Research on High-Pressure Hydrochloric Acid Leaching of Scandium, Aluminum and Other Valuable Components from the Non-Magnetic Tailings Obtained from Red Mud after Iron Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
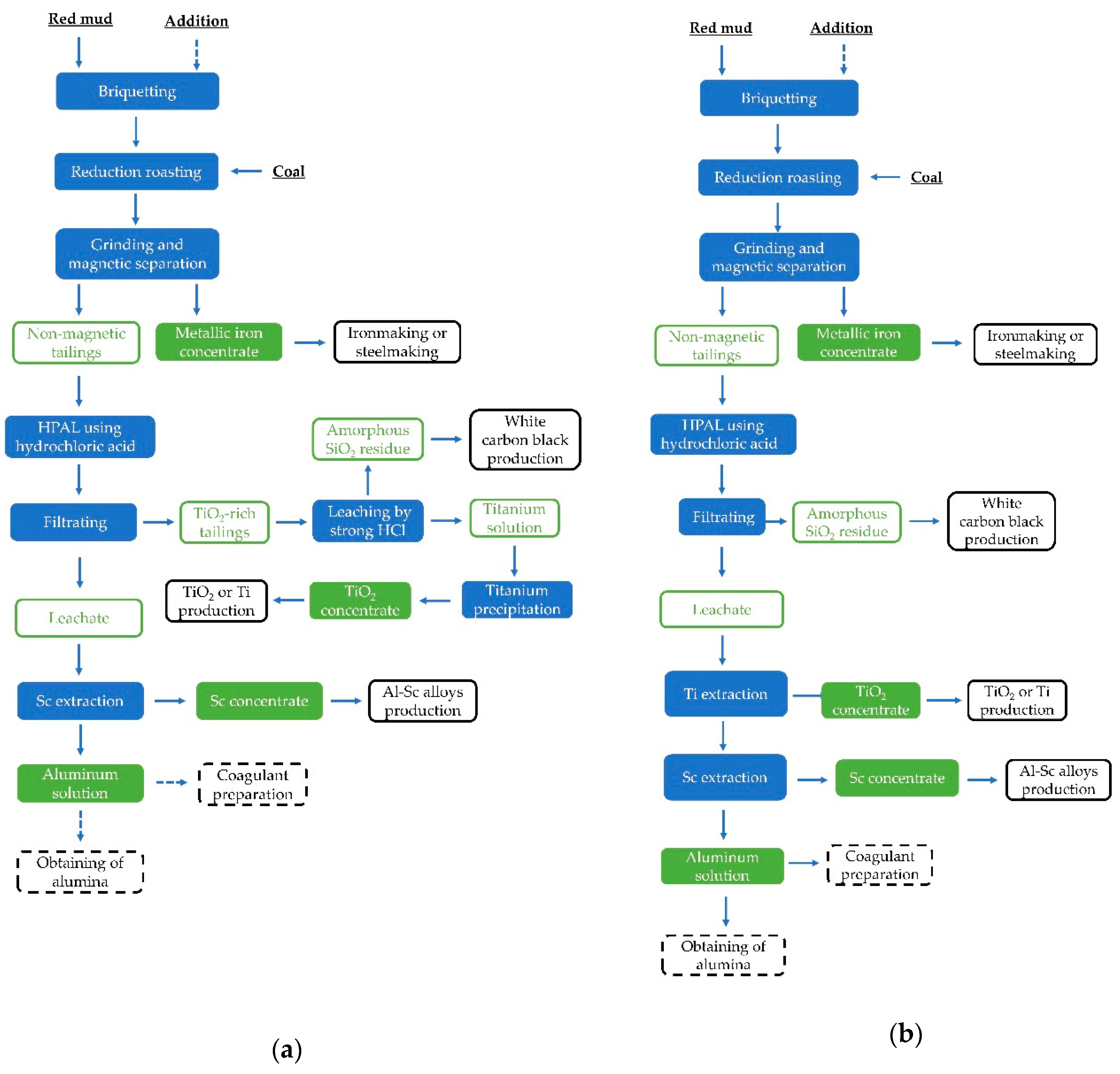
2.2. Experimental Procedure
3. Results
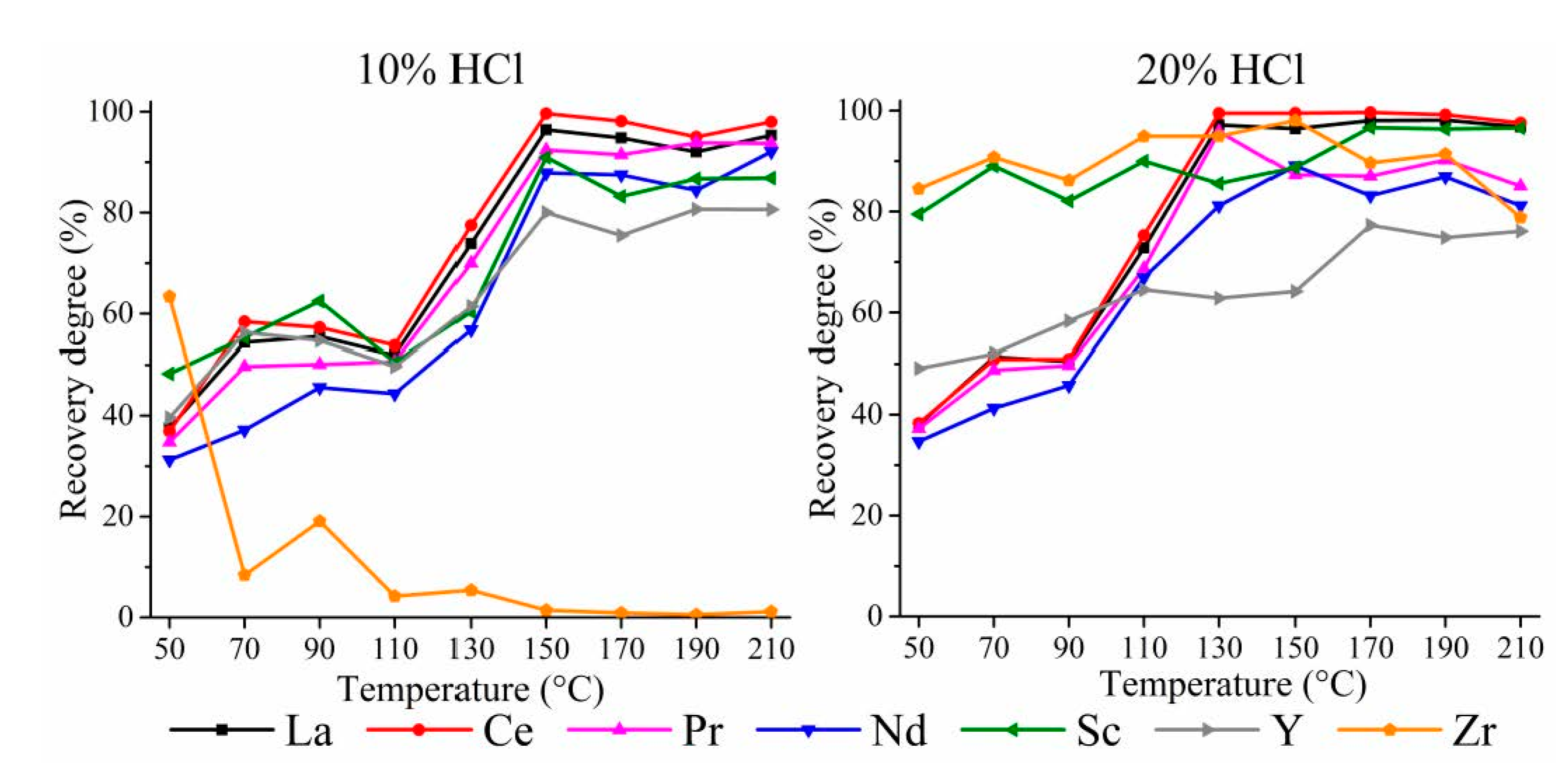
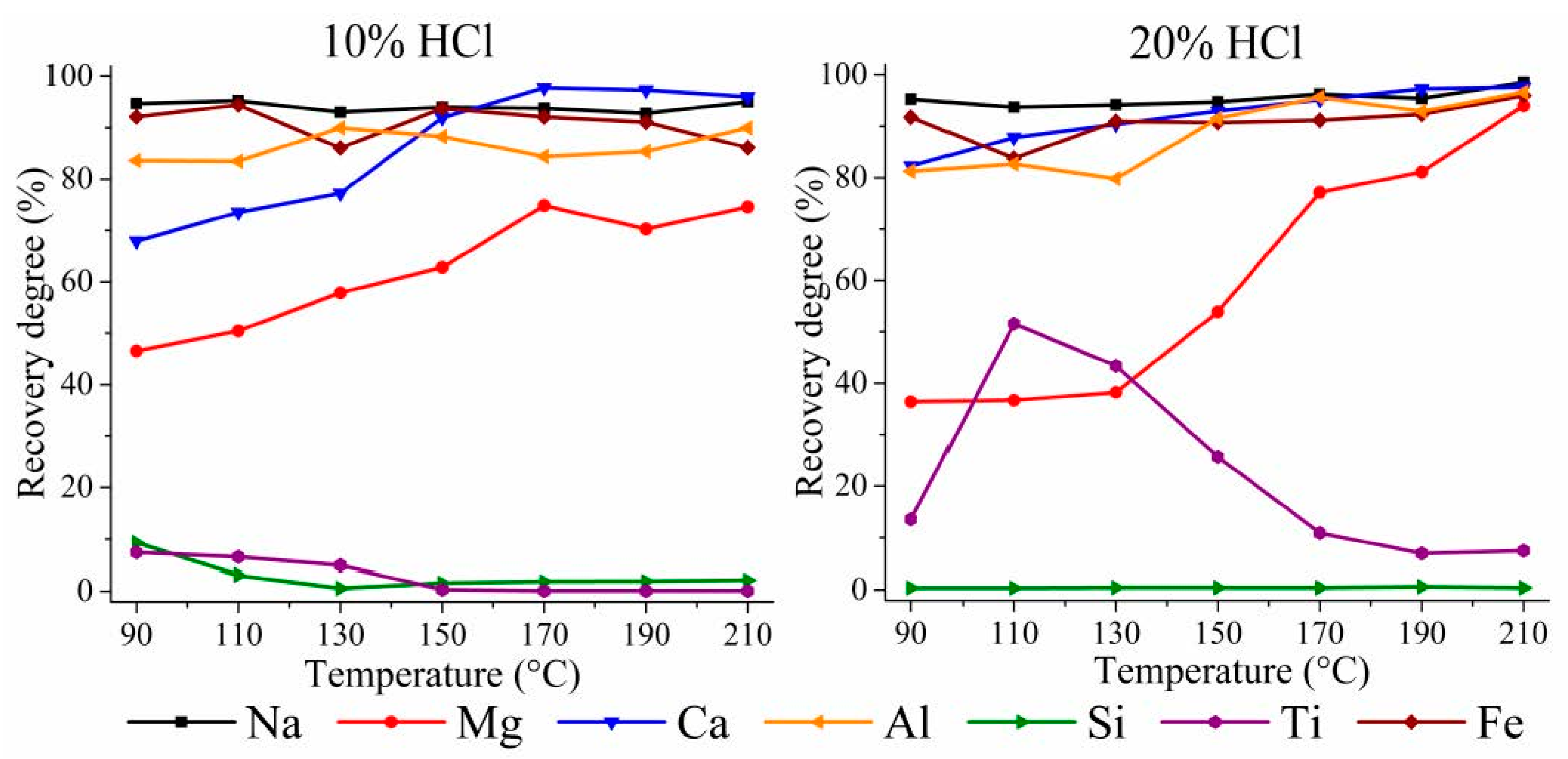
