Google Scholar] [CrossRef]
- Goh FW, T.; Liu, Z.; Ge, X.; Zong, Y.; Du, G.; Hor, T.S.A. Ag nanoparticle-modified MnO2 nanorods catalyst for use as an air electrode in zinc–air battery. Electrochim. Acta 2013, 114, 598–604. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Lou, Z.; Qiao, J.; Ren, B.; Sun, K. Preparation and characterization of silver-modified La0.8Sr0.2MnO3 cathode powders for solid oxide fuel cells by chemical reduction method. Int. J. Hydrog. Energy 2013, 38, 1074–1081. [Google Scholar] [CrossRef]
- Van Cleve, T.; Gibara, E.; Linic, S. Electrochemical Oxygen Reduction Reaction on Ag Nanoparticles of Different Shapes. ChemCatChem 2015, 8, 256–261. [Google Scholar] [CrossRef]
- Hu, J.; Shi, L.; Liu, Q.; Huang, H.; Jiao, T. Improved oxygen reduction activity on silver-modified LaMnO3–graphene via shortens the conduction path of adsorbed oxygen. RSC Adv. 2015, 5, 92096–92106. [Google Scholar] [CrossRef]
- Phuoca, L.T.; Viet, C.D.; Doh, W.H.; Bonnefont, A.; Janowska, I.; Begin, D.; Savinova, E.R.; Granger, P.; Huu, C.P. Influence of the reaction temperature on the oxygen reduction reaction on nitrogen-doped carbon nanotube catalysts. Catal. Today 2015, 249, 236–243. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, M.G.; Park, H.W.; Seo, M.H.; Ismayilov, V.; Ahmed, R.; Chen, Z. Highly active Co-doped LaMnO3 perovskite oxide and N-doped carbon nanotube hybrid bi-functional catalyst for rechargeable zinc–air batteries. Electrochem. Commun. 2015, 60, 38–41. [Google Scholar] [CrossRef]
- Rahman, A.; Wang, X.; Wen, C.; Rani, J.V.; Kanakaiah, V.; Dadmal, T.; Rao, M.S.; Bhavanarushi, S. High Energy Density Metal-Air Batteries: A Review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Heuveln, F.H.; Bouwmeester, H.J.M. Electrode Properties of Sr-Doped LaMnO3 on Yttria-Stabilized Zirconia II: Electrode kinetics. J. Electrochem. Soc. 1997, 144, 134–140. [Google Scholar] [CrossRef]
