Corrosion Behavior, Microstructure and Mechanical Properties of Novel Mg-Zn-Ca-Er Alloy for Bio-Medical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing
2.2. Microstructural Characterization
2.3. Mechanical Properties
2.3.1. Microhardness
2.3.2. Tensile Test
2.3.3. Damping Characteristics
2.4. Corrosion Behavior
3. Results
3.1. Microstructural Characterization
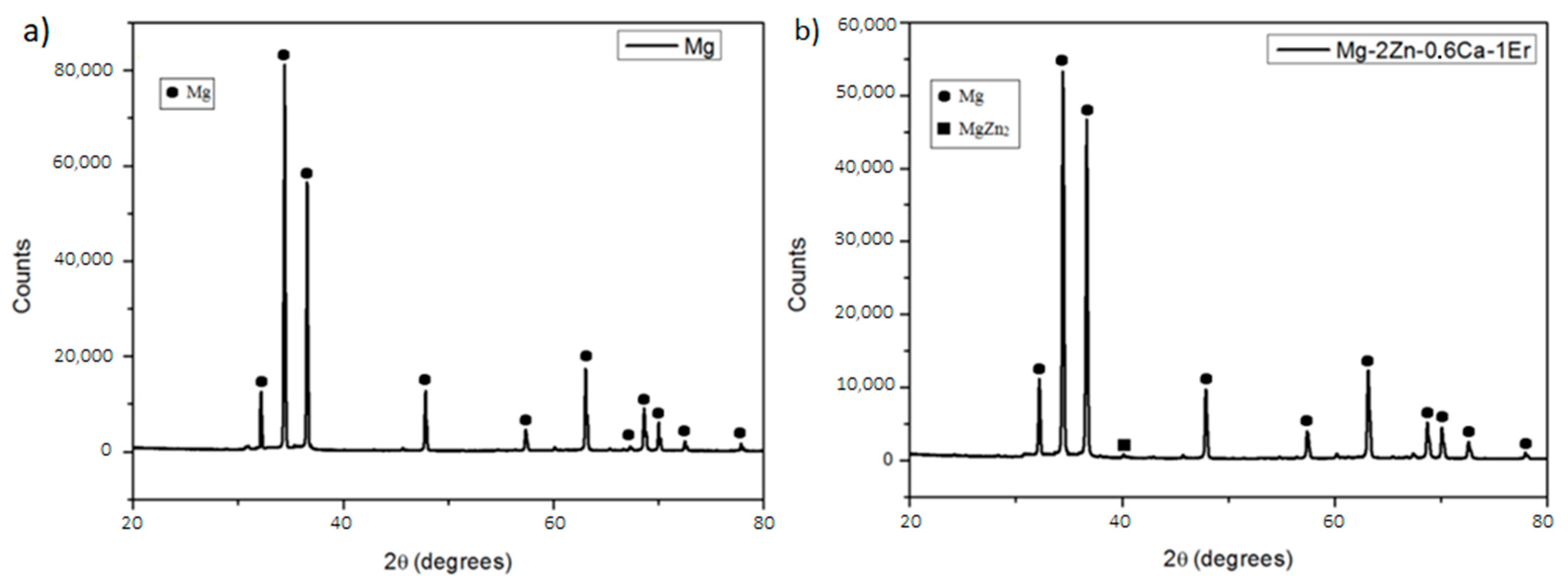
3.1.1. X-ray Diffraction
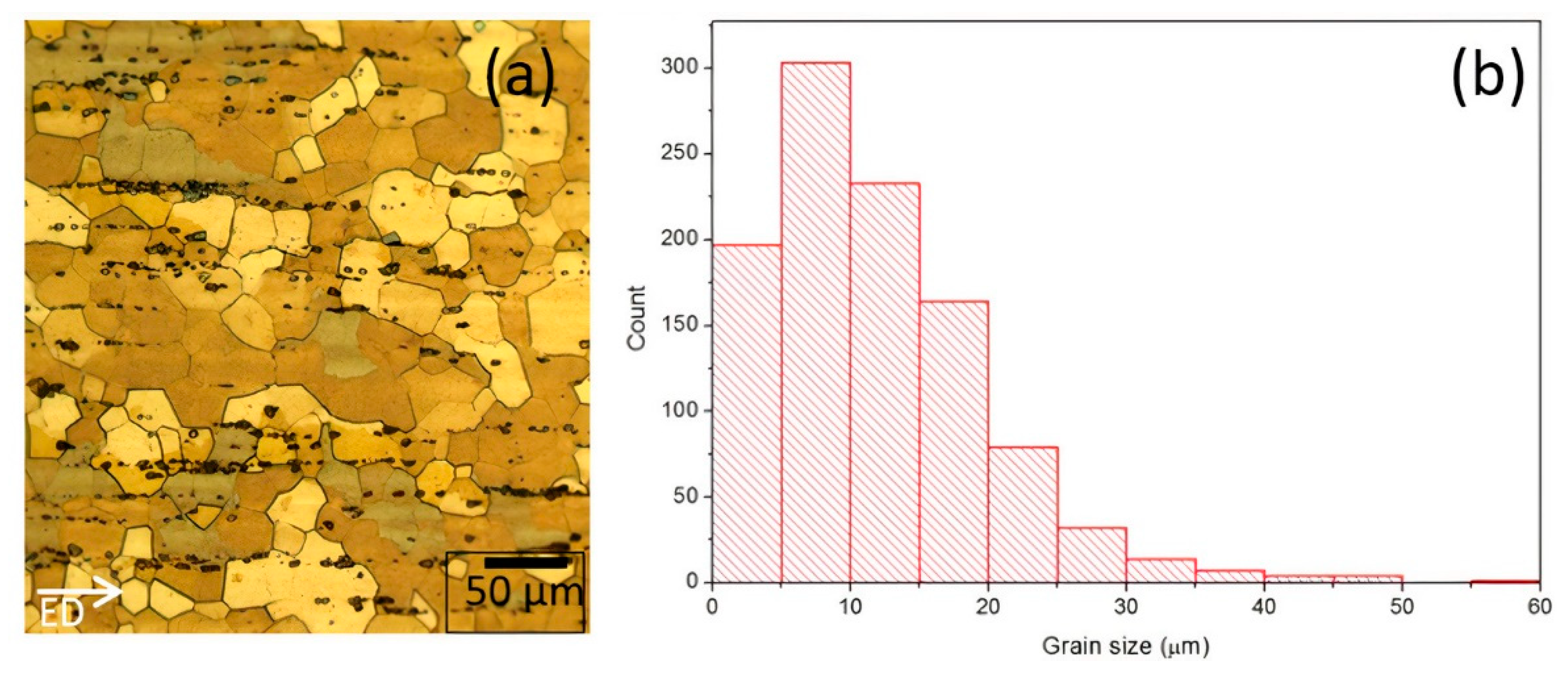
3.1.2. Optical Microscopy
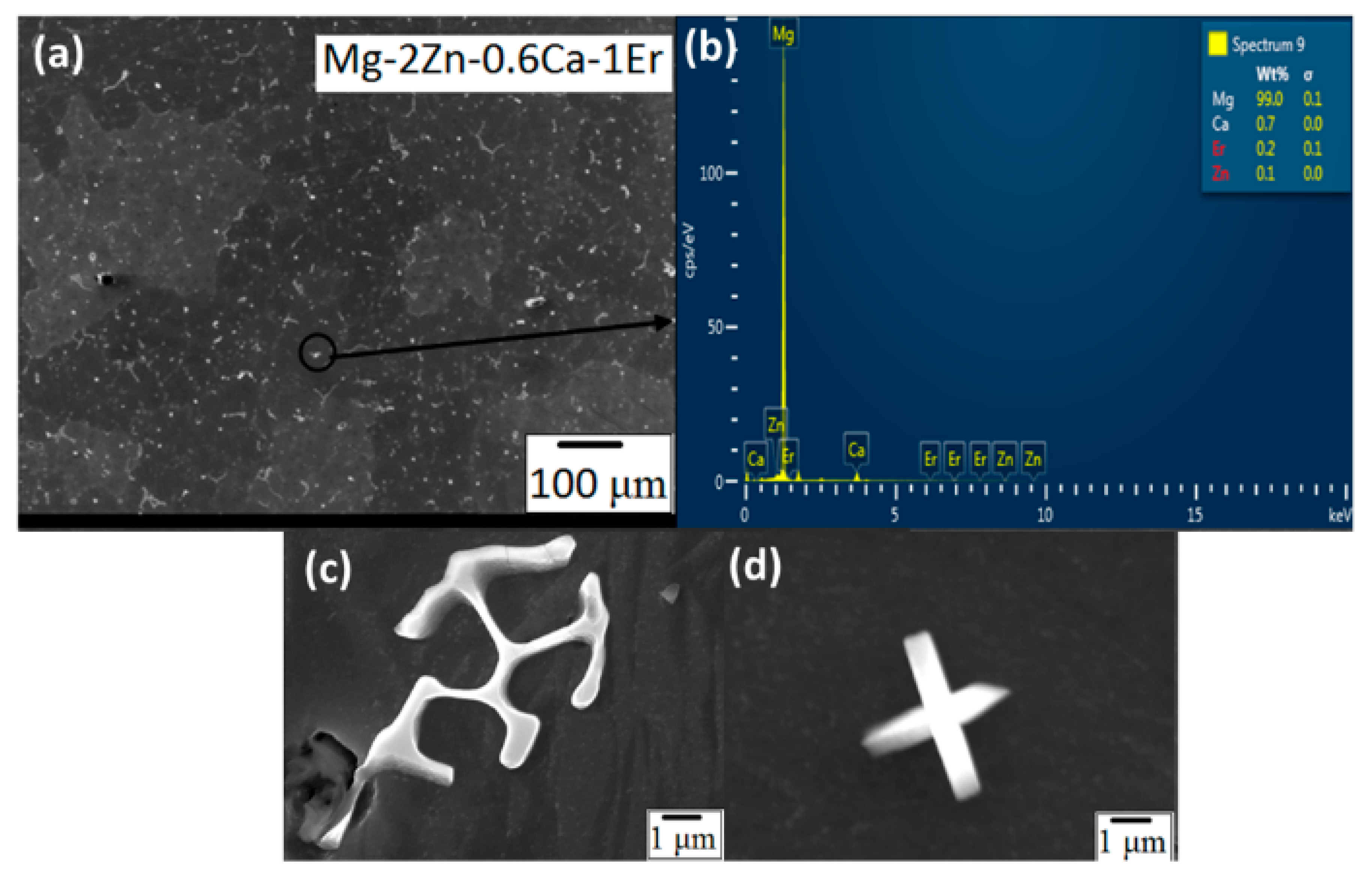
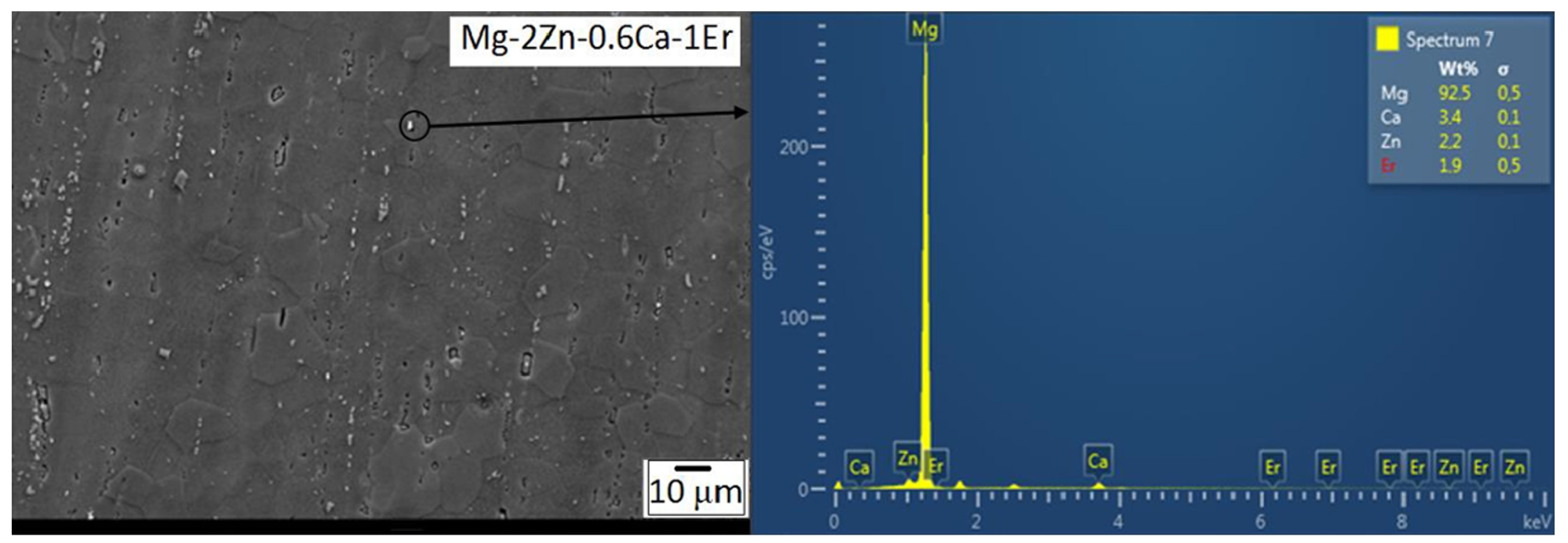
3.1.3. Scanning Electron Microscopy
3.1.4. EBSD Studies
3.2. Mechanical Properties
3.2.1. Microhardness
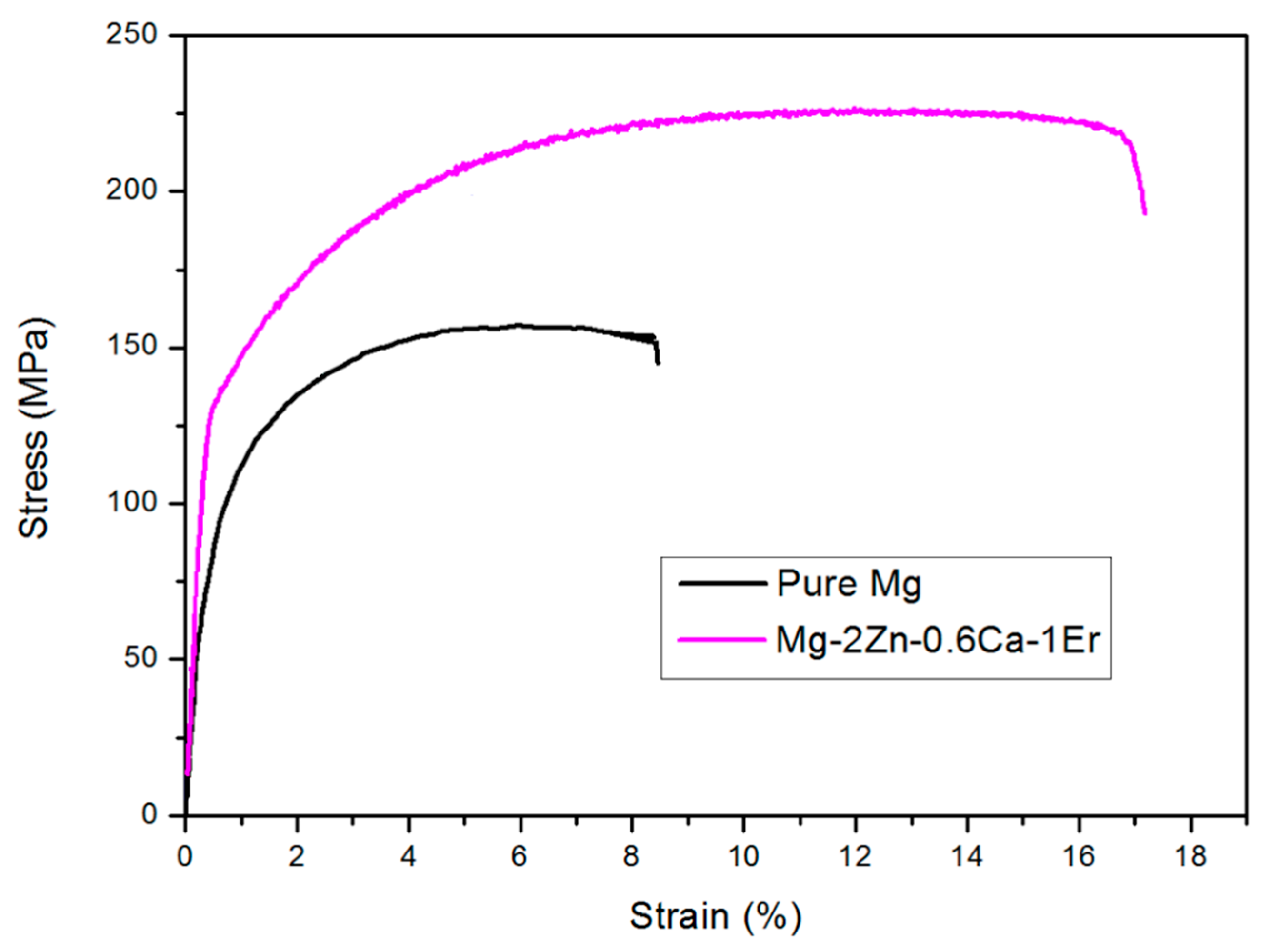
3.2.2. Tensile Properties
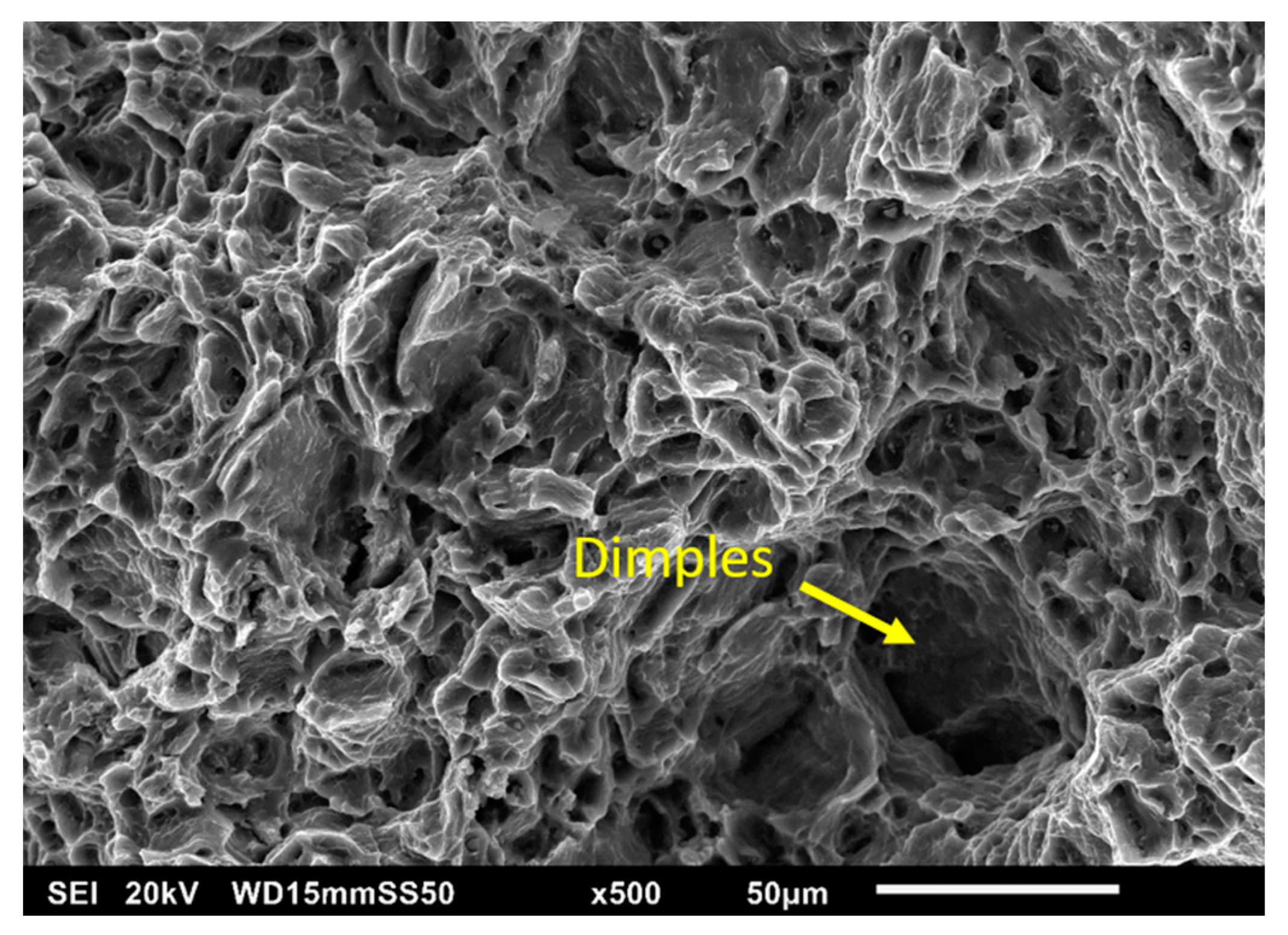
3.2.3. Fractography
3.2.4. Damping Properties
3.3. Corrosion Behavior
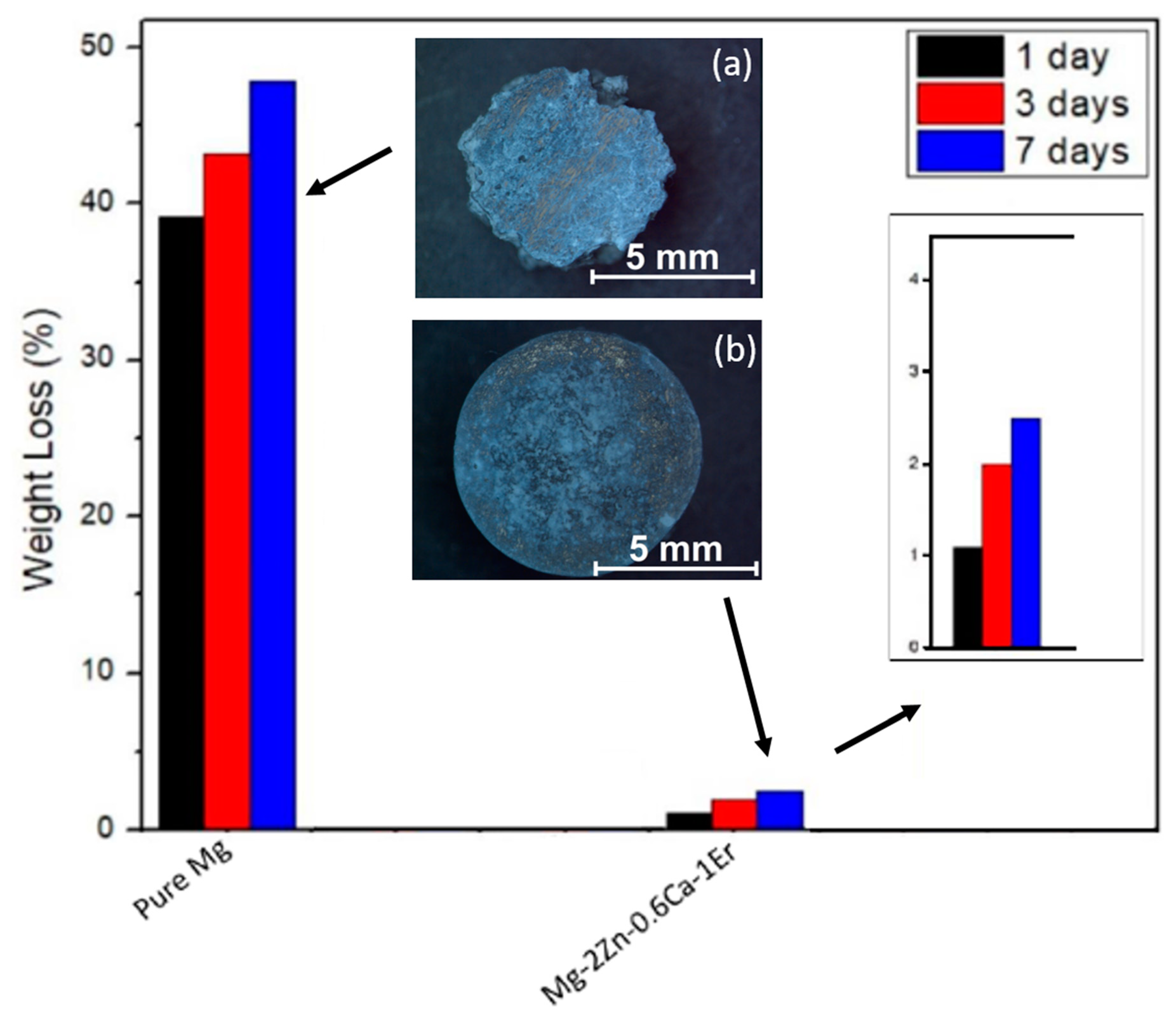
3.3.1. Immersion Test
3.3.2. Hydrogen Evolution Test
4. Discussion
4.1. Grain Size
4.2. Extrusion and Mechanical Properties
Grain Size, Solid Solution Strengthening, and Particle Strengthening
4.3. Corrosion Behavior
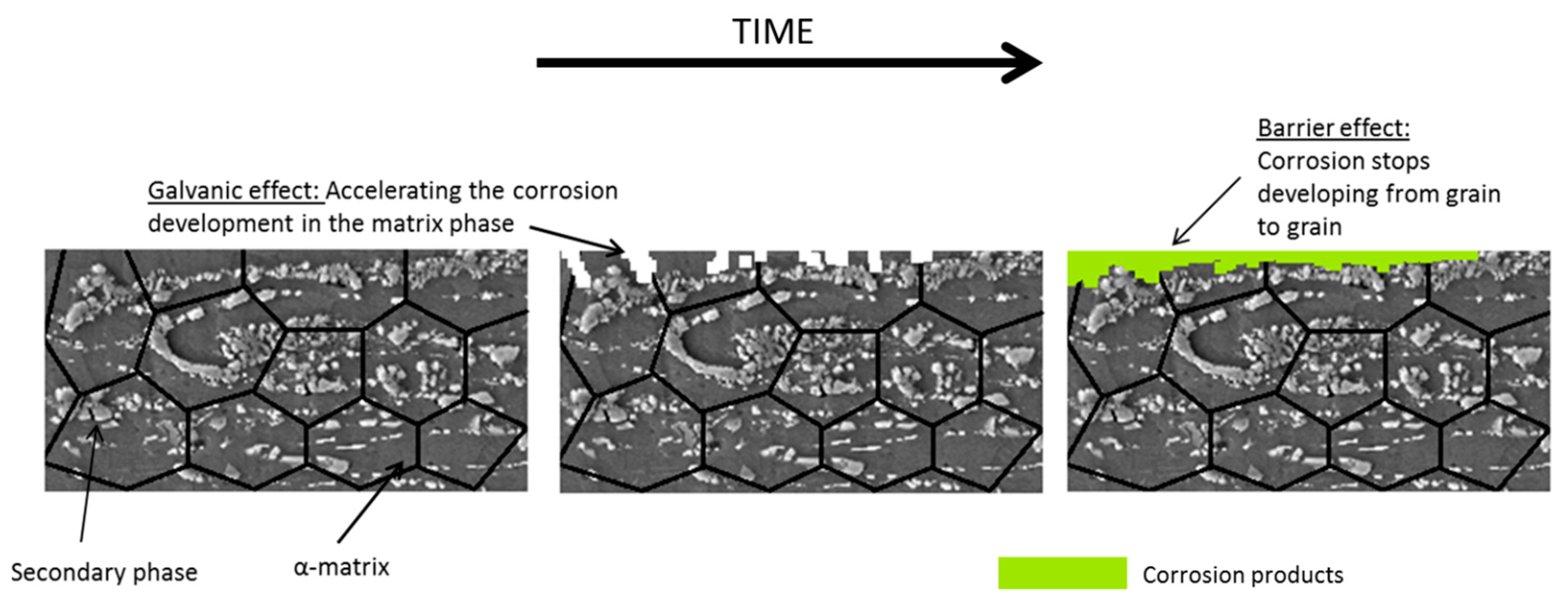
4.3.1. Grain Size and Secondary Phases
4.3.2. Effect of Alloying Elements
4.4. Biocompatibility and Biomechanical Properties of the Alloys
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable Biomaterials Based on Magnesium Corrosion; Elsevier: Amsterdam, The Netherlands, 2008; Volume 12, ISBN 4951153548. [Google Scholar]