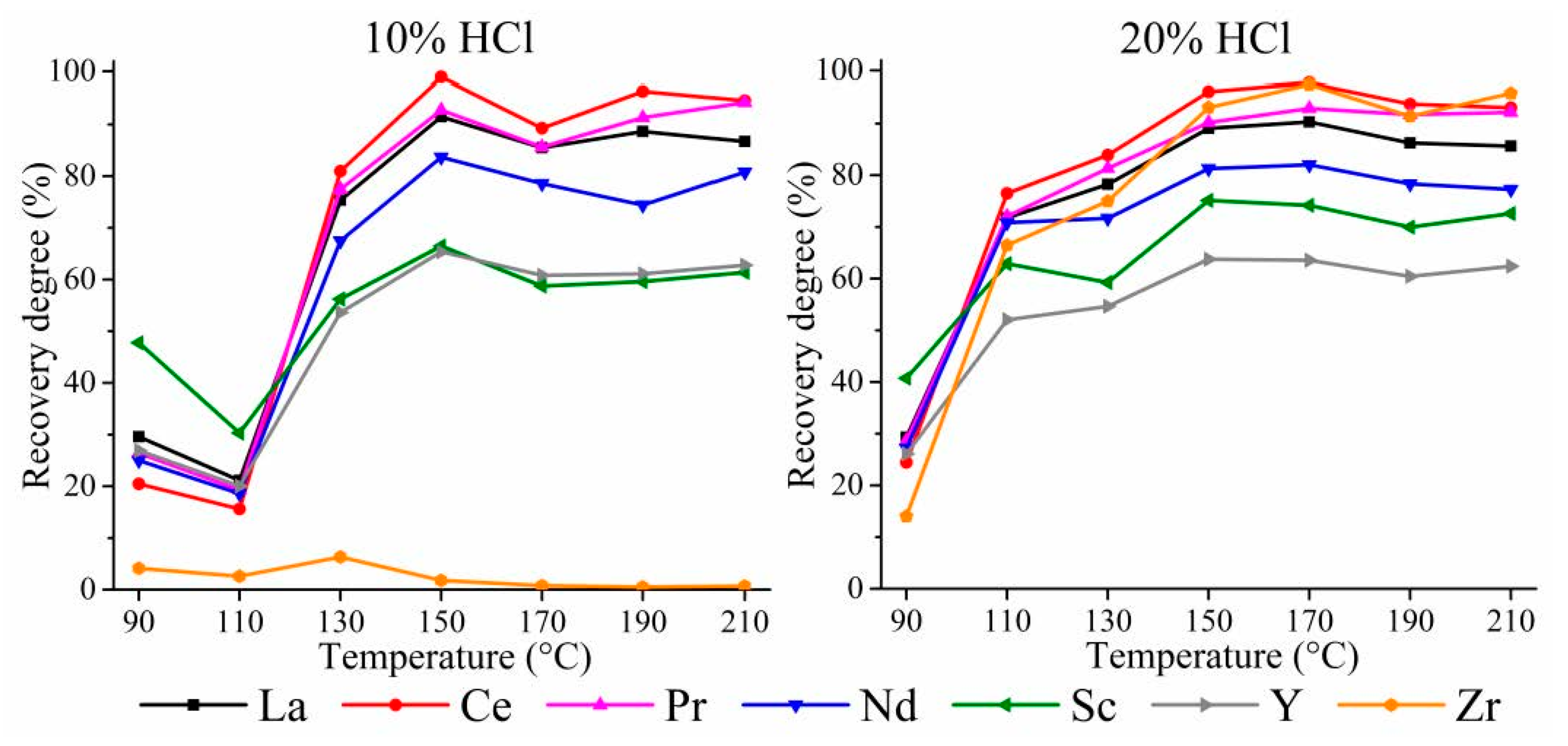
3.1. HPAL Experiments with the Variation of Leaching Temperature and HCl Concentration
3.1.1. HPAL for the WAT Sample
3.1.2. HPAL for the SSAT Sample
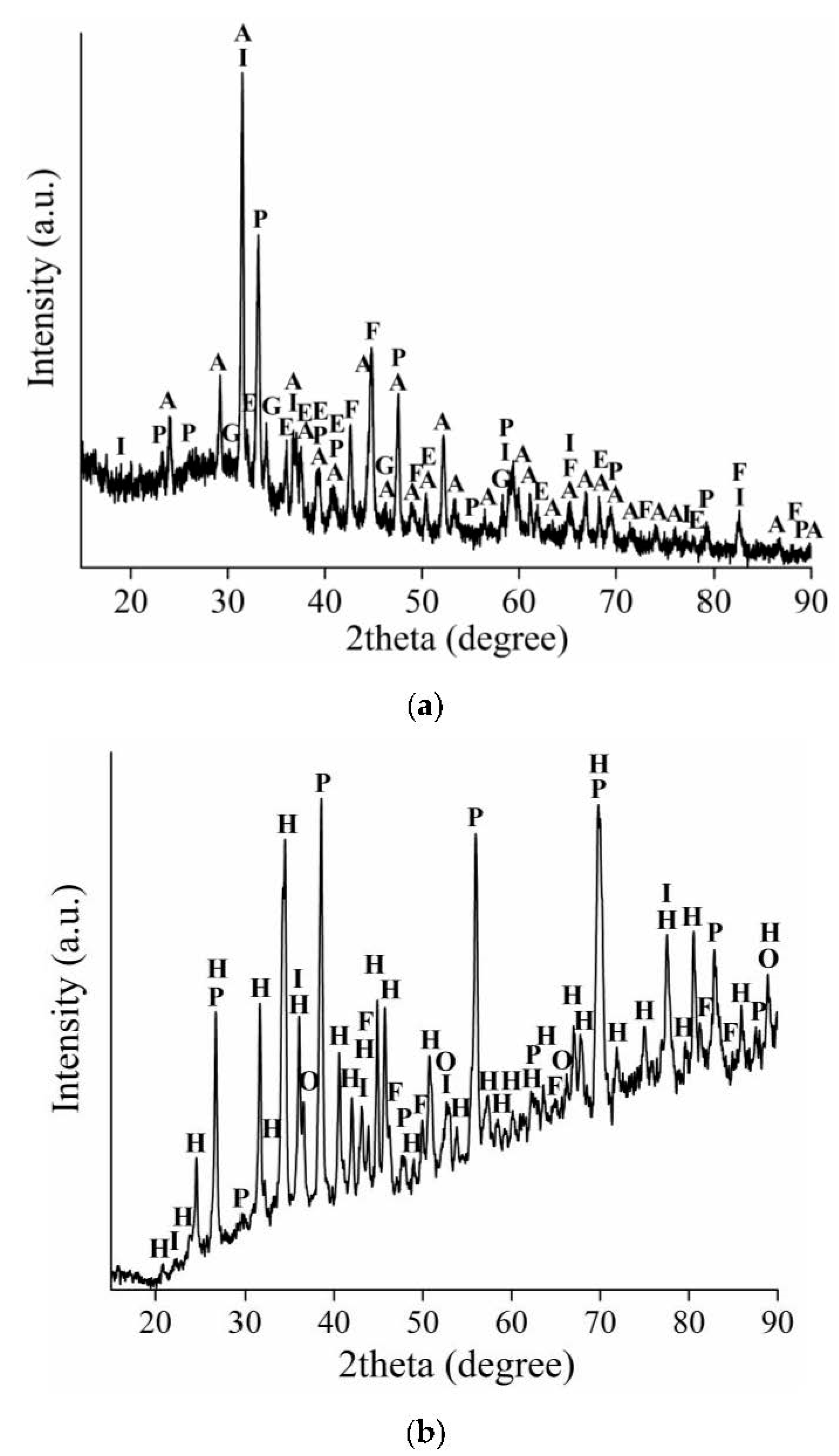
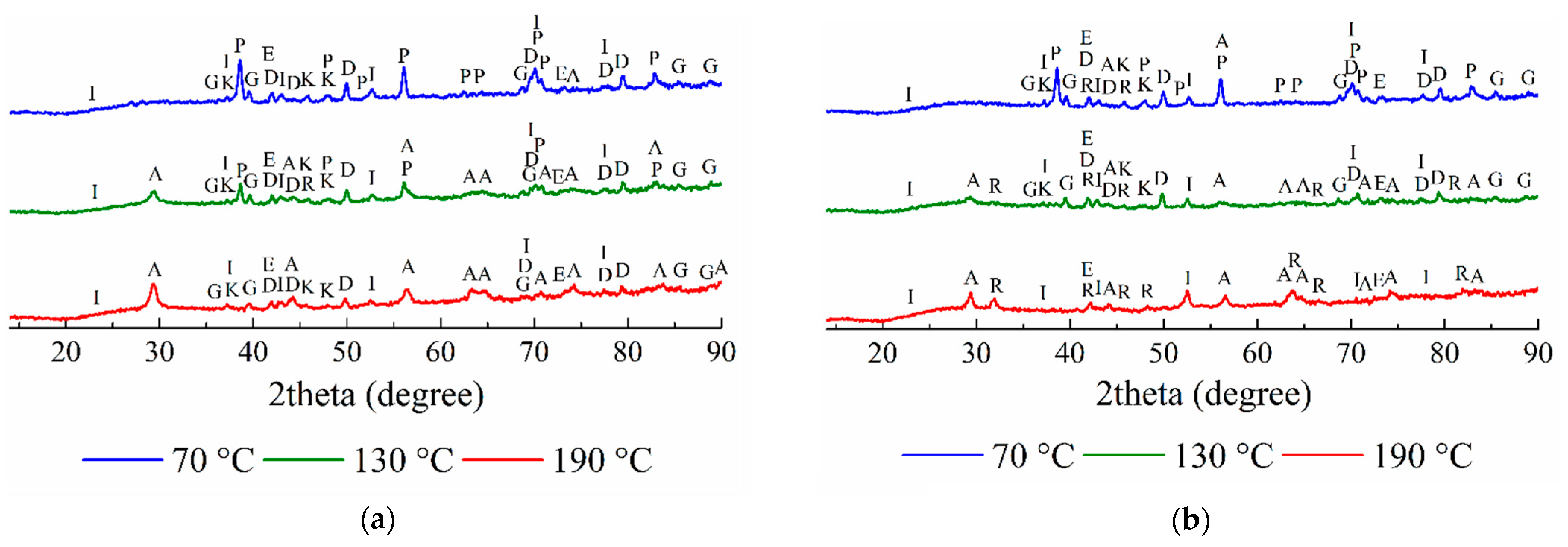
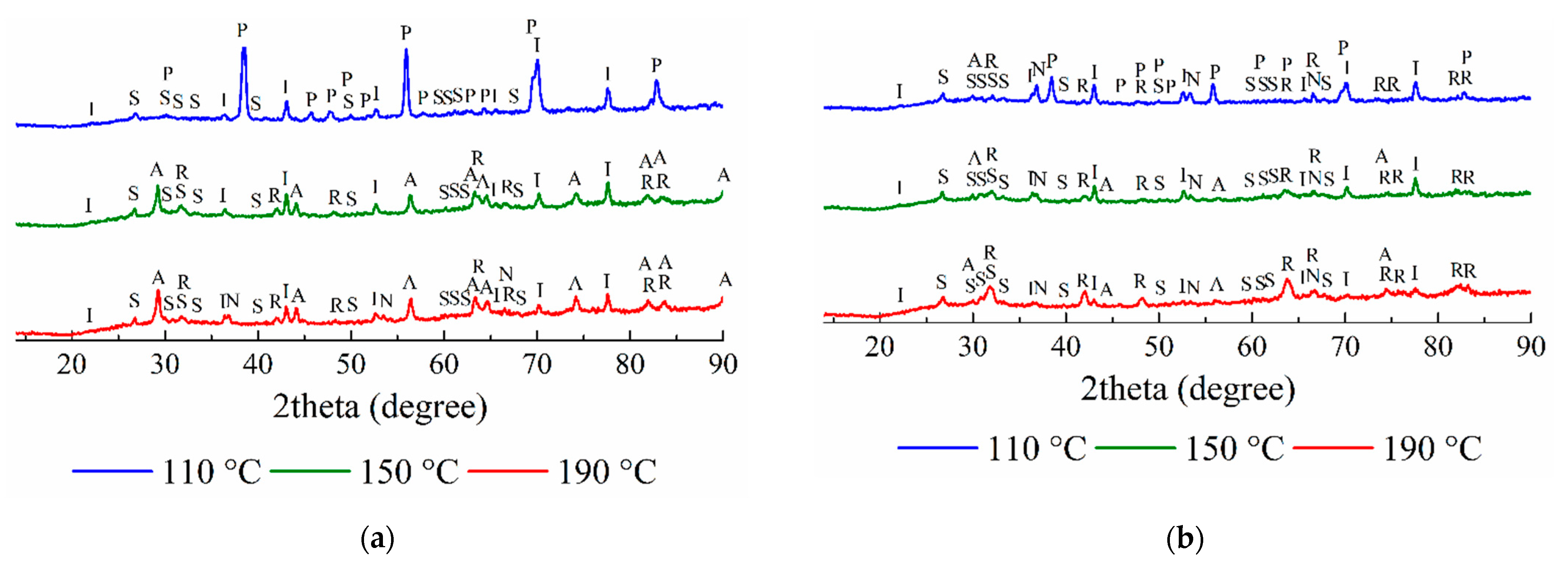
3.2. The Analysis of the Solid Residues
3.2.1. The Solid Residues Obtained from the WAT Sample
