Austenitic Stainless-Steel Reinforcement for Seawater Sea Sand Concrete: Investigation of Stress Corrosion Cracking
Abstract
1. Introduction
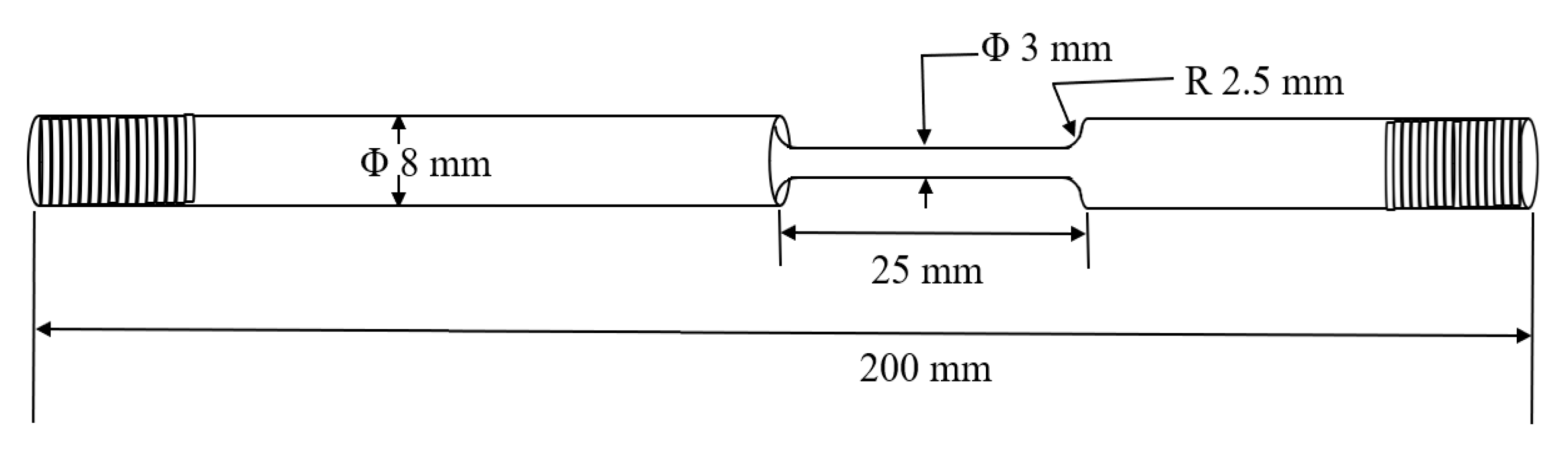
2. Experimental Procedure
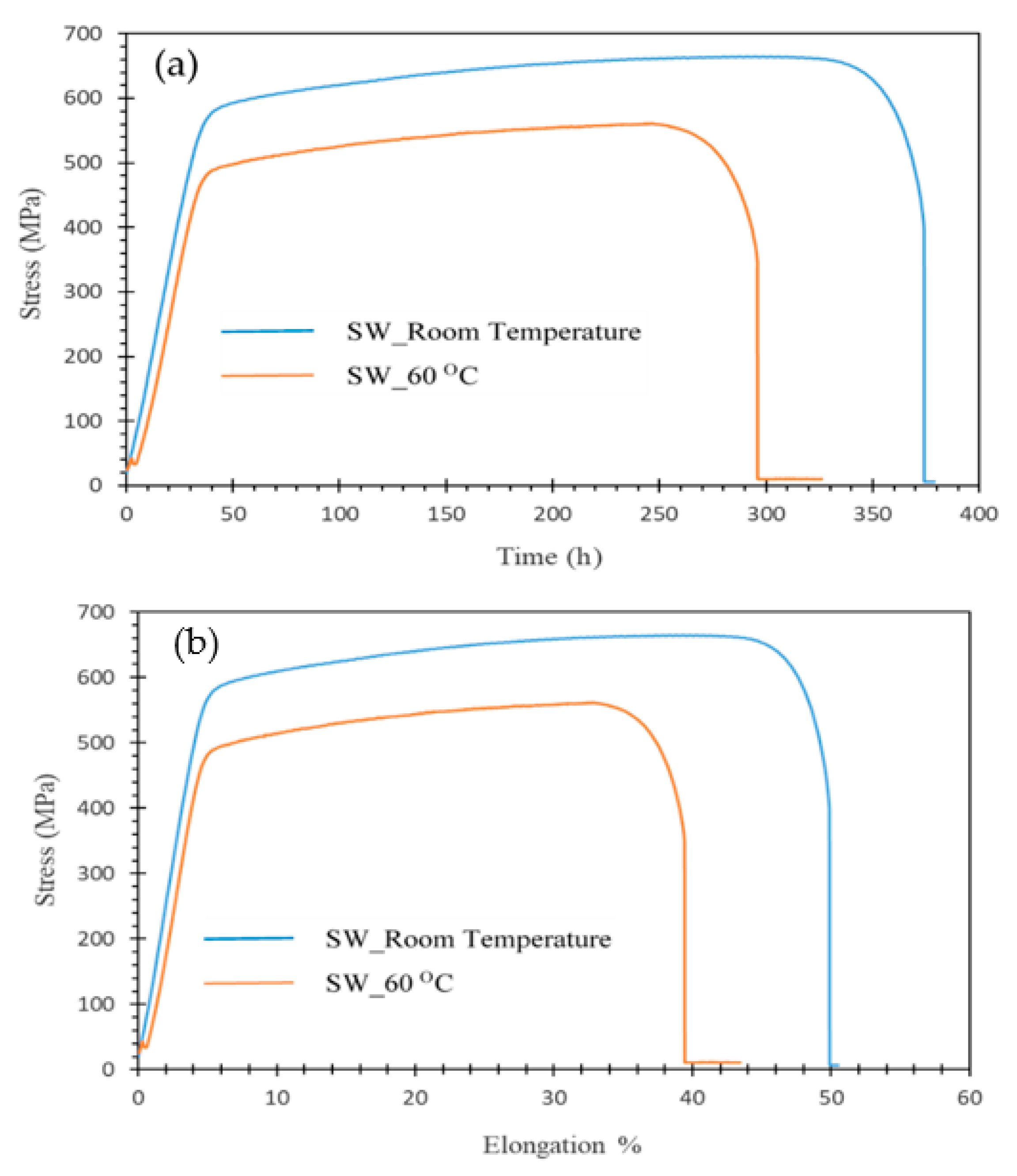
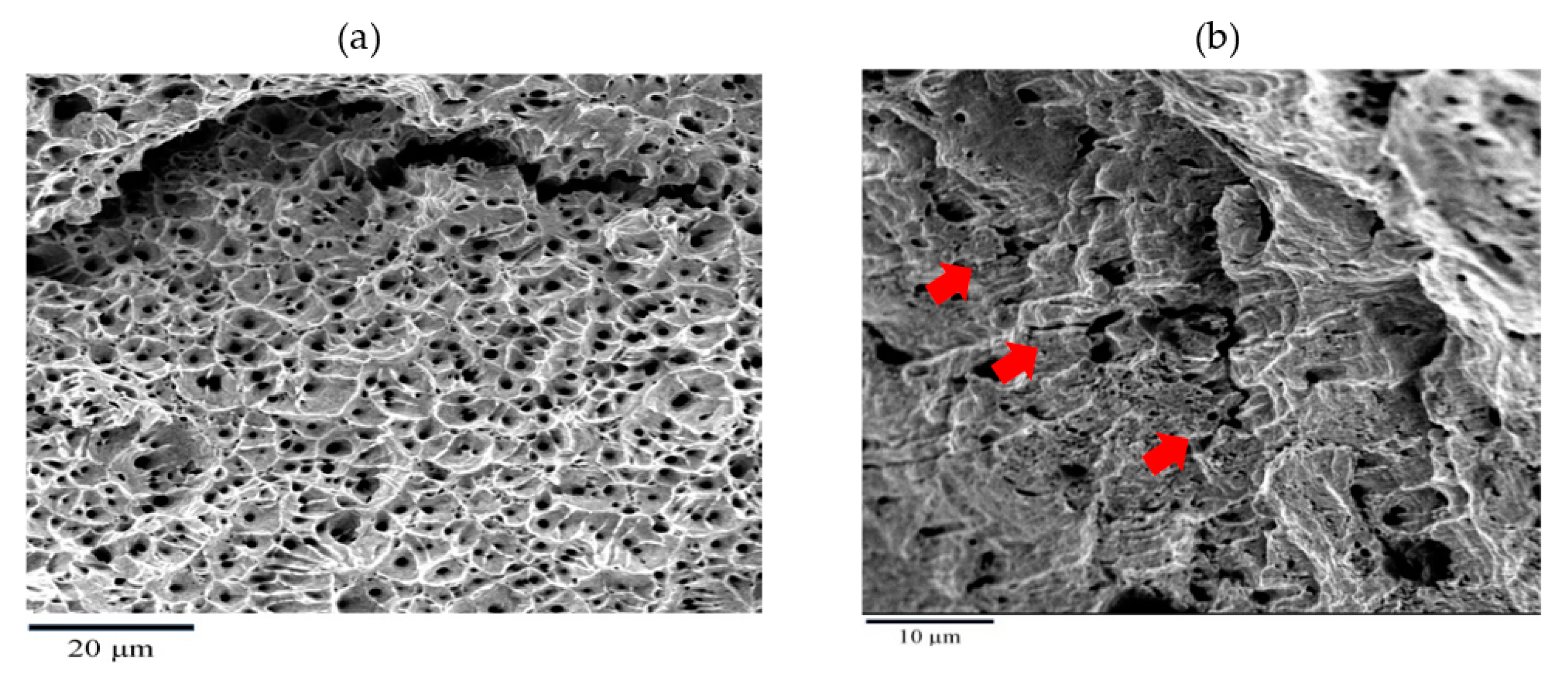
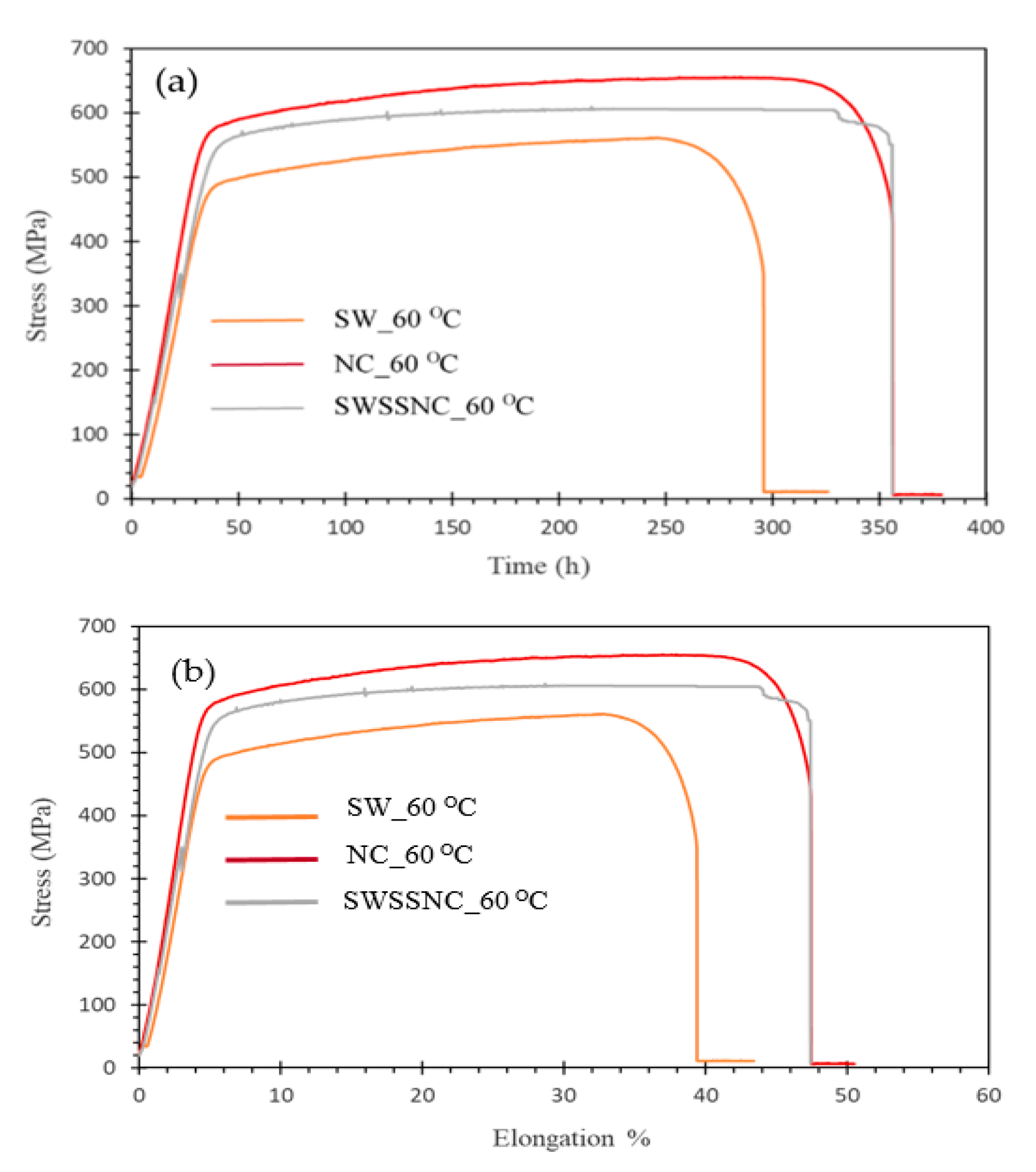
3. Results and Discussion
4. Conclusions
- (a)
- SS showed no SCC at 25 °C in any of the test solutions;
- (b)
- At 60 °C, some indications of SCC were seen in SW, but no SCC in SWSSC or NC;
- (c)
- Highly passivating alkaline condition accounts for the absence of SCC in SWSSC and NC at 60 °C. The absence of SCC in SWSSC also suggests ineffectiveness of chloride content of SWSSC in causing SCC;
- (d)
- The addition of silicate to SWSSC or NC caused transgranular SCC to SS at 60 °C, which may be the first finding of the role of silica in causing SCC to SS in concrete environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teng, J.G. Performance enhancement of structures through the use of fiber reinforced polymer (FRP) composites. In Proceedings of the 23rd Australasian Conference on the Mechanics of Structures and Materials (ACMSM23), Lismore, NSW, Australia, 9 December 2014. [Google Scholar]
- Qi, X.; Huang, Y.; Li, X.; Hu, Z.; Ying, J.; Li, D. Mechanical Properties of Sea Water Sea Sand Coral Concrete Modified with Different Cement and Fiber Types. J. Renew. Mater. 2020, 8, 915–937. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Singh, R.R.; Al-Saadi, S. Experimental study on seawater and sea sand concrete filled GFRP and stainless steel tubular stub columns. Thin Walled Struct. 2016, 106, 390–406. [Google Scholar] [CrossRef]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Mohammed, T.U.; Hamada, H.; Yamaji, T. Performance of seawater-mixed concrete in the tidal environment. Cem. Concr. Res. 2004, 34, 593–601. [Google Scholar] [CrossRef]
- Nishida, T.; Otsuki, N.; Ohara, H.; Garba-Say, Z.M.; Nagata, T. Some Considerations for Applicability of Seawater as Mixing Water in Concrete. J. Mater. Civ. Eng. 2015, 27, 4014004. [Google Scholar] [CrossRef]
- Zhang, G.L.; Chen, J.B.; Mo, L.W.; Liu, J.Z.; He, Z.M. Research on Influence of Fly Ash on the Microstructural Characteristics of Sea Sand Concrete. Appl. Mech. Mater. 2012, 204, 3831–3834. [Google Scholar] [CrossRef]
- Japan Concrete Institute Technical Committee. Report on the Use of Seawater in Concrete Japan; Japan Concrete Institute: Tokyo, Japan, 2015. [Google Scholar]
- Swamy, R.N.; All-Asali, M.M. Effect of Alkali-Silica Reaction on the Structural Behavior of Reinforced Concrete Beams. ACI Struct. J. 1989, 86, 451–459. [Google Scholar]
- Multon, S.; Sellier, A. Multi-scale analysis of alkali–silica reaction (ASR): Impact of alkali leaching on scale effects affecting expansion tests. Cem. Concr. Res. 2016, 81, 122–133. [Google Scholar] [CrossRef]
- Verbruggen, H.; Terryn, H.; De Graeve, I. Inhibitor evaluation in different simulated concrete pore solution for the protection of steel rebars. Constr. Build. Mater. 2016, 124, 887–896. [Google Scholar] [CrossRef]