- Song, G.; Atrens, A.; Stjohn, D.; Nairn, J.; Li, Y. The electrochemical corrosion of pure magnesium in 1 N NaCl. Corros. Sci. 1997, 39, 855–875. [Google Scholar] [CrossRef]
- Yan, T.; Tan, L.; Xiong, D.; Liu, X.; Zhang, B.; Yang, K. Fluoride treatment and in vitro corrosion behavior of an AZ31B magnesium alloy. Mater. Sci. Eng. C 2010, 30, 740–748. [Google Scholar] [CrossRef]
- Rettig, R.; Virtanen, S. Composition of corrosion layers on a magnesium rare-earth alloy in simulated body fluids. J. Biomed. Mater. Res. Part A 2009, 88, 359–369. [Google Scholar] [CrossRef]
- Yuen, C.K.; Ip, W.Y. Theoretical risk assessment of magnesium alloys as degradable biomedical implants. Acta Biomater. 2010, 6, 1808–1812. [Google Scholar] [CrossRef]
- Li, J.; Du, W.; Li, S.; Wang, Z. Effect of aging on microstructure of Mg-Zn-Er alloys. J. Rare Earths 2009, 27, 1042–1045. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, C.; Jing, Y.; Lv, S.; Liu, S.; Fang, D.; Zhuang, J.; Zhang, M.; Wu, R. New horizon for high performance Mg-based biomaterial with uniform degradation behavior: Formation of stacking faults. Sci. Rep. 2015, 5, 13933. [Google Scholar] [CrossRef] [PubMed]
- Boehlert, C.J.; Knittel, K. The microstructure, tensile properties, and creep behavior of Mg–Zn alloys containing 0–4.4wt.% Zn. Mater. Sci. Eng. A 2006, 417, 315–321. [Google Scholar] [CrossRef]