3.2.2. The Solid Residues Obtained from the SSAT Sample
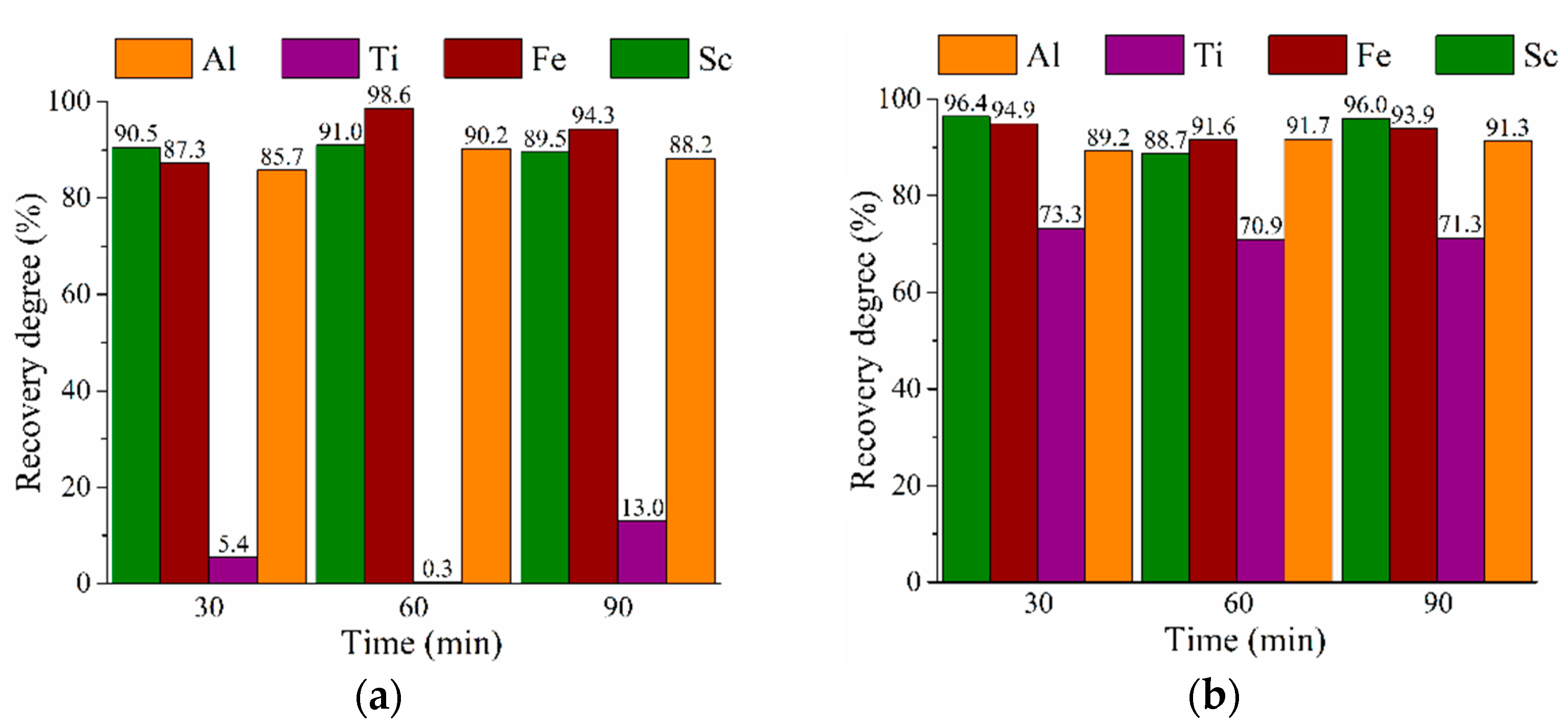
3.3. HPAL Experiments with Variation of Leaching Time, S:L Ratio, and HCl Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, H.; Lyu, F.; Sun, W.; Zhang, H.; Wang, L.; Wang, Y. Progress on the industrial applications of red mud with a focus on China. Minerals 2020, 10, 773. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A Review on Comprehensive Utilization of Red Mud. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Service, R.F. Red Mud Is Piling up. Can Scientists Figure out What to Do with It? Available online: https://www.sciencemag.org/news/2020/08/red-mud-piling-can-scientists-figure-out-what-do-it (accessed on 25 November 2020).
