Effect of Cd2+ on Electrodeposition of Copper in Cyclone Electrodeposition
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Experimental Procedure
2.2.1. Cu Cyclone Electrowinning
2.2.2. Process Flow
2.2.3. Calculation Method
2.2.4. Characterization and Analyses
3. Results and Discussion
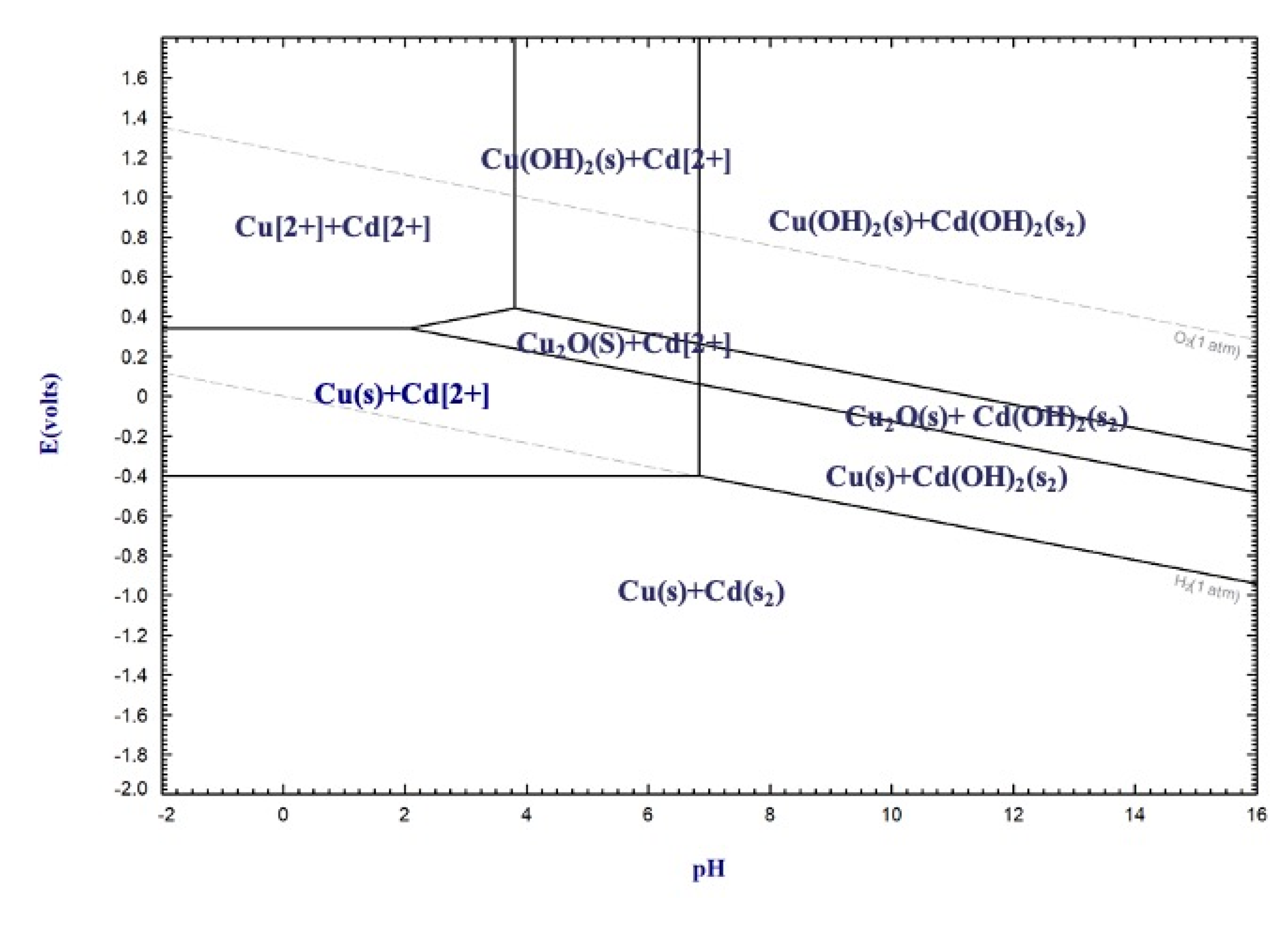
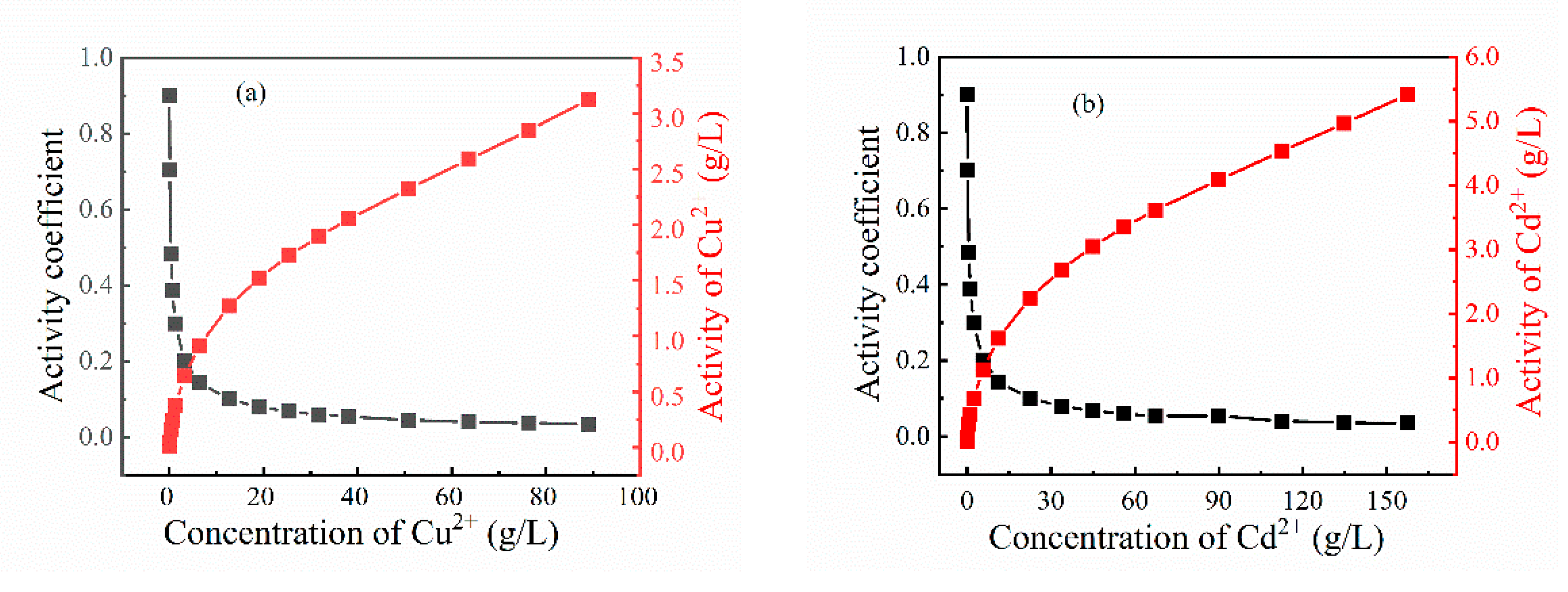
3.1. Thermodynamic Analysis
3.2. Cu Cyclone Electrowinning