- Li, C.Q.; Zheng, J.J.; Lawanwisut, W.; Melchers, R.E. Concrete Delamination Caused by Steel Reinforcement Corrosion. J. Mater. Civ. Eng. 2007, 19, 591–600. [Google Scholar] [CrossRef]
- Vacek, V.; Kolisko, J.; Pokorný, P.; Kostelecká, M. Steel Reinforcement Corrosion—Its Impact on Features of Steel PSC Strand. Key Eng. Mater. 2020, 868, 57–64. [Google Scholar] [CrossRef]
- Alhozaimy, A.; Hussain, R.R.; Al-Zaid, R.; Al Negheimish, A. Investigation of severe corrosion observed at intersection points of steel rebar mesh in reinforced concrete construction. Constr. Build. Mater. 2012, 37, 67–81. [Google Scholar] [CrossRef]
- Cramer, S.; Covino, B.; Bullard, S.; Holcomb, G.; Russell, J.; Nelson, F.; Laylor, H.; Soltesz, S. Corrosion prevention and remediation strategies for reinforced concrete coastal bridges. Cem. Concr. Compos. 2002, 24, 101–117. [Google Scholar] [CrossRef]
- Jones, D. The Technology and Evaluation of Corrosion, Principles and Prevention of Corrosion, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1996; pp. 3–4. [Google Scholar]
- Xie, Y.; Zhang, J.; Aldemir, T.; Denning, R. Multi-state Markov modeling of pitting corrosion in stainless steel exposed to chloride-containing environment. Reliab. Eng. Syst. Saf. 2018, 172, 239–248. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering; Tata McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Briz, E.; Biezma, M.; Bastidas, D. Stress corrosion cracking of new 2001 lean–duplex stainless steel reinforcements in chloride contained concrete pore solution: An electrochemical study. Constr. Build. Mater. 2018, 192, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Raman, R.S.; Yu, X. Axial compression tests on seawater and sea sand concrete-filled double-skin stainless steel circular tubes. Eng. Struct. 2018, 176, 426–438. [Google Scholar] [CrossRef]
- Raman, R.S.; Siew, W. Role of nitrite addition in chloride stress corrosion cracking of a super duplex stainless steel. Corros. Sci. 2010, 52, 113–117. [Google Scholar] [CrossRef]
- Parrott, R.; Pitts, H.; Hill, H.D. Chloride Stress Corrosion Cracking in Austenitic Stainless Steel; The Health and Safety Laboratory for the Health and Safety Executive: Buxton, UK, 2011.