- Archambo, M.; Kawatra, S.K. Red Mud: Fundamentals and New Avenues for Utilization. Miner. Process. Extr. Metall. Rev. 2020, 1–24. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ippolito, N.M.; Ferro, S.; Dovi, V.G.; Vocciante, M. Removal of Mn and As from drinking water by red mud and pyrolusite. J. Environ. Manag. 2019, 237, 526–533. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef]
- Joseph, C.G.; Taufiq-Yap, Y.H.; Krishnan, V.; Li Puma, G. Application of modified red mud in environmentally-benign applications: A review paper. Environ. Eng. Res. 2020, 25, 795–806. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Meshram, A.; Meshram, P. Overview On Extraction and Separation of Rare Earth Elements from Red Mud: Focus on Scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Bandopadhyay, A. Innovative methodologies for the utilisation of wastes from metallurgical and allied industries. Resour. Conserv. Recycl. 2006, 48, 301–314. [Google Scholar] [CrossRef]
- Roy, S.K.; Nayak, D.; Rath, S.S. A review on the enrichment of iron values of low-grade Iron ore resources using reduction roasting-magnetic separation. Powder Technol. 2020, 367, 796–808. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Avdibegović, D.; Regadío, M.; Binnemans, K. Efficient separation of rare earths recovered by a supported ionic liquid from bauxite residue leachate. RSC Adv. 2018, 8, 11886–11893. [Google Scholar] [CrossRef]
- Meng, F.; Li, X.; Wang, P.; Yang, F.; Liang, D.; Gao, F.; He, C.; Wei, Y. Recovery of Scandium from Bauxite Residue by Selective Sulfation Roasting with Concentrated Sulfuric Acid and Leaching. JOM 2020, 72, 816–822. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Kazantzis, N.K.; Emmert, M.H. Selective Process Steps for the Recovery of Scandium from Jamaican Bauxite Residue (Red Mud). ACS Sustain. Chem. Eng. 2018, 6, 1478–1488. [Google Scholar] [CrossRef]
- Bayca, S.U.; Kisik, H. Optimization of leaching parameters of aluminum hydroxide extraction from bauxite waste using the Taguchi method. Environ. Prog. Sustain. Energy 2018, 37, 196–202. [Google Scholar] [CrossRef]
- Vaylert, A.V.; Pyagay, I.N.; Kozhevnikov, V.L.; Pasechnik, L.A.; Yatsenko, S.P. Autoclave hydrometallurgical processing of alumina production red mud. Tsvetnye Met. 2014, 3, 27–31. [Google Scholar]
- Shoppert, A.A.; Loginova, I.V. Red Mud as an Additional Source of Titanium Raw Materials. KnE Mater. Sci. 2017, 2, 150–157. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Investigation of mechanical and thermal activation on metal extraction from red mud. Sustain. Mater. Technol. 2021, 27, e00246. [Google Scholar] [CrossRef]
- Reid, S.; Tam, J.; Yang, M.; Azimi, G. Technospheric Mining of Rare Earth Elements from Bauxite Residue (Red Mud): Process Optimization, Kinetic Investigation, and Microwave Pretreatment. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Ochsenkuehn-Petropoulou, M.; Tsakanika, L.-A.; Lymperopoulou, T.; Ochsenkuehn, K.-M.; Hatzilyberis, K.; Georgiou, P.; Stergiopoulos, C.; Serifi, O.; Tsopelas, F. Efficiency of Sulfuric Acid on Selective Scandium Leachability from Bauxite Residue. Metals 2018, 8, 915. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, N.; Li, J.; Zhou, F.; Ma, X.; Gu, G.; Zhang, W. Optimization of condition for extraction of aluminum and iron from red mud by hydrochloric acid leaching. Chin. J. Environ. Eng. 2017, 11, 5677–5682. [Google Scholar] [CrossRef]
- Alkan, G.; Schier, C.; Gronen, L.; Stopic, S.; Friedrich, B. A Mineralogical Assessment on Residues after Acidic Leaching of Bauxite Residue (Red Mud) for Titanium Recovery. Metals 2017, 7, 458. [Google Scholar] [CrossRef]
- Zhu, X.; Niu, Z.; Li, W.; Zhao, H.; Tang, Q. A novel process for recovery of aluminum, iron, vanadium, scandium, titanium and silicon from red mud. J. Environ. Chem. Eng. 2019, 8, 103528. [Google Scholar] [CrossRef]
- Gu, H.; Li, W.; Li, Z.; Guo, T.; Wen, H.; Wang, N. Leaching Behavior of Lithium from Bauxite Residue Using Acetic Acid. Min. Metall. Explor. 2020, 37, 443–451. [Google Scholar] [CrossRef]
- Atalay Kalsen, T.S.; Karadağ, H.B.; Eker, Y.R.; Kerti, I. Chemical Composition Simplification of the Seydişehir (Konya, Turkey) Alumina Plant Waste. J. Sustain. Metall. 2019, 5, 482–496. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Onghena, B.; Binnemans, K. Recovery of Scandium(III) from aqueous solutions by solvent extraction with the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide. Ind. Eng. Chem. Res. 2015, 54, 1887–1898. [Google Scholar] [CrossRef]
- Rivera, R.M.; Xakalashe, B.; Ounoughene, G.; Binnemans, K.; Friedrich, B.; Van Gerven, T. Selective rare earth element extraction using high-pressure acid leaching of slags arising from the smelting of bauxite residue. Hydrometallurgy 2019, 184, 162–174. [Google Scholar] [CrossRef]