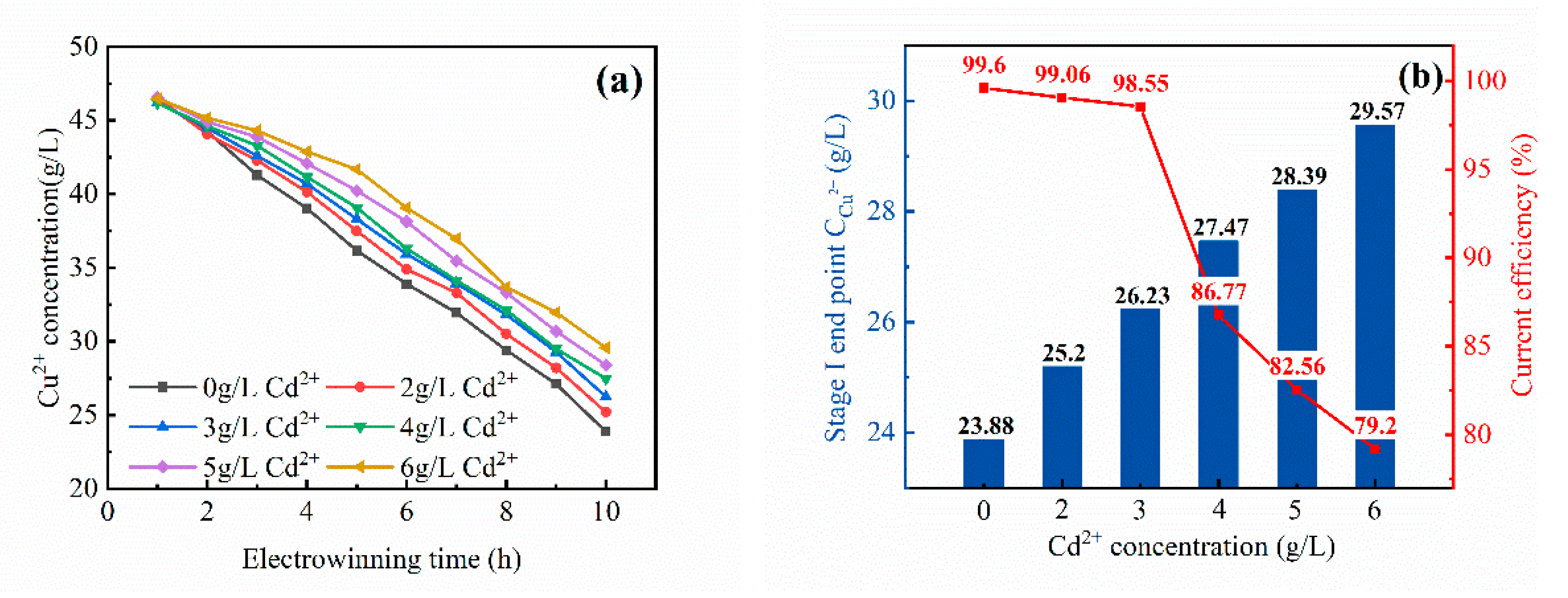
3.2.1. First Stage of Cyclone Electrowinning
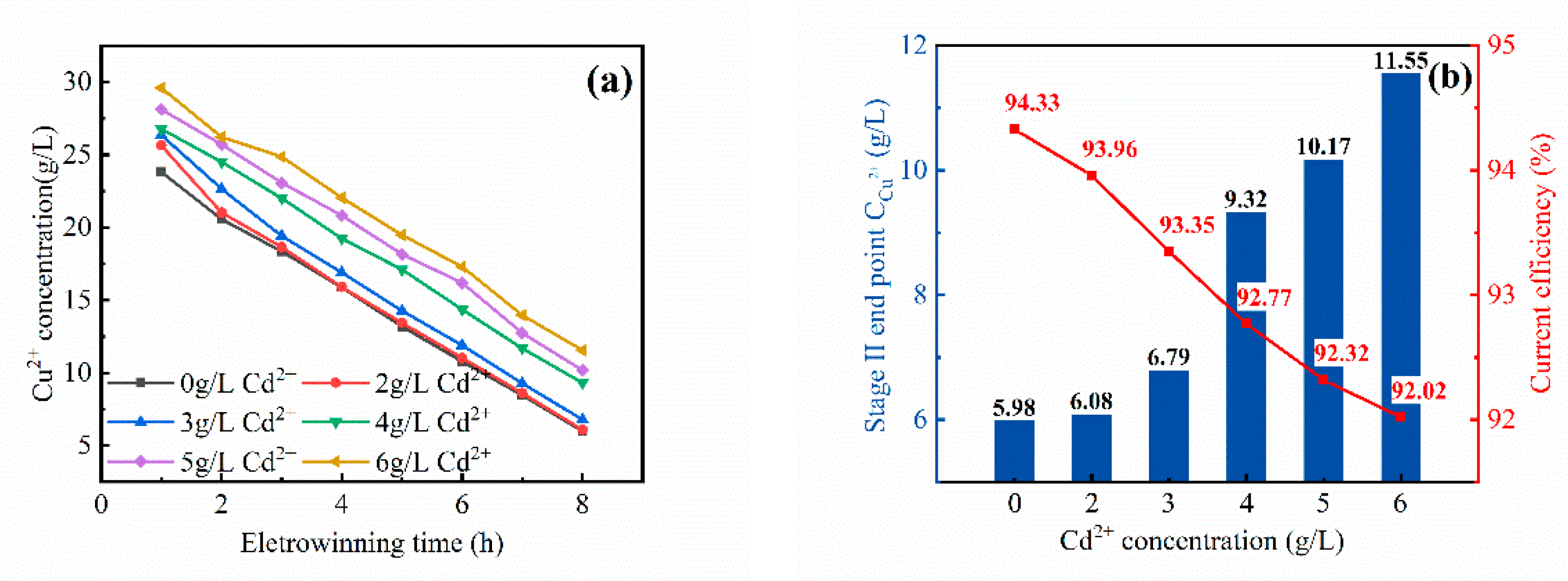
3.2.2. Second Stage of Cyclone Electrowinning
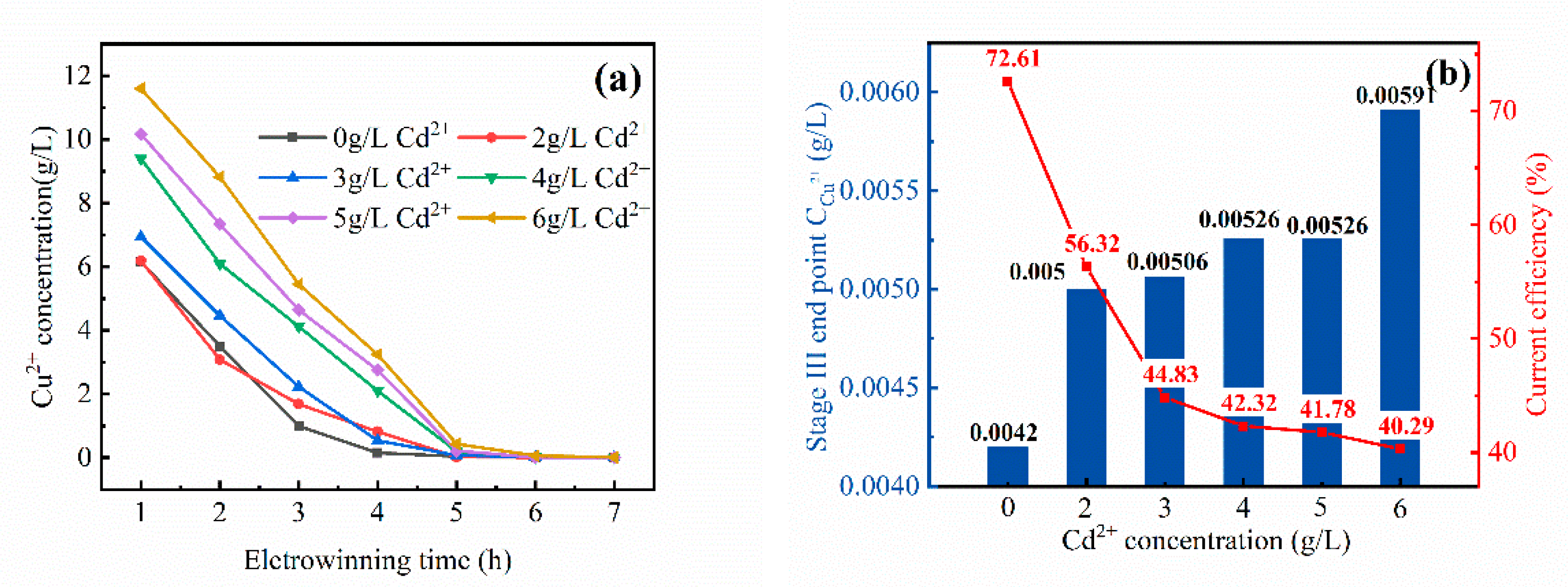
3.2.3. Third Stage of Cyclone Electrowinning
4. Conclusions
- The addition of Cd2+ had little effect on the purity of the cathode copper sheet produced by cyclone electrodeposition of a copper electrolyte. High-purity cathode copper, standard cathode copper, and coarse copper powder with purities over 99.9, 99, and 98%, respectively, were obtained by three-stage electrodeposition, which realized the separation of Cu and Cd.
- With the increase of the Cd2+ content in the electrolyte, the current efficiency of cyclone electrodeposition and the electrodeposition rate of the copper electrolyte showed different rules. The current efficiency of the first stage decreased from 99.6 to 79.20%. The current efficiency of the second stage had no change from 94.33 to 92.02%. The three-stage current efficiency decreased from 72.61 to 40.29%. The electrodeposition rate of copper in the first stage decreased from 52 to 40%. The second-stage electrodeposition rate decreased from 88 to 77%. The electrodeposition rate of the three sections had basically no change, can reach 99%.