- Vu, N.A.; Castel, A.; François, R. Effect of stress corrosion cracking on stress–strain response of steel wires used in prestressed concrete beams. Corros. Sci. 2009, 51, 1453–1459. [Google Scholar] [CrossRef]
- Raman, R.K.S. Evaluation of caustic embrittlement susceptibility of steels by slow strain rate testing. Met. Mater. Trans. 2005, 36, 1817–1823. [Google Scholar] [CrossRef]
- Truman, J. The influence of chloride content, pH and temperature of test solution on the occurrence of stress corrosion cracking with austenitic stainless steel. Corros. Sci. 1977, 17, 737–746. [Google Scholar] [CrossRef]
- Chen, Y.; Davalos, J.F.; Ray, I.; Kim, H.-Y. Accelerated aging tests for evaluations of durability performance of FRP reinforcing bars for concrete structures. Compos. Struct. 2007, 78, 101–111. [Google Scholar] [CrossRef]
- Guo, F.; Khoo, W.; Al-Saadi, S.H.M.; Li, Y.; Singh, R.R.K.; Zhao, X.L. Preliminary study on durability of FRP and stainless steel in seawater and sea sand concrete (SWSSC) environment. In Proceedings of the ASCCS International Conference on Steel-Concrete Composite and Hybrid Structures 2015, Beijing, China, 3–5 December 2015; pp. 1–8. [Google Scholar]
- Guo, F.; Al-Saadi, S.; Raman, R.S.; Zhao, X. Durability of fiber reinforced polymer (FRP) in simulated seawater sea sand concrete (SWSSC) environment. Corros. Sci. 2018, 141, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.-L.; Xian, G.; Wu, G.; Raman, R.S.; Al-Saadi, S.; Haque, A. Long-term durability of basalt- and glass-fibre reinforced polymer (BFRP/GFRP) bars in seawater and sea sand concrete environment. Constr. Build. Mater. 2017, 139, 467–489. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Xian, G.; Wu, G.; Raman, R.S.; Al-Saadi, S. Tensile properties of basalt-fibre reinforced polymer (BFRP) bars within seawater and sea sand concrete environment. In Proceedings of the 7th International Conference on Fibre-Reinforced Polymer (FRP) Composites in Civil Engineering (CICE 2016), Hong Kong, China, 14–16 December 2016. [Google Scholar]