- Zinoveev, D.V.; Grudinskii, P.I.; Dyubanov, V.G.; Kovalenko, L.V.; Leont’ev, L.I. Global recycling experience of red mud—A review. Part I: Pyrometallurgical methods. Izv. Ferr. Metall. 2018, 61, 843–858. [Google Scholar] [CrossRef]
- Alkan, G.; Xakalashe, B.; Yagmurlu, B.; Kaußen, F.; Friedrich, B. Conditioning of red mud for subsequent titanium and scandium recovery—A conceptual design study. World Metall. Erzmetall 2017, 70, 5–12. [Google Scholar]
- Balomnenos, E.; Kastritis, D.; Panias, D.; Paspaliaris, I.; Boufounos, D. The Enexal Bauxite Residue Treatment Process: Industrial Scale Pilot Plant Results. In Light Metals 2014; Grandfield, J., Ed.; Springer: Cham, Switzerland, 2014; pp. 143–147. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Ragona, M.; Pisu, M.; Viola, A. Characterization of cast iron and slag produced by red muds reduction via Arc Transferred Plasma (ATP) reactor under different smelting conditions. J. Environ. Chem. Eng. 2020, 8, 104293. [Google Scholar] [CrossRef]
- Ning, G.; Zhang, B.; Liu, C.; Li, S.; Ye, Y.; Jiang, M. Large-Scale Consumption and Zero-Waste Recycling Method of Red Mud in Steel Making Process. Minerals 2018, 8, 102. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Major Metals from Bauxite Residue (Red Mud) by Alkali Roasting, Smelting, and Leaching. J. Sustain. Metall. 2017, 3, 393–404. [Google Scholar] [CrossRef]
- Chun, T.J.; Zhu, D.Q.; Pan, J.; He, Z. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Q. 2014, 53, 183–189. [Google Scholar] [CrossRef]
- Ding, W.; Xiao, J.-H.; Peng, Y.; Shen, S.-Y.; Chen, T. Iron Extraction from Red Mud using Roasting with Sodium Salt. Miner. Process. Extr. Metall. Rev. 2019, 1–9. [Google Scholar] [CrossRef]
- Zinoveev, D.; Grudinsky, P.; Zakunov, A.; Semenov, A.; Panova, M.; Valeev, D.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Influence of Na2CO3 and K2CO3 addition on iron grain growth during carbothermic reduction of red mud. Metals 2019, 9, 1313. [Google Scholar] [CrossRef]
- Chun, T.; Li, D.; Di, Z.; Long, H.; Tang, L.; Li, F.; Li, Y. Recovery of iron from red mud by high temperature reduction of carbon bearing briquettes. J. South. Afr. Inst. Min. Metall. 2017, 117, 361–364. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Chun, T.J.; Pan, J.; He, Z. Recovery of Iron From High-Iron Red Mud by Reduction Roasting with Adding Sodium Salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Ding, W.; Peng, Y.; Wu, Q.; Shen, S.; Xiao, J.; Liang, G.; Huang, W. Increase of iron concentration and reduction of impurities in red mud from the Wenshan area of Yunnan Province by segregation roasting-low intensity magnetic separation. J. Mines Met. Fuels 2019, 67, 377–384. [Google Scholar]
- Gao, F.; Zhang, J.; Deng, X.; Wang, K.; He, C.; Li, X.; Wei, Y. Comprehensive Recovery of Iron and Aluminum from Ordinary Bayer Red Mud by Reductive Sintering–Magnetic Separation–Digesting Process. JOM 2019, 71, 2936–2943. [Google Scholar] [CrossRef]
- Li, G.; Luo, J.; Jiang, T.; Li, Z.; Peng, Z.; Zhang, Y. Digestion of alumina from non-magnetic material obtained from magnetic separation of reduced iron-rich diasporic bauxite with sodium salts. Metals 2016, 6, 294. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Rao, M.; Jiang, T.; Zhuang, J.; Zhang, Y. Stepwise extraction of valuable components from red mud based on reductive roasting with sodium salts. J. Hazard. Mater. 2014, 280, 774–780. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K. Behaviour of Silica during Metal Recovery from Bauxite Residue by Acidic Leaching. In Proceedings of the 35th International ICSOBA Conference, Hamburg, Germany, 2–5 October 2017; Académie Croate Des Sciences Et Des Arts: Hamburg, Germany, 2017; pp. 547–556. [Google Scholar]
- Deng, B.; Li, G.; Luo, J.; Ye, Q.; Liu, M.; Peng, Z.; Jiang, T. Enrichment of Sc2O3 and TiO2 from bauxite ore residues. J. Hazard. Mater. 2017, 331, 71–80. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- Valeev, D.; Kunilova, I.; Alpatov, A.; Mikhailova, A.; Goldberg, M.; Kondratiev, A. Complex utilisation of ekibastuz brown coal fly ash: Iron & carbon separation and aluminum extraction. J. Clean. Prod. 2019, 218, 192–201. [Google Scholar] [CrossRef]
- Valeev, D.V.; Lainer, Y.A.; Pak, V.I. Autoclave leaching of boehmite-kaolinite bauxites by hydrochloric acid. Inorg. Mater. Appl. Res. 2016, 7, 272–277. [Google Scholar] [CrossRef]
- Valeev, D.V.; Lainer, Y.A.; Mikhailova, A.B.; Dorofievich, I.V.; Zheleznyi, M.V.; Gol’dberg, M.A.; Kutsev, S.V. Reaction of Bauxite with Hydrochloric Acid Under Autoclave Conditions. Metallurgist 2016, 60, 204–211. [Google Scholar] [CrossRef]
- Li, S.; Qin, S.; Kang, L.; Liu, J.; Wang, J.; Li, Y. An Efficient Approach for Lithium and Aluminum Recovery from Coal Fly Ash by Pre-Desilication and Intensified Acid Leaching Processes. Metals 2017, 7, 272. [Google Scholar] [CrossRef]