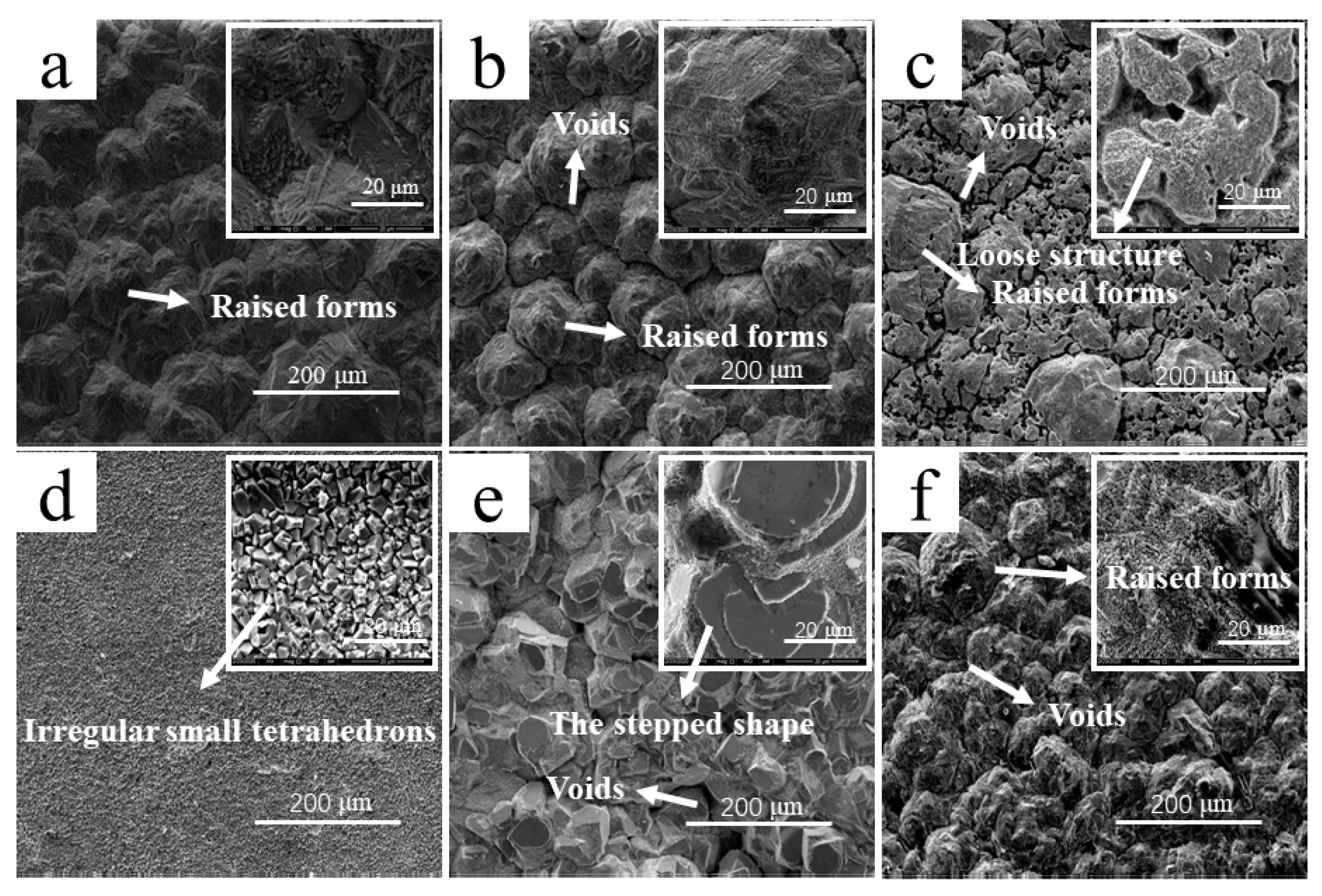
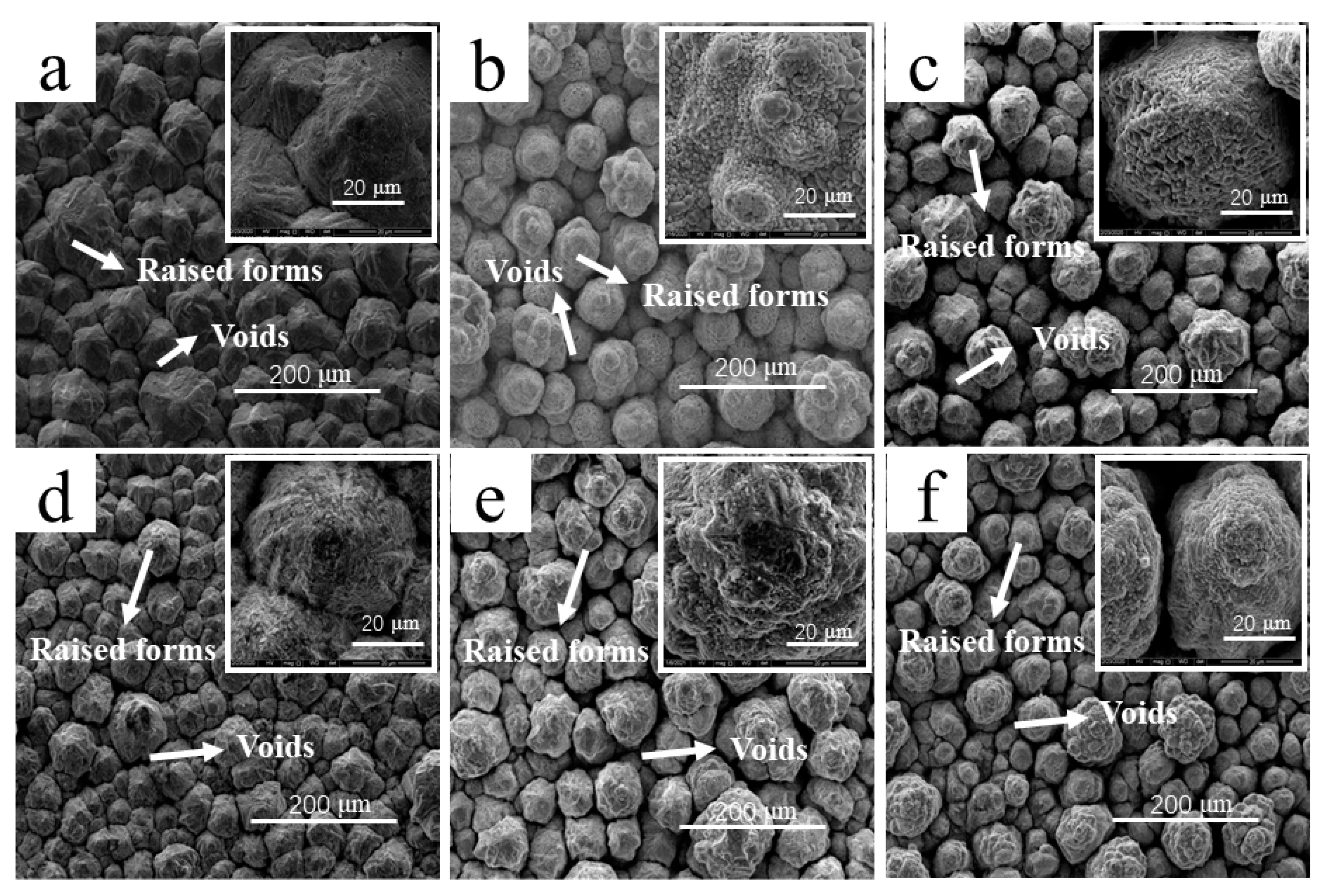
- The addition of Cd2+ had a great influence on the microstructure of the cathode copper. The cathode copper products with a high Cd2+ concentration had more small grains, and their overall density decreased. This may have been because the concentration of Cd2+ affected the electrodeposition state of the cathode and led to the decrease of the copper plate mass.
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Tian-Cong, Z. Heavy Metal Metallurgy; Metallurgical Industry Press: Beijing, China, 1981. [Google Scholar]
- Zhai, X. Heavy Metal Metallurgy; Metallurgical Industry Press: Beijing, China, 2011. [Google Scholar]
- Dupeux, M. Science des Matériaux; Dunod: Paris, France, 2004. [Google Scholar]
- Barragan, J.A.; De León, C.P.; Castro, J.R.A.; Peregrina-Lucano, A.; Gómez-Zamudio, F.; Larios-Durán, E.R. Copper and Antimony Recovery from Electronic Waste by Hydrometallurgical and Electrochemical Techniques. ACS Omega 2020, 5, 12355–12363. [Google Scholar] [CrossRef]
- Guo, X.; Qin, H.; Tian, Q.; Li, D. Recovery of Metals from Waste Printed Circuit Boards by Selective Leaching Combined with Cyclone Electrowinning Process. J. Hazard. Mater. 2020, 384, 121355. [Google Scholar] [CrossRef]
- Oishi, T.; Yaguchi, M.; Takai, Y. Hydrometallurgical Recovery of High-Purity Copper Cathode from Highly Impure Crude Copper. Resour. Conserv. Recycl. 2021, 167, 105382. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chang, C.-T.; Fan, K.-S.; Chang, T.-C. An Overview of Recycling and Treatment of Scrap Computers. J. Hazard. Mater. 2004, 114, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Brar, K.K.; Magdouli, S.; Etteieb, S.; Zolfaghari, M.; Fathollahzadeh, H.; Calugaru, L.; Komtchou, S.-P.; Tanabene, R.; Brar, S.K. Integrated Bioleaching-Electrometallurgy for Copper Recovery—A Critical Review. J. Clean. Prod. 2020, 291, 125257. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J. Recovery of Copper and the Deportment of other Base Metals from Alkaline Glycine Leachates Derived from Waste Printed Circuit Boards (WPCBs). Hydrometallurgy 2020, 199, 105540. [Google Scholar] [CrossRef]
- Jiang, Q.; Song, X.; Liu, J.; Shao, Y.; Feng, Y. In-situ Cu (II) Enrichment and Recovery from Low-Strength Copper-Laden Wastewater Using a Novel Electrically Enhanced Microbial Copper Recovery Cell (MCRC). Chem. Eng. J. 2020, 382, 122788. [Google Scholar] [CrossRef]
