Properties of Passive Films Formed on Ferrite-Martensite and Ferrite-Pearlite Steel Microstructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Microstructure Characterisation
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Microstructure Characterisation
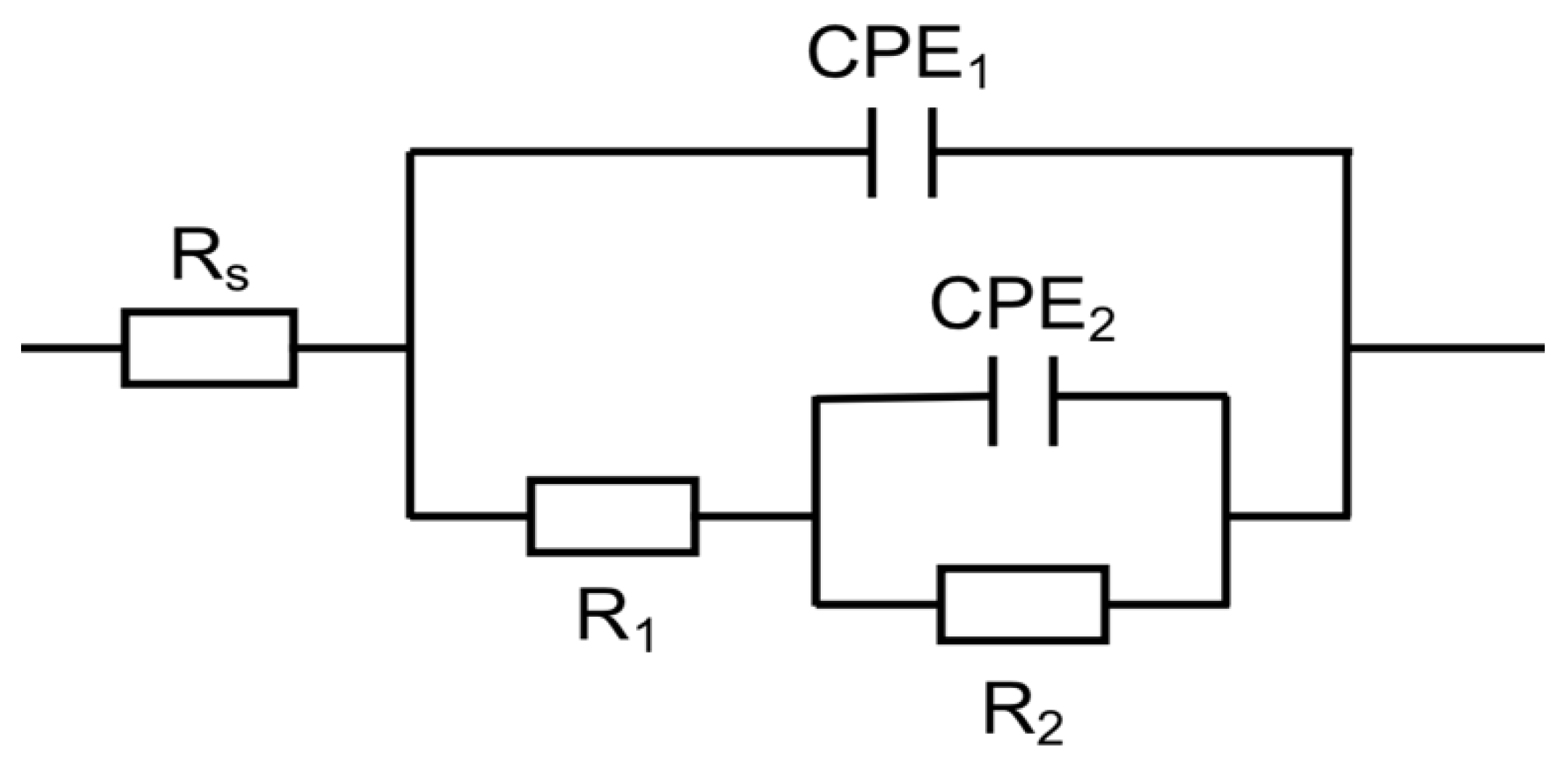
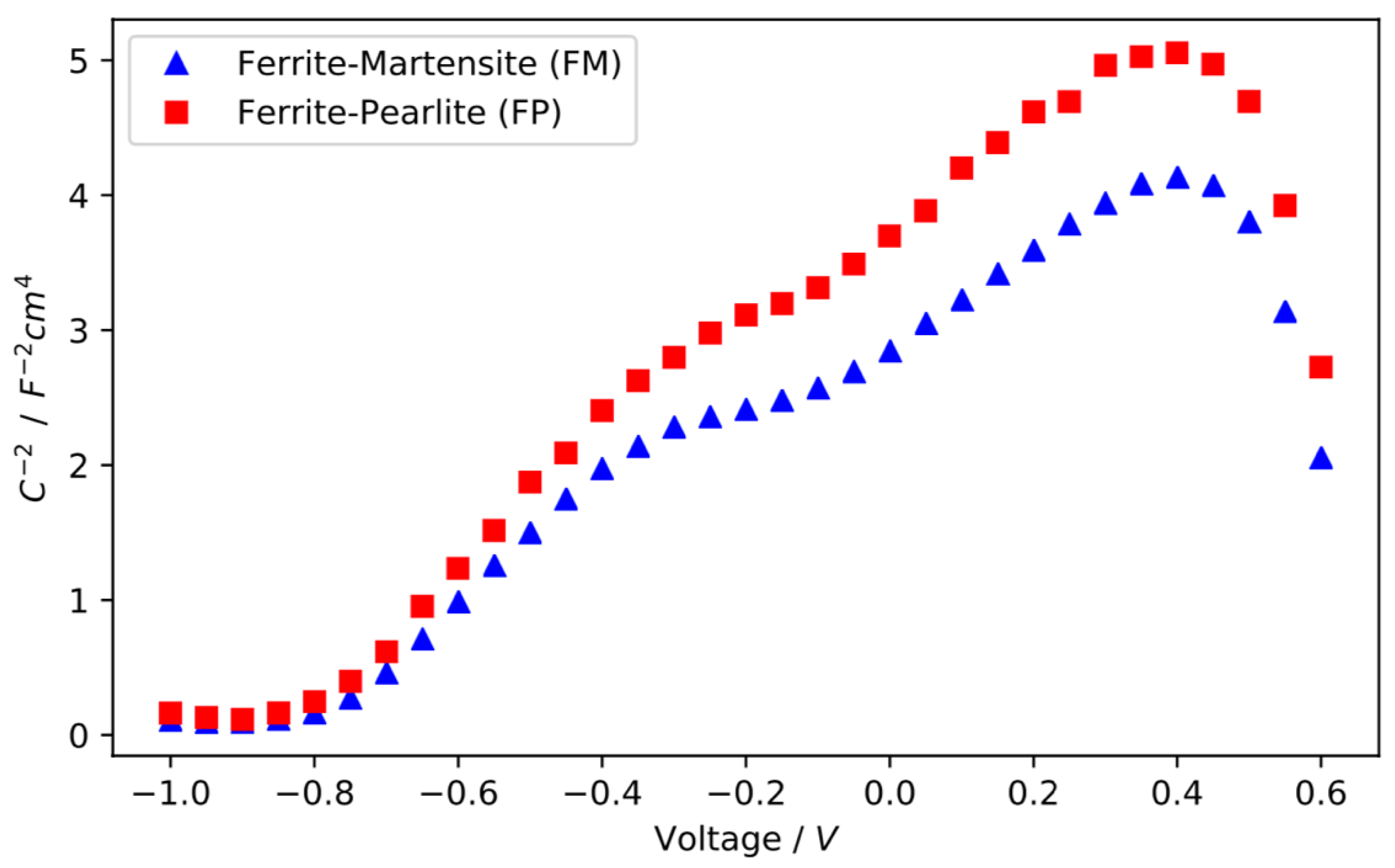
3.2. Electrochemical Characterisation of the Passive Layer
4. Conclusions
- (a)
- The current density during steady-state potentiostatic polarisation is higher for the ferrite-martensite microstructure than for the ferrite-pearlite microstructure. The evolution of the logarithmic slopes with time indicates an immense degree of ferric to ferrous oxide transformation for the passive film formed on the ferrite-martensite microstructure.