- Grudinsky, P.; Zinoveev, D.; Pankratov, D.; Semenov, A.; Panova, M.; Kondratiev, A.; Zakunov, A.; Dyubanov, V.; Petelin, A. Influence of Sodium Sulfate Addition on Iron Grain Growth during Carbothermic Roasting of Red Mud Samples with Different Basicity. Metals 2020, 10, 1571. [Google Scholar] [CrossRef]
- Match! Version 3.11; Software for Identification of Powder Diffraction Patterns; Crystal Impact: Bonn, Germany, 2021.
- Falayi, T.; Ntuli, F.; Ndubisi, F. Kinetic and thermodynamic parameters of silica leaching from Camden power station fly ash. In Proceedings of the 4th NAUN International Conference on Energy Systems, Environment, Entrepreneurship and Innovation (ICESEEI′15), Dubai, United Arab Emirates, 22–24 February 2015; WSEAS: Dubai, United Arab Emirates, 2017; pp. 241–248. [Google Scholar]
- Han, J.; Zhang, J.; Feng, W.; Chen, X.; Zhang, L.; Tu, G. A clean process to prepare high-quality acid-soluble titanium slag from titanium middling ore. Minerals 2019, 9, 460. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, D.; Wang, W.; Yi, L.; Chen, D.; Zhao, H.; Wu, J.; Qi, T.; Cao, C. Preparation of rutile TiO2 by hydrolysis of TiOCl2 solution: Experiment and theory. RSC Adv. 2016, 6, 59541–59549. [Google Scholar] [CrossRef]
- Deng, B.; Li, G.; Luo, J.; Ye, Q.; Liu, M.; Rao, M.; Jiang, T.; Bauman, L.; Zhao, B. Selectively leaching the iron-removed bauxite residues with phosphoric acid for enrichment of rare earth elements. Sep. Purif. Technol. 2019, 227, 115714. [Google Scholar] [CrossRef]
- Wajima, T. Effects of step-wise acid leaching with HCl on synthesis of zeolitic materials from paper sludge ash. Minerals 2020, 10, 402. [Google Scholar] [CrossRef]
- Rivera, R.M.; Ulenaers, B.; Ounoughene, G.; Binnemans, K.; Van Gerven, T. Extraction of rare earths from bauxite residue (red mud) by dry digestion followed by water leaching. Miner. Eng. 2018, 119, 82–92. [Google Scholar] [CrossRef]
- Yu, W.; Wen, X.; Chen, J.; Tang, Q.; Dong, W.; Zhong, J. Effect of Sodium Borate on the Preparation of TiN from Titanomagnetite Concentrates by Carbothermic Reduction—Magnetic Separation and Acid Leaching Process. Minerals 2019, 9, 675. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; He, H.; Sun, Z.; Li, A. Effective aluminum extraction using pressure leaching of bauxite reaction residue from coagulant industry and leaching kinetics study. J. Environ. Chem. Eng. 2020, in press. [Google Scholar] [CrossRef]
- Digne, M.; Sautet, P.; Raybaud, P.; Toulhoat, H.; Artacho, E. Structure and stability of aluminum hydroxides: A theoretical study. J. Phys. Chem. B 2002, 106, 5155–5162. [Google Scholar] [CrossRef]
- Shrivastava, O.P.; Kumar, N.; Sharma, I.B. Solid state synthesis and structural refinement of polycrystalline LaxCa1-xTiO3 ceramic powder. Bull. Mater. Sci. 2004, 27, 121–126. [Google Scholar] [CrossRef][Green Version]
- Anawati, J.; Azimi, G. Recovery of scandium from Canadian bauxite residue utilizing acid baking followed by water leaching. Waste Manag. 2019, 95, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Steijns, M.; Derks, F.; Verloop, A.; Mars, P. The mechanism of the catalytic oxidation of hydrogen sulfide. II. Kinetics and mechanism of hydrogen sulfide oxidation catalyzed by sulfur. J. Catal. 1976, 42, 87–95. [Google Scholar] [CrossRef]
- Kshumaneva, E.S.; Kasikov, A.G.; Neradovskii, Y.N. Behavior of sulfides of nonferrous metals in hydrochloric-acid leaching of residues formed in synthesis of carbonyl nickel. Russ. J. Appl. Chem. 2005, 78, 178–183. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Bonomi, C.; Paiste, P.; Sajó, I.E.; Blanpain, B.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Modes of occurrences of scandium in Greek bauxite and bauxite residue. Miner. Eng. 2018, 123, 35–48. [Google Scholar] [CrossRef]
- Huang, F.; Liao, Y.; Zhou, J.; Wang, Y.; Li, H. Selective recovery of valuable metals from nickel converter slag at elevated temperature with sulfuric acid solution. Sep. Purif. Technol. 2015, 156, 572–581. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef]
- Zablotskaya, Y.V.; Sadykhov, G.B.; Gocharenko, T.V. Autoclave leaching kinetics of a leucoxene concentrate with alkaline solutions. Russ. Metall. 2015, 2015, 3–7. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Cao, X.; Wu, S.; Liu, C.; Li, G.; Jiang, W.; Wang, H.; Wang, N.; Ding, W. Preparation and characterization of TiO2 nanoparticles by two different precipitation methods. Ceram. Int. 2020, 46, 15333–15341. [Google Scholar] [CrossRef]
- Yuan, W.; Yao, Z.; Zhang, Q.; Li, J. Characterization of residue from leached cathode ray tube funnel glass: Reutilization as white carbon black. J. Mater. Cycles Waste Manag. 2014, 16, 629–634. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Recovery of Rare Earths and Other Valuable Metals From Bauxite Residue (Red Mud): A Review. J. Sustain. Metall. 2016, 2, 365–386. [Google Scholar] [CrossRef]