- Rudnik, E.; Nikiel, M. Hydrometallurgical Recovery of Cadmium and Nickel from Spent Ni–Cd Batteries. Hydrometallurgy 2007, 89, 61–71. [Google Scholar] [CrossRef]
- Jajuli, M.N.; Mohamed, N.; Suah, F.B.M. Electrochemical Removal of Cadmium from a Sulphate Solution Using a Three-Dimensional Electrode. Alex. Eng. J. 2020, 59, 4237–4245. [Google Scholar] [CrossRef]
- Tran, T.K.; Kim, N.; Le, Q.C.; Nguyen, M.T.; Leu, H.J.; Thi, K.N. Electrochemical preparation and characterization of polyaniline enhanced electrodes: An application for the removal of cadmium metals in industrial wastewater. Mater. Chem. Phys. 2021, 261, 124221. [Google Scholar] [CrossRef]
- Li, H.; Zha, J.; Zhang, H.; Zheng, N.; Guo, G. Design Method for Cadmium and Arsenic Contamination Control during the Mining-Selecting-Backfilling Integrated Technology. J. Clean. Prod. 2020, 261, 121259. [Google Scholar] [CrossRef]
- Tang, S.; Chen, K.; Wan, N.; Ni, T.; Zhang, Y. Study on Extraction of Sponge Cadmium from Copper Cadmium Slag. Min. Metall. 2014, 23, 65–68. [Google Scholar]
- Yang, W.; Sun, L.; Hu, Y.; Yang, Y.; Jiang, X.; Wang, H. Cyclone Electrowinning of Antimony from Antimonic Gold Concentrate Ores. In TMS Annual Meeting & Exhibition; Springer: Berlin, Germany, 2018; pp. 143–154. [Google Scholar]
- Xu, H.; Li, B.; Wei, Y.; Wang, H. Extracting of Copper from Simulated Leaching Solution of Copper-Cadmium Residues by Cyclone Electrowinning Technology. Hydrometallurgy 2020, 194, 105298. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Su, J.; Zheng, S.; Lei, H.; Cai, C.; Jin, W. Efficient Electrochemical Recovery of Dilute Selenium by Cyclone Electrowinning. Hydrometallurgy 2018, 179, 232–237. [Google Scholar] [CrossRef]
- Jin, W. Simultaneous Phenol Detoxification and Dilute Metal Recovery in Cyclone Electrochemical Reactor. Ind. Eng. Chem. Res. 2019, 58, 12642–12649. [Google Scholar]
- Mooiman, M.B.; Ewart, I.; Robinson, J. Electrowinning Precious Metals from Cyanide Solution Using EMEW Technology. In Proceedings of the 142nd Annual Meeting of Society of Mining and Metallurgical Engineers, Denver, CO, USA, 24–27 February 2019. [Google Scholar]
- Junaedi, A.; Handoko, A.; Amin, M.; Sumardi, S.; Mufakhir, F.; Nurjaman, F.; Tandoko, A.; Supriyatna, Y. Nickel Production from Laterites Using Electro Metal Electrowinning (EMEW) Process. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012016. [Google Scholar]