- Peng, Q.; Li, X.; Ma, N.; Liu, R.; Zhang, H. Effects of backward extrusion on mechanical and degradation properties of Mg-Zn biomaterial. J. Mech. Behav. Biomed. Mater. 2012, 10, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Fereidouni-Lotfabadi, A.; Daroonparvar, M.; Yajid, M.A.M.; Mezbahul-Islam, M.; Kasiri-Asgarani, M.; Medraj, M. Microstructure and bio-corrosion behavior of Mg-Zn and Mg-Zn-Ca alloys for biomedical applications. Mater. Corros. 2014, 65, 1178–1187. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Kopparthy, S.D.S.; Gupta, M. Utilizing Low-Cost Eggshell Particles to Enhance the Mechanical Response of Mg-2.5Zn Magnesium Alloy Matrix. Adv. Eng. Mater. 2017, 1700919. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Hoehn, R.; Perz, A.; Bach, F.-W.; Peuster, M. Development and biocompatibility of a novel corrodible fluoride-coated magnesium-calcium alloy with improved degradation kinetics and adequate mechanical properties for cardiovascular applications. J. Biomed. Mater. Res. Part A 2010, 93, 763–775. [Google Scholar] [CrossRef]
- Kannan, M.B.; Raman, R.K.S. In vitro degradation and mechanical integrity of calcium-containing magnesium alloys in modified-simulated body fluid. Biomaterials 2008, 29, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Mordike, B.L.; Lukac, P. Physical Metallurgy. In Magnesium Technology—Metallurgy, Design Data, Applications; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- You, B.-S.; Park, W.-W.; Chung, I.-S. The Effect of Calcium Addition to Magnesium on the Microstructure and Compositional Changes of Oxide Film Formed at High Temperature. Mater. Trans. 2001, 42, 1139–1141. [Google Scholar] [CrossRef]