- Zhang, B.; Hao, S.; Wu, J.; Li, X.; Li, C.; Di, X.; Huang, Y. Direct evidence of passive film growth on 316 stainless steel in alkaline solution. Mater. Charact. 2017, 131, 168–174. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Pourbaix Diagrams for the Ternary System of Iron-Chromium-Nickel. Corrosion 1999, 55, 1077–1087. [Google Scholar] [CrossRef]
- Saremi, M.; Mahallati, E. A study on chloride-induced depassivation of mild steel in simulated concrete pore solution. Cem. Concr. Res. 2002, 32, 1915–1921. [Google Scholar] [CrossRef]
- Leek, D.S.; Poole, A.B. The Breakdown of the Passive Film on High Yield Mild Steel by Chloride Ions. Corrosion of Reinforcement in Concrete. In Proceedings of the Third International Symposium on Corrosion of Reinforcement in Concrete Construction, Wishaw, Warwickshire, UK, 21–24 May 1990. [Google Scholar]
- Elfström, B.-O. The effect of chloride ions on passive layers on stainless steels. Mater. Sci. Eng. 1980, 42, 173–180. [Google Scholar] [CrossRef]
- Figueira, R.; Sousa, R.; Coelho, L.; Azenha, M.; De Almeida, J.; Jorge, P.; Silva, C. Alkali-silica reaction in concrete: Mechanisms, mitigation and test methods. Constr. Build. Mater. 2019, 222, 903–931. [Google Scholar] [CrossRef]
- Santos, M.B.; De Brito, J.; Silva, A.S. A Review on Alkali-Silica Reaction Evolution in Recycled Aggregate Concrete. Materials 2020, 13, 2625. [Google Scholar] [CrossRef]
- Diamond, S. A review of alkali-silica reaction and expansion mechanisms Alkalies in cements and in concrete pore solutions. Cem. Concr. Res. 1975, 5, 329–345. [Google Scholar] [CrossRef]
- Tang, Y.; Miao, Y.; Zuo, Y.; Zhang, G.; Wang, C. Corrosion behavior of steel in simulated concrete pore solutions treated with calcium silicate hydrates. Constr. Build. Mater. 2012, 30, 252–256. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; Liu, L.; She, W.; Yu, J. Experimental and molecular dynamics studies on the transport and ab-sorption of chloride ions in the nano-pores of calcium silicate phase: The influence of calcium to silicate ratios. Microporous Mesoporous Mater. 2018, 255, 23–35. [Google Scholar] [CrossRef]