- Xu, Z.; Guo, X.; Tian, Q.; Li, D.; Zhang, Z.; Zhu, L. Electrodeposition of Tellurium from Alkaline Solution by Cyclone Electrowinning. Hydrometallurgy 2020, 193, 105316. [Google Scholar] [CrossRef]
- Wang, S. Recovering Copper Using a Combination of Electrolytic Cells. JOM 2002, 54, 51–54. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Wei, Y.; Wang, H.; Barati, M. Extraction of Copper from Copper and Cadmium Residues of Zinc Hydrometallurgy by Oxidation Acid Leaching and Cyclone Electrowinning. Miner. Eng. 2018, 128, 247–253. [Google Scholar] [CrossRef]
- Sudibyo; Darmansyah; Junaedi, A.; Handoko, A.S.; Mufakhir, F.K.; Nurjaman, F.; Amin, M.; Supriyatna, Y.I.; Sumardi, S.; Salsabila, P. Nickel recovery from electrocoagulation sludge of hydrometallurgy wastewater using electrowinning. In Proceedings of the 3rd International Seminar on Metallurgy and Materials (ISMM2019): Exploring New Innovation in Metallurgy and Materials, Tangerang Selatan, Indonesia, 23–24 October 2019. [Google Scholar]
- Bae, M.; Kim, S.; Lee, J.-J. Gold Recovery from Waste Solutions of PCBs Gold Plating Process Using Hydro Cyclone Reactor for Demonstration Study. In Proceedings of the 3rd Pan American Materials Congress; Springer: Berlin, Germany, 2017; pp. 519–527. [Google Scholar]
- Jin, W.; Su, J.; Chen, S.; Li, P.; Moats, M.S.; Maduraiveeran, G.; Lei, H. Efficient Electrochemical Recovery of Fine Tellurium Powder from Hydrochloric Acid Media via Mass Transfer Enhancement. Sep. Purif. Technol. 2018, 203, 117–123. [Google Scholar] [CrossRef]
- Lu, X.H.; Maurer, G. Model for Describing Activity Coefficients in Mixed Electrolyte Aqueous Solutions. AIChE J. 1993, 39, 1527–1538. [Google Scholar]
- Pitzer, K.S. Thermodynamic Properties of Aqueous Solutions of Bivalent Sulphates. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1972, 68, 101–113. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamics of Electrolytes. I. Theoretical Basis and General Equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Mayorga, G. Thermodynamics of Electrolytes. III. Activity and Osmotic Coefficients for 2–2 Electrolytes. J. Solut. Chem. 1974, 3, 539–546. [Google Scholar] [CrossRef]
- Clegg, S.L.; Pitzer, K.S. Thermodynamics of Multicomponent, Miscible, Ionic Solutions: Generalized Equations for Symmetrical Electrolytes. J. Phys. Chem. 1992, 96, 3513–3520. [Google Scholar] [CrossRef]