- Nie, J.F.; Muddle, B.C. Precipitation hardening of Mg-Ca(-Zn) alloys. Scr. Mater. 1997, 37, 1475–1481. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef]
- Leng, Z.; Zhang, J.; Yin, T.; Zhang, L.; Guo, X.; Peng, Q.; Zhang, M.; Wu, R. Influence of biocorrosion on microstructure and mechanical properties of deformed Mg-Y-Er-Zn biomaterial containing 18R-LPSO phase. J. Mech. Behav. Biomed. Mater. 2013, 28, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Blawert, C.; Ng, B.M.; Wong, W.L.E.; Goh, C.S.; Hort, N.; Gupta, M. Effect of erbium modification on the microstructure, mechanical and corrosion characteristics of binary Mg-Al alloys. J. Alloys Compd. 2015, 648, 759–770. [Google Scholar] [CrossRef]
- Du, X.; Wei, C.; Wu, B.; Hong, M.; Xia, Y.; Li, W. A high-performance Mg-Er-Zn-Zr alloy with low Er/Zn mass ratio. J. Cent. South Univ. 2015, 22, 4123–4127. [Google Scholar] [CrossRef]
- Li, H.; Du, W.; Li, S.; Wang, Z. Effect of Zn/Er weight ratio on phase formation and mechanical properties of as-cast Mg-Zn-Er alloys. Mater. Des. 2012, 35, 259–265. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wang, Q.; Du, W.; Liu, K. Effects of heat treatment on microstructure and mechanical properties of Mg-5Zn-0.63Er alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 59–65. [Google Scholar] [CrossRef]