- (b)
- Electrochemical Impedance Spectroscopy measurements of the passive layers show a similar electrochemical response with two-time constants for both microstructures; however, the passive layer of the ferrite-pearlite microstructure displays a distinctly more resistive behaviour.
- (c)
- The ferrite-martensite microstructure passive layer has a higher donor density than the layer on the ferrite-pearlite microstructure, which results in a more defective film.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sietsma, J. Physical modelling the microstructure formation in advanced high-strength steels. Mater. Sci. Forum 2013, 762, 194–209. [Google Scholar] [CrossRef]
- Kalhor, A.; Soleimani, M.; Mirzadeh, H.; Uthaisangsuk, V. A review of recent progress in mechanical and corrosion properties of dual phase steels. Arch. Civ. Mech. Eng. 2020, 20, 85. [Google Scholar] [CrossRef]
- Astafurova, E.G.; Zakharova, G.G.; Naydenkin, E.V.; Raab, G.I.; Dobatkin, S. V Structure and mechanical properties of low-carbon ferrite-pearlite steel after severe plastic deformation and subsequent high-temperature annealing. Phys. Mesomech. 2011, 14, 195–203. [Google Scholar] [CrossRef]
- Fushimi, K.; Nakagawa, R.; Kitagawa, Y.; Hasegawa, Y. Micro- and Nano-Scopic Aspects of Passive Surface on Pearlite Structure of Carbon Steel in pH 8.4 Boric Acid-Borate Buffer. J. Electrochem. Soc. 2019, 166, C3409–C3416. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Nakanishi, T.; Hasegawa, Y.; Fushimi, K. Passivity of dual-phase carbon steel with ferrite and martensite phases in pH 8.4 boric acid-borate buffer solution. J. Electrochem. Soc. 2015, 162, C322–C326. [Google Scholar] [CrossRef]
- Takabatake, Y.; Kitagawa, Y.; Nakanishi, T.; Hasegawa, Y.; Fushimi, K. Grain Dependency of a Passive Film Formed on Polycrystalline Iron in {pH} 8.4 Borate Solution. J. Electrochem. Soc. 2017, 164, C349–C355. [Google Scholar] [CrossRef]
- Macdonald, D.D. PassivityÐthe key to our metals-based civilization. Pure Appl. Chem 1999, 71, 951–978. [Google Scholar] [CrossRef]
- Burstein, G.T.; Davenport, A.J. The Current-Time Relationship during Anodic Oxide Film Growth under High Electric Field. J. Electrochem. Soc. 1989, 136, 936–941. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fushimi, K.; Miura, S.; Konno, H. Influence of Substrate Dislocation on Passivation of Pure Iron in pH 8.4 Borate Buffer Solution. J. Electrochem. Soc. 2010, 157, 231–237. [Google Scholar] [CrossRef]
- Freire, L.; Nóvoa, X.R.; Montemor, M.F.; Carmezim, M.J. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Joiret, S.; Keddam, M.; Nóvoa, X.R.; Pérez, M.C.; Rangel, C.; Takenouti, H. Use of EIS, ring-disk electrode, EQCM and Raman spectroscopy to study the film of oxides formed on iron in 1 M NaOH. Cem. Concr. Compos. 2002, 24, 7–15. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Concernng the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Azumi, K. Mott-Schottky Plot of the Passive Film Formed on Iron in Neutral Borate and Phosphate Solutions. J. Electrochem. Soc. 1987, 134, 1352. [Google Scholar] [CrossRef]
- Azumi, K.; Ohtsuka, T.; Sato, N. Impedance of Iron Electrode Passivated in Borate and Phosphate Solutions. Trans. Jpn. Inst. Met. 1986, 27, 382–392. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Frese, K.W. Flatband Potentials and Donor Densities of Polycrystalline α-Fe2O3 Determined from Mott-Schottky Plots. J. Electrochem. Soc. 1978, 125, 723–726. [Google Scholar] [CrossRef]
- Wielant, J.; Goossens, V.; Hausbrand, R.; Terryn, H. Electronic properties of thermally formed thin iron oxide films. Electrochim. Acta 2007, 52, 7617–7625. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Characterisation of passive films formed on low carbon steel in borate buffer solution (pH 9.2) by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2005, 252, 1510–1519. [Google Scholar] [CrossRef]
- Schmuki, P.; Böhni, H. Illumination effects on the stability of the passive film on iron. Electrochim. Acta 1995, 40, 775–783. [Google Scholar] [CrossRef]
- Schottky, W. Halbleitertheorie der Sperrschicht. Naturwissenschaften 1938, 26, 843. [Google Scholar] [CrossRef]