- Chai, L.; Xiong, C.; Zeng, W.; Wu, L.; Hu, H. The Role of Chelators in Electrodeposition of Cu-As Alloys from Copper Electrolyte. JOM 2020, 72, 3876–3886. [Google Scholar] [CrossRef]
- Jacobs, J.C.; Groot, D.R. Improving Cathode Morphology at a Copper Electrowinning Plant by Optimizing Magnafloc 333 and Chloride Concentrations. J. South. Afr. Inst. Min. Metall. 2019, 119, 983–987. [Google Scholar] [CrossRef]
- Nikoli, N.D.; Avramovi, L.; Ivanovi, E.R.; Maksimovi, V.M.; Ignjatovi, N. Comparative Morphological and Crystallographic Analysis of Copper Powders Obtained Under Different Electrolysis Conditions. Trans. Nonferrous Met. Soc. China 2019, 29, 1275–1284. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, H. Recovery of Ni from the Acid Leaching Solution of Electroplating Sludge through Preparing Ni-Fe Alloy with the Addition of Saccharin Na First and then Thiourea. Electrochemistry 2018, 87, 8–13. [Google Scholar] [CrossRef]



| Metal Elements | 0 g/L | 2 g/L | 3 g/L | 4 g/L | 5 g/L | 6 g/L |
|---|---|---|---|---|---|---|
| Cu | >99.7 | >99.7 | >99.7 | >99.7 | >99.7 | 99.63 |
| Cd | <0.001 | <0.001 | 0.0011 | 0.0023 | 0.0026 | 0.0041 |
| Metal Elements | 0 g/L | 2 g/L | 3 g/L | 4 g/L | 5 g/L | 6 g/L |
|---|---|---|---|---|---|---|
| Cu | >99.7 | >99.7 | >99.7 | >99.7 | 99.51 | 97.7 |
| Cd | <0.001 | <0.001 | 0.0023 | 0.004 | <0.01 | 0.012 |
| Metal Elements | 0 g/L | 2 g/L | 3 g/L | 4 g/L | 5 g/L | 6 g/L |
|---|---|---|---|---|---|---|
| Cu | 97.47 | 96.18 | 94.82 | 94.23 | 93.56 | 92.98 |
| Cd | 0.0015 | 0.016 | 0.019 | 0.0234 | 0.041 | 0.056 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, B.; Xu, H.; Guo, J. Effect of Cd2+ on Electrodeposition of Copper in Cyclone Electrodeposition. Metals 2021, 11, 529. https://doi.org/10.3390/met11040529
Wang Y, Li B, Xu H, Guo J. Effect of Cd2+ on Electrodeposition of Copper in Cyclone Electrodeposition. Metals. 2021; 11(4):529. https://doi.org/10.3390/met11040529
Chicago/Turabian StyleWang, Yan, Bo Li, Hongao Xu, and Jihao Guo. 2021. "Effect of Cd2+ on Electrodeposition of Copper in Cyclone Electrodeposition" Metals 11, no. 4: 529. https://doi.org/10.3390/met11040529
APA StyleWang, Y., Li, B., Xu, H., & Guo, J. (2021). Effect of Cd2+ on Electrodeposition of Copper in Cyclone Electrodeposition. Metals, 11(4), 529. https://doi.org/10.3390/met11040529