- Gupta, M.; Wong, W.L.E. Magnesium-based nanocomposites: Lightweight materials of the future. Mater. Charact. 2015, 105, 30–46. [Google Scholar] [CrossRef]
- Panemangalore, D.B.; Shabadi, R.; Gupta, M.; Ji, G. Effect of fluoride coatings on the corrosion behavior of Mg-Zn-Er alloys. Surf. Interfaces 2019, 14, 72–81. [Google Scholar] [CrossRef]
- Chino, Y.; Huang, X.; Suzuki, K.; Sassa, K.; Mabuchi, M. Influence of Zn concentration on stretch formability at room temperature of Mg-Zn-Ce alloy. Mater. Sci. Eng. A 2010, 528, 566–572. [Google Scholar] [CrossRef]
- Styczynski, A.; Hartig, C.; Bohlen, J.; Letzig, D. Cold rolling textures in AZ31 wrought magnesium alloy. Scr. Mater. 2004, 50, 943–947. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Gupta, M. Synthesis and characterization of high performance low volume fraction TiC reinforced Mg nanocomposites targeting biocompatible/structural applications. Mater. Sci. Eng. A 2015, 627, 306–315. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Q.; Pan, F. Effects of trace Er addition on the microstructure and mechanical properties of Mg-Zn-Zr alloy. Mater. Des. 2010, 31, 4043–4049. [Google Scholar] [CrossRef]
- Zhang, E.; Yin, D.; Xu, L.; Yang, L.; Yang, K. Microstructure, mechanical and corrosion properties and biocompatibility of Mg-Zn-Mn alloys for biomedical application. Mater. Sci. Eng. C 2009, 29, 987–993. [Google Scholar] [CrossRef]