- Sasaki, K.; Hosoda, K.; Lan, T.; Yasumoto, K.; Wang, S.; Dokiya, M. Ag–Zr(Sc)O cermet cathode for reduced temperature SOFCs. Solid State Ion. 2004, 174, 97–102. [Google Scholar] [CrossRef]
- Lee, K.T.; Manthiram, A. Electrochemical performance of Nd0.6Sr0.4Co0.5Fe0.5O3−δ–Ag composite cathodes in intermediate temperatur solid oxide fuel cells. J. Power Sources 2006, 160, 903–908. [Google Scholar] [CrossRef]
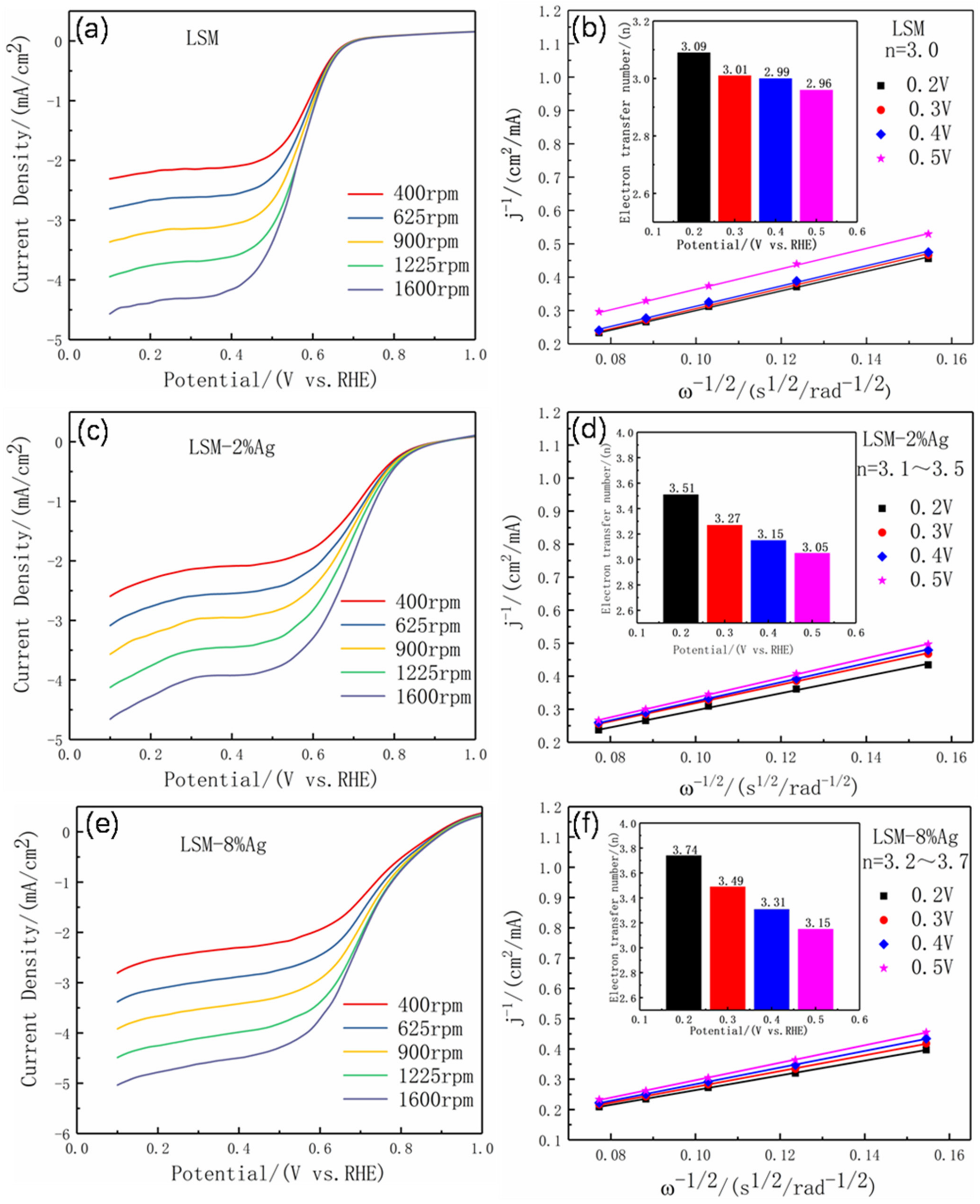
| Sample | Eonset | EHALF-WAVE | Jlim | n | Refs |
|---|---|---|---|---|---|
| (V vs. RHE) | (V vs. RHE) | (mA/cm2) | |||
| LSM | 0.70 | 0.56 | −4.62 | 3.09 | This work |
| LSM-2%Ag | 0.88 | 0.68 | −4.73 | 3.51 | |
| LSM-8%Ag | 0.89 | 0.69 | −5.01 | 3.74 | |
| LSM-14%Ag | 0. 91 | 0.688 | −5.42 | 3.90 | |
| LSM-20%Ag | 0.90 | 0.695 | −5.54 | 3.91 | |
| 20 wt% Pt/C | 0.96 | 0.62 | −5.5 | 4.02 | |
| LaMnO3 | 0.728 | 0.558 | −3.14 | 2.63 | [29] |
| LSM nanorods | 0.785 | 0.638 | −4.54 | 3.01 | [22] |
| Ag/LaMnO3 | 0.85 | 0.725 | −5.13 | 3.42 | [26] |
| Ag/LaMnO3-RGO | 0.81 | 0.58 | −5.42 | 3.90 | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Liu, H.; Zhang, J.; Song, J.; Huang, J.; Xu, W.; Yan, Y.; Yu, K. Synthesis of Ag-La0.8Sr0.2MnO3 (LSM-Ag) Composite Powder and Its Application in Magnesium Air Battery. Metals 2021, 11, 633. https://doi.org/10.3390/met11040633
Wu X, Liu H, Zhang J, Song J, Huang J, Xu W, Yan Y, Yu K. Synthesis of Ag-La0.8Sr0.2MnO3 (LSM-Ag) Composite Powder and Its Application in Magnesium Air Battery. Metals. 2021; 11(4):633. https://doi.org/10.3390/met11040633
Chicago/Turabian StyleWu, Xiaohan, Hui Liu, Jiaxi Zhang, Juemin Song, Jiefeng Huang, Wanli Xu, Yang Yan, and Kun Yu. 2021. "Synthesis of Ag-La0.8Sr0.2MnO3 (LSM-Ag) Composite Powder and Its Application in Magnesium Air Battery" Metals 11, no. 4: 633. https://doi.org/10.3390/met11040633
APA StyleWu, X., Liu, H., Zhang, J., Song, J., Huang, J., Xu, W., Yan, Y., & Yu, K. (2021). Synthesis of Ag-La0.8Sr0.2MnO3 (LSM-Ag) Composite Powder and Its Application in Magnesium Air Battery. Metals, 11(4), 633. https://doi.org/10.3390/met11040633