- Świerczyńska, A.; Fydrych, D.; Landowski, M.; Rogalski, G.; Łabanowski, J. Hydrogen embrittlement of X2CrNiMoCuN25-6-3 super duplex stainless steel welded joints under cathodic protection. Constr. Build. Mater. 2020, 238, 117697. [Google Scholar] [CrossRef]


| Simulated Solution Nomenclature | Constituents (g/L) | pH | ||||
|---|---|---|---|---|---|---|
| NaOH | KOH | Ca(OH)2 | NaCl | Na2SiO3 | ||
| NC a | 2.4 | 19.6 | 2.0 | 13.4 | ||
| SWSSNC b | 2.4 | 19.6 | 2.0 | 35 | 13.4 | |
| NC_Sil | 2.4 | 19.6 | 2.0 | 3.0 | 13.4 | |
| SWSSNC_Sil | 2.4 | 19.6 | 2.0 | 35 | 3.0 | 13.4 |
| SW | 35 | 7.5 | ||||
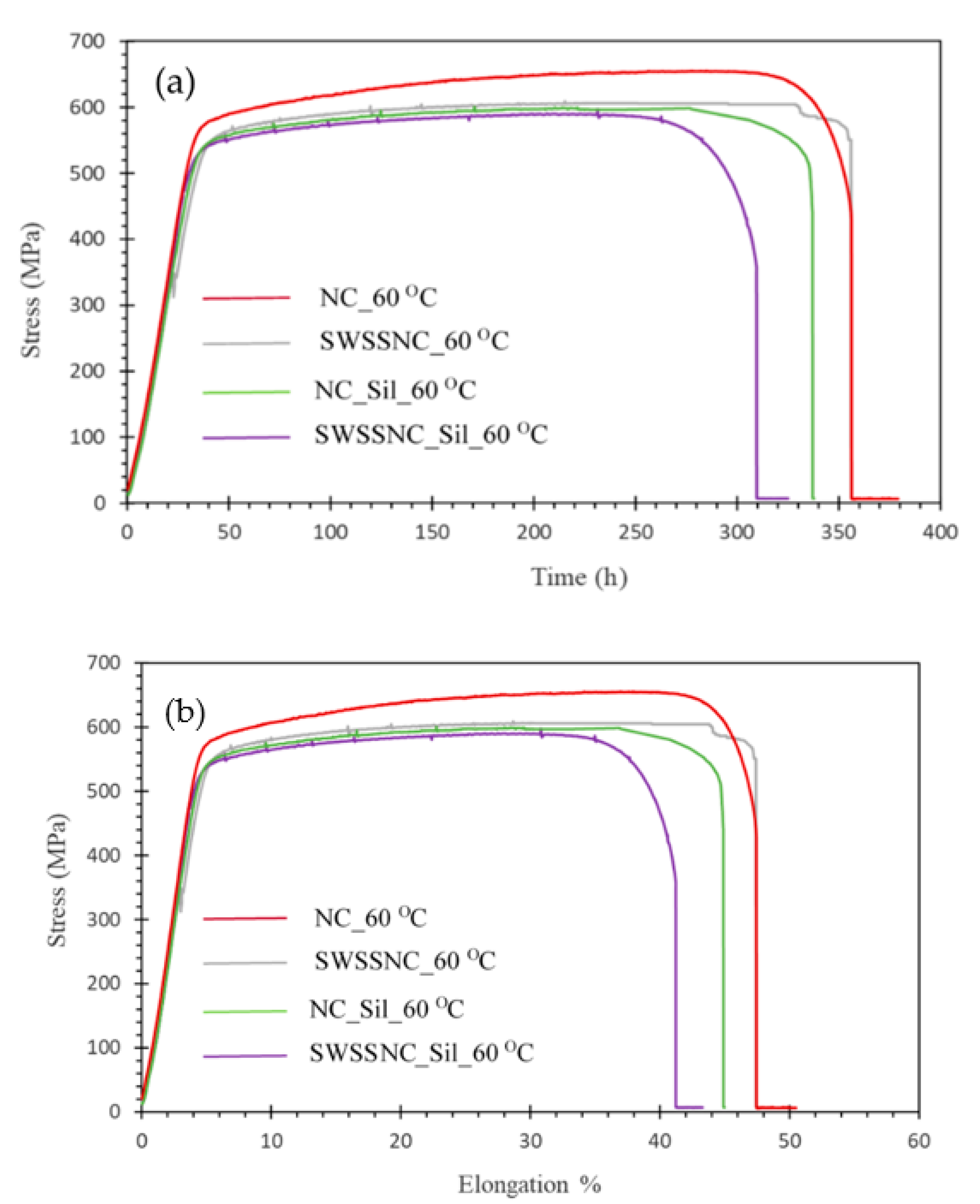
| Simulated Solution | Time to Failure (tf, h) | Elongation to Failure (Ԑf, %) | Ultimate Tensile Strength (UTS, MPa) |
|---|---|---|---|
| NC | 356 ± 6.8 | 47.4 ± 2.0 | 652 ± 6.6 |
| SWSSNC | 356 ± 2.4 | 47.4 ± 0.3 | 606 ± 1.9 |
| NC_Sil | 337 ± 5.2 | 44.9 ± 1.1 | 598 ± 3.9 |
| SWSSNC_Sil | 309 ± 6.5 | 41.2 ± 1.8 | 596 ± 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Al-Saadi, S.; Kohli, I.; Zhao, X.-L.; Singh Raman, R.K. Austenitic Stainless-Steel Reinforcement for Seawater Sea Sand Concrete: Investigation of Stress Corrosion Cracking. Metals 2021, 11, 500. https://doi.org/10.3390/met11030500
Yu X, Al-Saadi S, Kohli I, Zhao X-L, Singh Raman RK. Austenitic Stainless-Steel Reinforcement for Seawater Sea Sand Concrete: Investigation of Stress Corrosion Cracking. Metals. 2021; 11(3):500. https://doi.org/10.3390/met11030500
Chicago/Turabian StyleYu, Xiang, Saad Al-Saadi, Isha Kohli, Xiao-Ling Zhao, and R. K. Singh Raman. 2021. "Austenitic Stainless-Steel Reinforcement for Seawater Sea Sand Concrete: Investigation of Stress Corrosion Cracking" Metals 11, no. 3: 500. https://doi.org/10.3390/met11030500
APA StyleYu, X., Al-Saadi, S., Kohli, I., Zhao, X.-L., & Singh Raman, R. K. (2021). Austenitic Stainless-Steel Reinforcement for Seawater Sea Sand Concrete: Investigation of Stress Corrosion Cracking. Metals, 11(3), 500. https://doi.org/10.3390/met11030500








