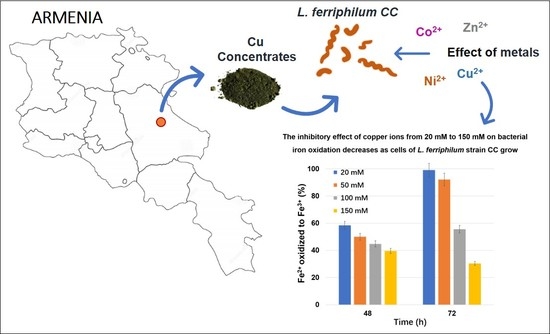
The Effect of Metal Ions on the Growth and Ferrous IronOxidation by Leptospirillum ferriphilum CC Isolated from Armenia Mine Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Cultivation
2.2. Bioleaching of Sulfide Minerals
2.3. The Influence of Fe2+/Fe3+ and Other Metal Ions on Growth and Fe2+ Oxidation
2.4. Physico–Chemical Analysis
3. Results and Discussion
3.1. Bioleaching of Sulfide Minerals
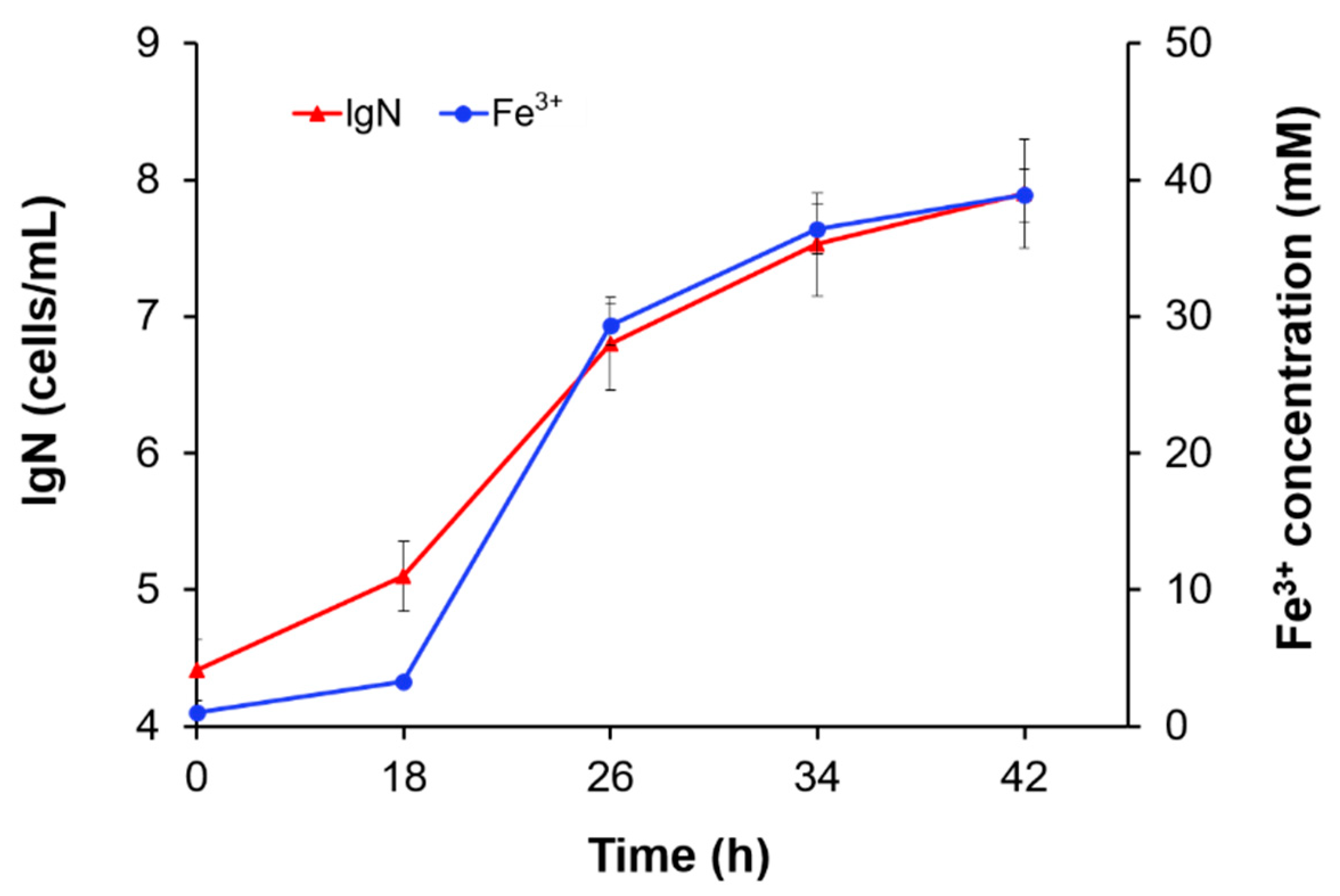
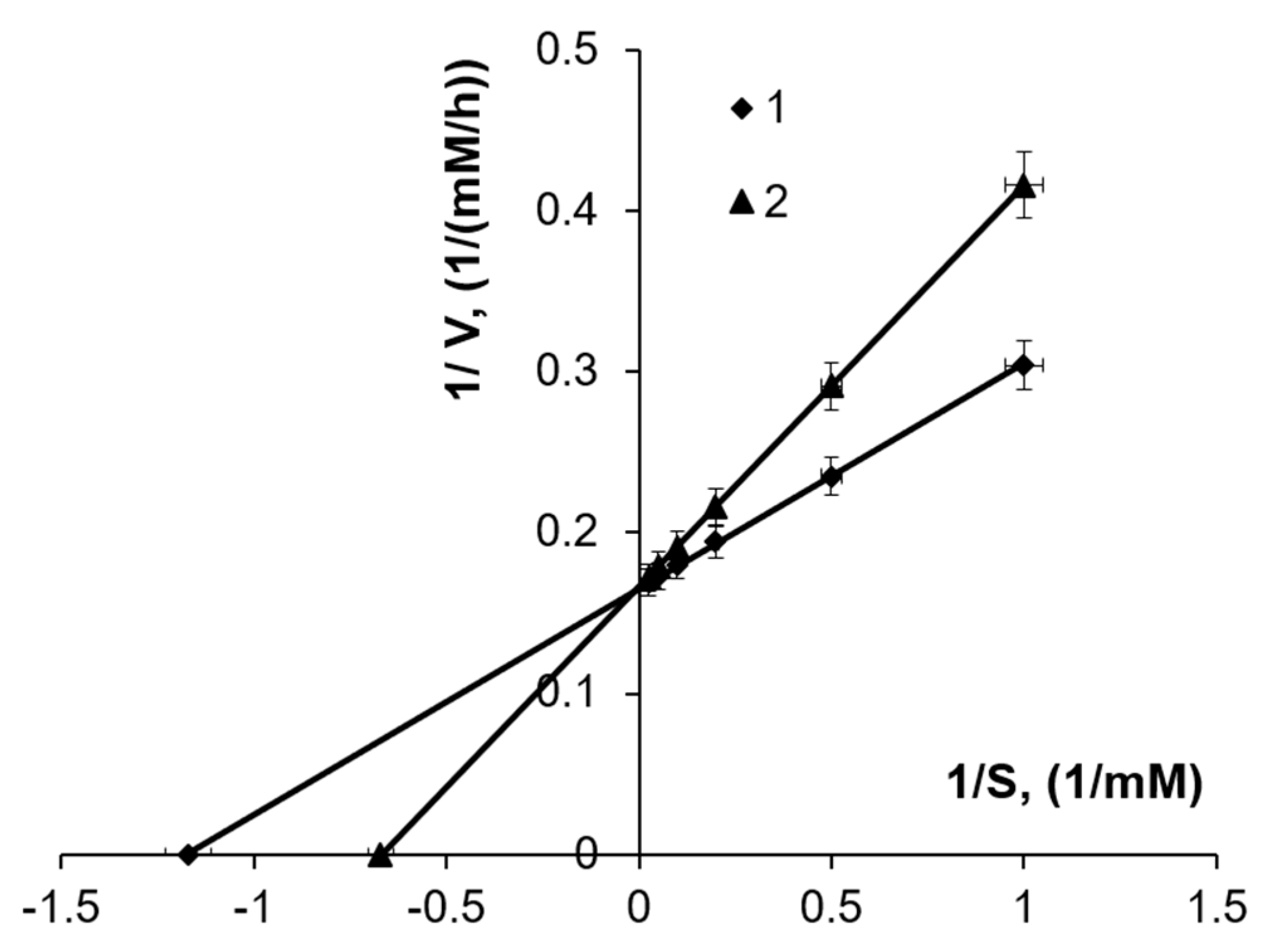
3.2. The Effect of Substrate Concentration