- Yilmaz, A.; Li, X.; Pletincx, S.; Hauffman, T.; Sietsma, J.; Gonzalez-Garcia, Y. Effect of microstructural defects on passive layer properties of interstitial free (IF) ferritic steels in alkaline environment. Corros. Sci. 2021, 182, 109271. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Takabatake, Y.; Fushimi, K.; Nakanishi, T.; Hasegawa, Y. Grain-Dependent Passivation of Iron in Sulfuric Acid Solution. J. Electrochem. Soc. 2014, 161, C594–C600. [Google Scholar] [CrossRef]
- Takabatake, Y.; Kitagawa, Y.; Nakanishi, T.; Hasegawa, Y.; Fushimi, K. Heterogeneity of a Thermal Oxide Film Formed on Polycrystalline Iron Observed by Two-Dimensional Ellipsometry. J. Electrochem. Soc. 2016, 163, C815–C822. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 3527302743. [Google Scholar]
- Moon, A.P.; Sangal, S.; Layek, S.; Giribaskar, S.; Mondal, K. Corrosion Behavior of High-Strength Bainitic Rail Steels. Metall. Mater. Trans. A 2015, 46, 1500–1518. [Google Scholar] [CrossRef]
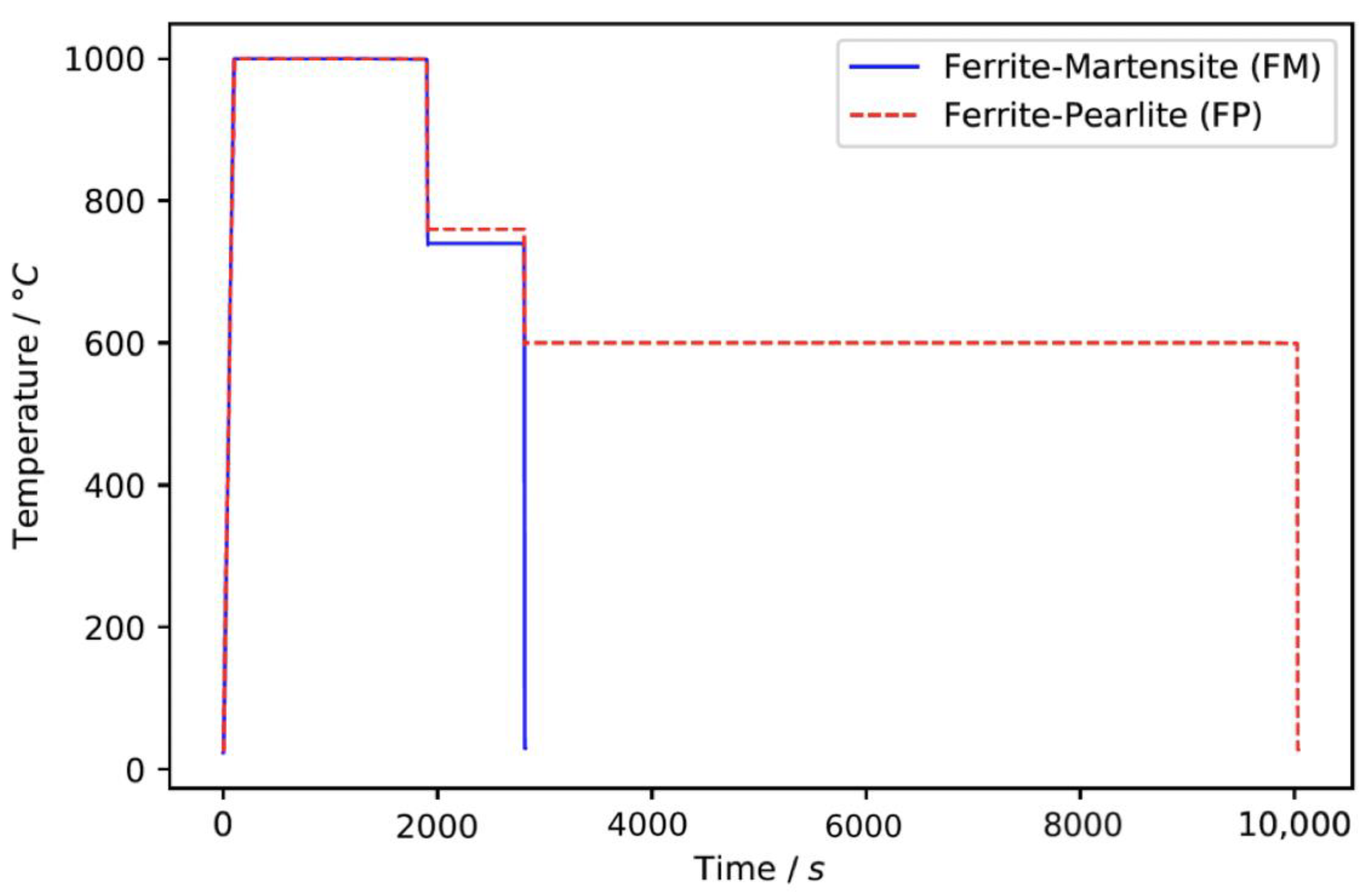
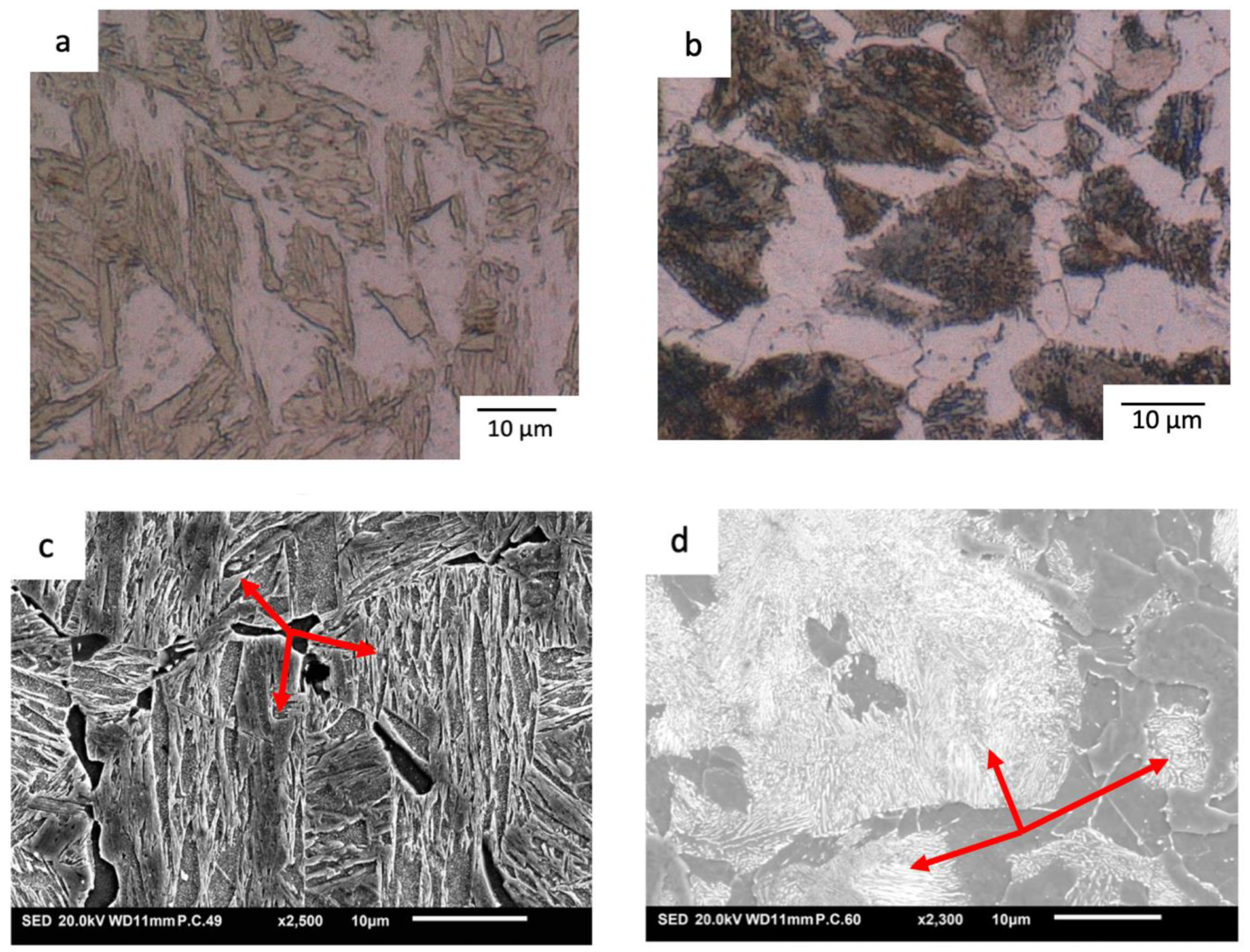

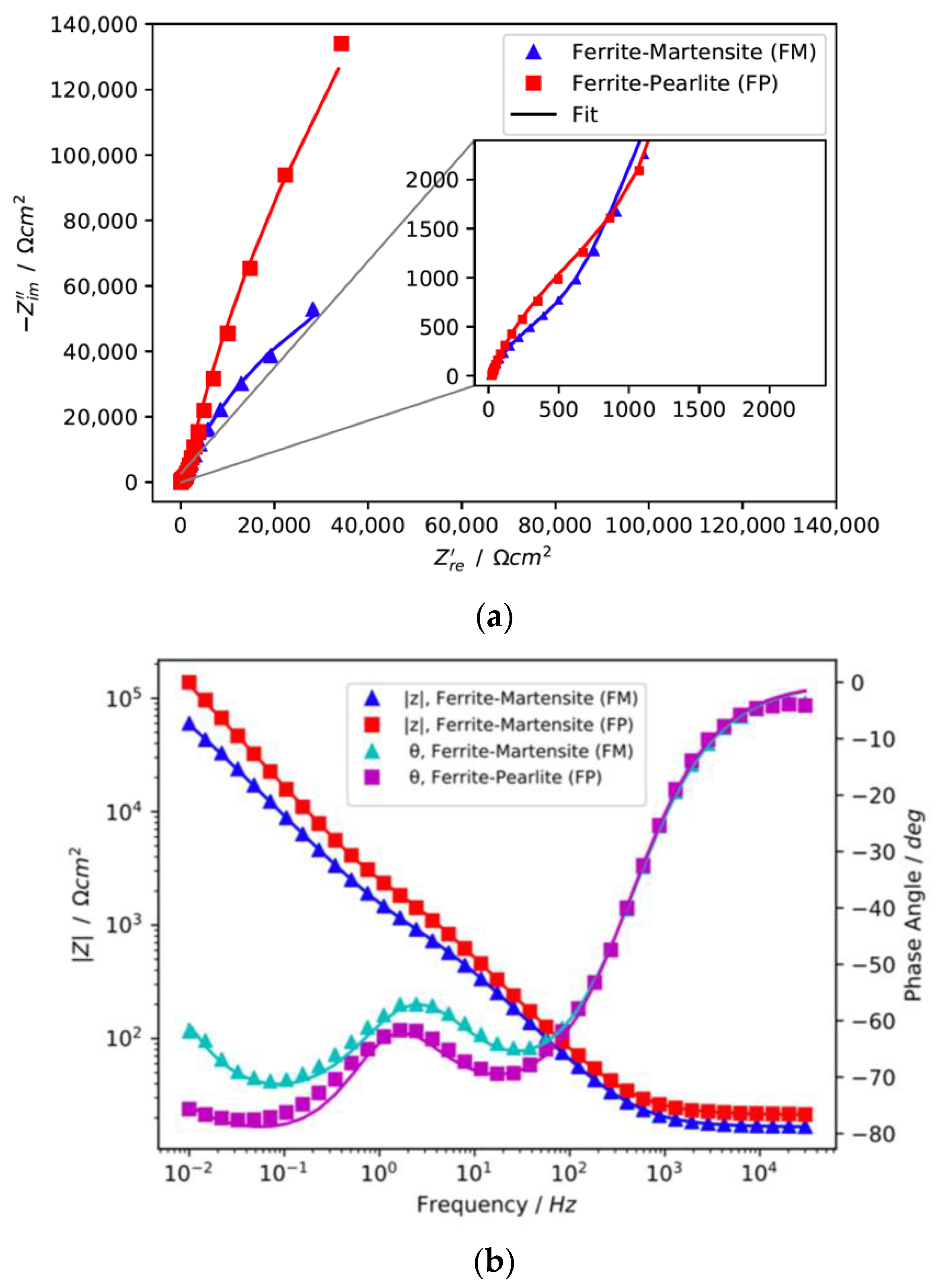
| Element | Fe | C | Si | Mn | P | S | Al | Cr | Cu | Mo | Ni | Sn | Ti | Ca | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 96.92 | 0.141 | 0.051 | 2.149 | 0.013 | 0.014 | 0.041 | 0.576 | 0.012 | 0.004 | 0.021 | 0.001 | 0.032 | 0.022 | 0.007 |
| Temperatures | Ac1 | Ac3 | Ms | Mf |
|---|---|---|---|---|
| °C | 720 | 840 | 400 | 140 |