- Valeev, D.; Kunilova, I.; Shoppert, A.; Salazar-Concha, C.; Kondratiev, A. High-pressure HCl leaching of coal ash to extract Al into a chloride solution with further use as a coagulant for water treatment. J. Clean. Prod. 2020, 276, 123206. [Google Scholar] [CrossRef]
- Valeev, D.; Shoppert, A.; Mikhailova, A.; Kondratiev, A. Acid and Acid-Alkali Treatment Methods of Al-Chloride Solution Obtained by the Leaching of Coal Fly Ash to Produce Sandy Grade Alumina. Metals 2020, 10, 585. [Google Scholar] [CrossRef]
- Azopkov, S.V.; Kuzin, E.N.; Kruchinina, N.E. Study of the Efficiency of Combined Titanium Coagulants in the Treatment of Formation Waters. Russ. J. Gen. Chem. 2020, 90, 1811–1816. [Google Scholar] [CrossRef]
- Kuzin, E.N.; Kruchinina, N.E. Purification of circulating and waste water in metallurgical industry using complex coagulants. CIS Iron Steel Rev. 2019, 18, 72–75. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Wang, M.; Wang, J.; Wu, S.; Yao, X.; Li, L. Separation of elemental sulfur from zinc concentrate direct leaching residue by vacuum distillation. Sep. Purif. Technol. 2014, 138, 41–46. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, K.; Zhang, B.; Dong, Z.; Zhang, F.; Wang, F.; Jiang, T.; Xu, B. Selective Flotation of Elemental Sulfur from Pressure Acid Leaching Residue of Zinc Sulfide. Minerals 2021, 11, 89. [Google Scholar] [CrossRef]
- Mombelli, D.; Barella, S.; Gruttadauria, A.; Mapelli, C. Iron recovery from Bauxite Tailings Red Mud by thermal reduction with blast furnace sludge. Appl. Sci. 2019, 9, 4902. [Google Scholar] [CrossRef]
- Yao, Z.T.; Xia, M.S.; Sarker, P.K.; Chen, T. A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 2014, 120, 74–85. [Google Scholar] [CrossRef]

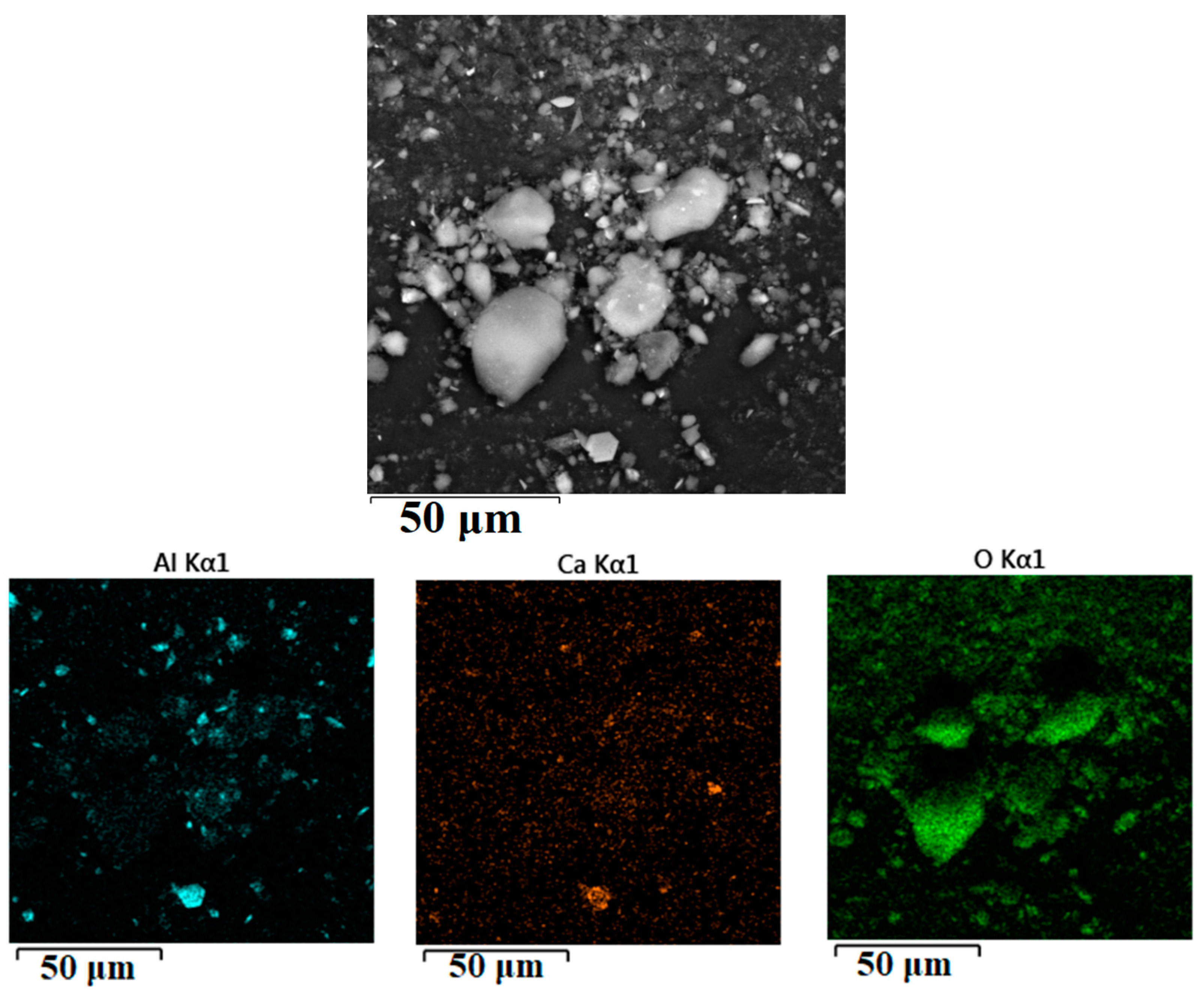

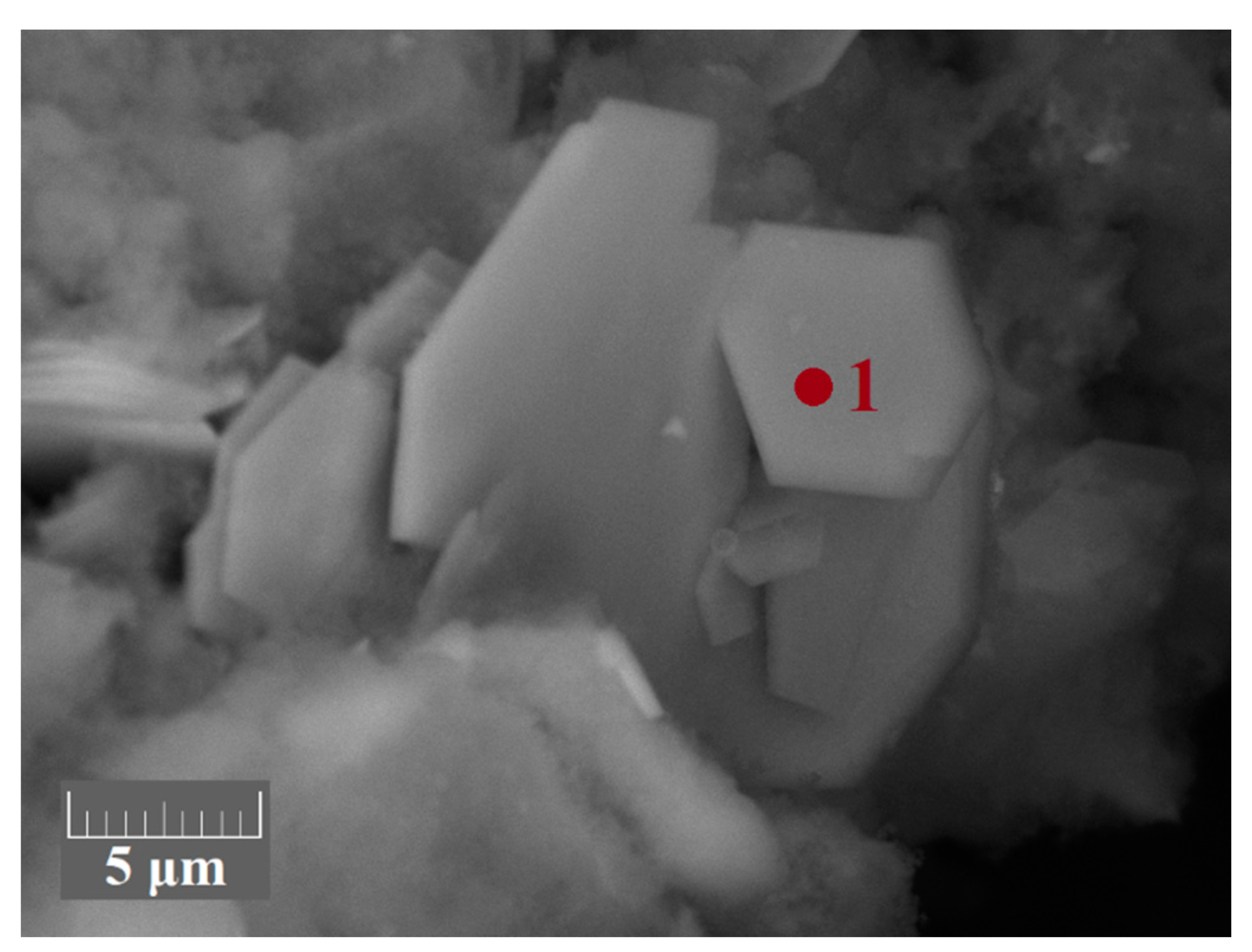



| Element | Unit | RM | WAT | SSAT |
|---|---|---|---|---|
| Fe | wt.% | 34.8 | 2.60 | 4.50 |
| Si | wt.% | 4.07 | 10.1 | 8.37 |
| Al | wt.% | 6.76 | 16.7 | 13.10 |
| Ti | wt.% | 2.80 | 6.16 | 5.50 |
| Ca | wt.% | 6.62 | 18.2 | 10.56 |
| Mg | wt.% | 0.390 | 1.59 | 1.08 |
| Mn | wt.% | 0.200 | 0.294 | 0.225 |
| Na | wt.% | 2.45 | 1.24 | 6.62 |
| P | wt.% | 0.370 | 0.0742 | 0.0570 |
| S | wt.% | 0.480 | 0.440 | 4.77 |
| Zr | mg/kg | 252 | 626 | 486 |
| Sc | mg/kg | 140 | 340 | 260 |
| Y | mg/kg | 460 | 1100 | 950 |
| Nd | mg/kg | 270 | 760 | 590 |
| Pr | mg/kg | 60.0 | 147 | 127 |
| Ce | mg/kg | 390 | 655 | 992 |
| La | mg/kg | 290 | 670 | 690 |
| Sign | Al | Si | Ti | Ca | Mg | Cl | O |
|---|---|---|---|---|---|---|---|
| 1 | 21.6 | 5.20 | 2.30 | 1.60 | 1.40 | 0.200 | 67.6 |
| HPAL Conditions | Al | Ca | Mg | Si | Ti | S |
|---|---|---|---|---|---|---|
| 10% HCl, 190 °C | 4.95 | 0.928 | 0.826 | 27.6 | 13.5 | 0.155 |
| 20% HCl, 150 °C | 4.48 | 1.02 | 1.05 | 33.5 | 5.78 | 0.244 |
| 20% HCl, 210 °C | 0.68 | 0.23 | 0.61 | 36.0 | 8.73 | 0.253 |
| HPAL Conditions | Al | Ca | Mg | Si | Ti | S |
|---|---|---|---|---|---|---|
| 10% HCl, 90 °C | 4.86 | 7.65 | 1.31 | 18.1 | 8.66 | 7.22 |
| 10% HCl, 210 °C | 3.75 | 1.20 | 0.785 | 23.6 | 12.0 | 4.02 |
| 20% HCl, 130 °C | 5.98 | 2.30 | 1.51 | 21.7 | 3.41 | 8.50 |
| 20% HCl, 210 °C | 1.56 | 0.880 | 0.229 | 24.7 | 9.92 | 8.70 |
| Sign | Al | Si | Ti | Ca | Mg | Zr | Sc | S | Cl | O |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2.60 | 5.20 | 20.7 | 5.40 | 0.80 | 3.20 | 1.40 | 0.600 | 0.300 | 59.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinoveev, D.; Grudinsky, P.; Zhiltsova, E.; Grigoreva, D.; Volkov, A.; Dyubanov, V.; Petelin, A. Research on High-Pressure Hydrochloric Acid Leaching of Scandium, Aluminum and Other Valuable Components from the Non-Magnetic Tailings Obtained from Red Mud after Iron Removal. Metals 2021, 11, 469. https://doi.org/10.3390/met11030469
Zinoveev D, Grudinsky P, Zhiltsova E, Grigoreva D, Volkov A, Dyubanov V, Petelin A. Research on High-Pressure Hydrochloric Acid Leaching of Scandium, Aluminum and Other Valuable Components from the Non-Magnetic Tailings Obtained from Red Mud after Iron Removal. Metals. 2021; 11(3):469. https://doi.org/10.3390/met11030469
Chicago/Turabian StyleZinoveev, Dmitry, Pavel Grudinsky, Ekaterina Zhiltsova, Darya Grigoreva, Anton Volkov, Valery Dyubanov, and Alexander Petelin. 2021. "Research on High-Pressure Hydrochloric Acid Leaching of Scandium, Aluminum and Other Valuable Components from the Non-Magnetic Tailings Obtained from Red Mud after Iron Removal" Metals 11, no. 3: 469. https://doi.org/10.3390/met11030469
APA StyleZinoveev, D., Grudinsky, P., Zhiltsova, E., Grigoreva, D., Volkov, A., Dyubanov, V., & Petelin, A. (2021). Research on High-Pressure Hydrochloric Acid Leaching of Scandium, Aluminum and Other Valuable Components from the Non-Magnetic Tailings Obtained from Red Mud after Iron Removal. Metals, 11(3), 469. https://doi.org/10.3390/met11030469