- Humphreys, J.; Rohrer, G.S.; Rollett, A.; Humphreys, J.; Rohrer, G.S.; Rollett, A. Chapter 9—Recrystallization of Two-Phase Alloys. In Recrystallization and Related Annealing Phenomena; Elsevier: Amsterdam, The Netherlands, 2017; pp. 321–359. ISBN 9780080982359. [Google Scholar]
- Robson, J.D.; Henry, D.T.; Davis, B. Particle effects on recrystallization in magnesium—Manganese alloys: Particle-stimulated nucleation. Acta Mater. 2009, 57, 2739–2747. [Google Scholar] [CrossRef]
- Chen, X.; Pan, F.; Mao, J.; Wang, J.; Zhang, D.; Tang, A.; Peng, J. Effect of heat treatment on strain hardening of ZK60 Mg alloy. Mater. Des. 2011, 32, 1526–1530. [Google Scholar] [CrossRef]
- Wang, Q.; Du, W.; Liu, K.; Wang, Z.; Li, S.; Wen, K. Microstructure, texture and mechanical properties of as-extruded Mg-Zn-Er alloys. Mater. Sci. Eng. A 2013, 581, 31–38. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.; Peng, S.; Xu, L.; He, C.; Qi, F.; Zhao, M.; Shuai, C. Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact. Mater. 2021, 6, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Birbilis, N.; Ralston, K.D.; Virtanen, S.; Fraser, H.L.; Davies, C.H.J. Grain character influences on corrosion of ECAPed pure magnesium. Corros. Eng. Sci. Technol. 2010, 45, 224–230. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kim, W.J. Enhancement of mechanical properties and corrosion resistance of Mg-Ca alloys through microstructural refinement by indirect extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Argade, G.R.; Panigrahi, S.K.; Mishra, R.S. Effects of grain size on the corrosion resistance of wrought magnesium alloys containing neodymium. Corros. Sci. 2012, 58, 145–151. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Song, Y.; Zhao, C.; Zhang, X.; Xie, C.; Zhang, Y.; Tao, H.; He, Y.; Jiang, Y.; et al. In vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg-Zn alloy. Mater. Sci. Eng. C 2009, 29, 1907–1912. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D. Structural characteristics and corrosion behavior of biodegradable Mg-Zn, Mg-Zn-Gd alloys. J. Mater. Sci. Mater. Med. 2013, 24, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Tekumalla, S.; Seetharaman, S.; Almajid, A.; Gupta, M. Mechanical Properties of Magnesium-Rare Earth Alloy Systems: A Review. Metals 2014, 5, 1. [Google Scholar] [CrossRef]


| Material/Alloy | Hardness (Hv) | Grain Size (µm) | % Hardness Increment |
|---|---|---|---|
| Pure Mg | 33.84 | 22.42 | - |
| Mg-2Zn-0.6Ca-1Er | 55.04 | 11.57 | 62.64 |
| Sl. No | Material | TYS (MPa) | UTS (MPa) | Elongation |
|---|---|---|---|---|
| 1 | Pure Mg | 96 ± 3 | 150 ± 3 | 8.3 ± 0.2 |
| 2 | Mg-2Zn-0.6Ca-1Er | 128 ± 4 (↑33.33%) | 225 ± 2 (↑50%) | 17.2 ± 0.3 (↑107.2%) |
| Material | Damping Loss Rate | Damping Capacity | Elastic Modulus (GPa) |
|---|---|---|---|
| Pure Mg | 7.2 | 0.000275 | 45.26 |
| Mg-2Zn-0.6Ca-1Er | 6.8 | 0.000261 | 44.45 |