| Sample | Rs (Ωcm2) | R1 (Ωcm2) | C1 (Fcm−2 × 10−5) | CPE1-Q (Ω−1 sncm2 × 10−5) | CPE1-n | R2 (Ωcm2 × 105) | C2 (Fcm−2 × 10−5) | CPE2-Q (Ω−1 sncm2 × 10−5) | CPE2-n | Chi-Squared (× 10−4) |
|---|---|---|---|---|---|---|---|---|---|---|
| FM | 13.2 ± 1.5 | 85 ± 30 | 13.1 ± 0.7 | 2.3 ± 1.2 | 0.87 ± 0.04 | 2.02 ± 0.12 | 3.2 ± 0.5 | 2.2 ± 0.5 | 0.82 ± 0.03 | 3 ± 2 |
| FP | 15 ± 2 | 250 ± 110 | 1.3 ± 0.5 | 1.91 ± 0.13 | 0.85 ± 0.02 | 11.0 ± 1.5 | 1.3 ± 0.4 | 1.1 ± 0.4 | 0.88 ± 0.08 | 4 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, A.; Ozkan, C.; Sietsma, J.; Gonzalez-Garcia, Y. Properties of Passive Films Formed on Ferrite-Martensite and Ferrite-Pearlite Steel Microstructures. Metals 2021, 11, 594. https://doi.org/10.3390/met11040594
Yilmaz A, Ozkan C, Sietsma J, Gonzalez-Garcia Y. Properties of Passive Films Formed on Ferrite-Martensite and Ferrite-Pearlite Steel Microstructures. Metals. 2021; 11(4):594. https://doi.org/10.3390/met11040594
Chicago/Turabian StyleYilmaz, Aytac, Can Ozkan, Jilt Sietsma, and Yaiza Gonzalez-Garcia. 2021. "Properties of Passive Films Formed on Ferrite-Martensite and Ferrite-Pearlite Steel Microstructures" Metals 11, no. 4: 594. https://doi.org/10.3390/met11040594
APA StyleYilmaz, A., Ozkan, C., Sietsma, J., & Gonzalez-Garcia, Y. (2021). Properties of Passive Films Formed on Ferrite-Martensite and Ferrite-Pearlite Steel Microstructures. Metals, 11(4), 594. https://doi.org/10.3390/met11040594