| Material | H2 Evolution Test | Immersion Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NaCl at 25 °C | PBS at 37 °C | NaCl at 25 °C | PBS at 37 °C | |||||||
| VH (mL) | CR (mm/y) | VH (mL) | CR (mm/y) | Weight Loss (g) | ΔW (mg cm−2 d−1) | CR (mm/y) | Weight Loss (g) | ΔW (mg cm−2 d−1) | CR (mm/y) | |
| Pure Mg | 19.2 | 43.76 | 8.75 | 19.94 | 0.1762 | 39.33 | 82.59 | 0.0446 | 9.955 | 20.91 |
| Mg-2Zn-0.6Ca-1Er | 4.32 | 9.85 | 1.75 | 3.99 | 0.0167 | 3.728 | 7.83 | 0.0033 | 0.7366 | 1.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panemangalore, D.B.; Shabadi, R.; Gupta, M. Corrosion Behavior, Microstructure and Mechanical Properties of Novel Mg-Zn-Ca-Er Alloy for Bio-Medical Applications. Metals 2021, 11, 519. https://doi.org/10.3390/met11030519
Panemangalore DB, Shabadi R, Gupta M. Corrosion Behavior, Microstructure and Mechanical Properties of Novel Mg-Zn-Ca-Er Alloy for Bio-Medical Applications. Metals. 2021; 11(3):519. https://doi.org/10.3390/met11030519
Chicago/Turabian StylePanemangalore, Devadas Bhat, Rajashekhara Shabadi, and Manoj Gupta. 2021. "Corrosion Behavior, Microstructure and Mechanical Properties of Novel Mg-Zn-Ca-Er Alloy for Bio-Medical Applications" Metals 11, no. 3: 519. https://doi.org/10.3390/met11030519
APA StylePanemangalore, D. B., Shabadi, R., & Gupta, M. (2021). Corrosion Behavior, Microstructure and Mechanical Properties of Novel Mg-Zn-Ca-Er Alloy for Bio-Medical Applications. Metals, 11(3), 519. https://doi.org/10.3390/met11030519