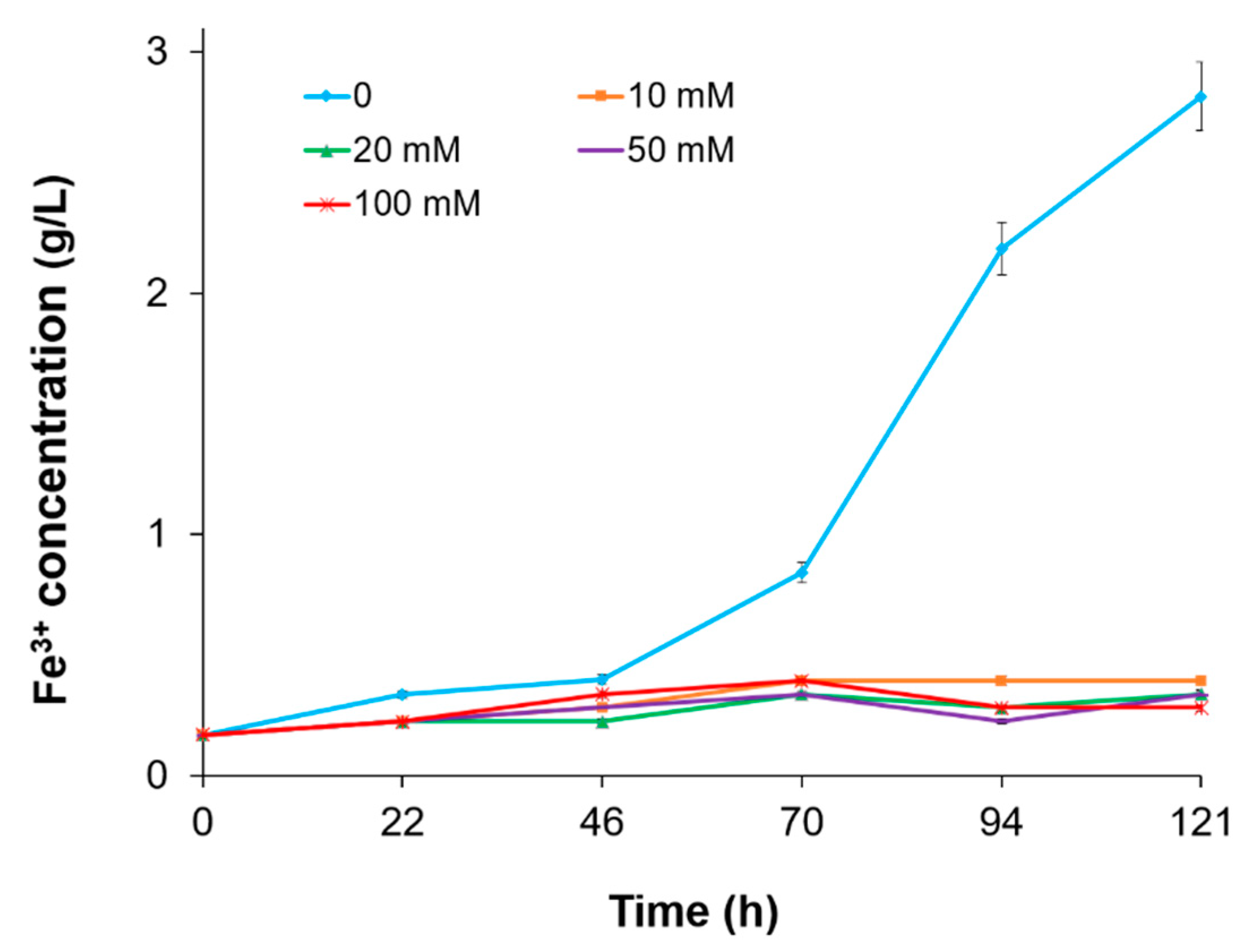
3.3. The Effect of Fe3+ Concentration
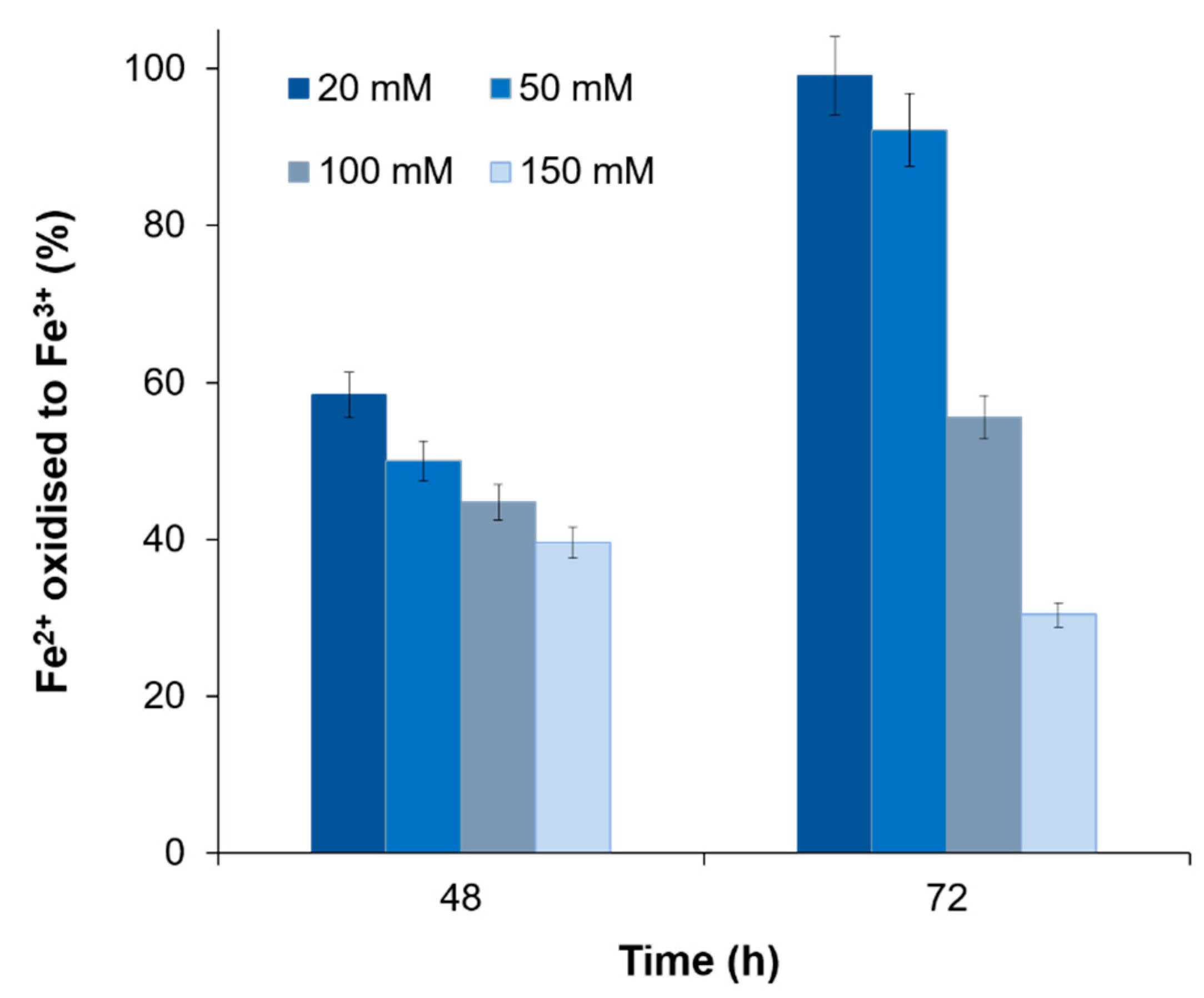
3.4. The Influence of Other Metal Ions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schippers, A.; Hedrich, S.; Vasters, J.; Drobe, M.; Sand, W.; Willscher, S. Biomining: Metal recovery from ores with microorganisms. In Geobiotechnology I. Advances in Biochemical Engineering/Biotechnology; Schippers, A., Glombitza, F., Sand, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 141, pp. 1–47. [Google Scholar]
- Johnson, D.B. The evolution, current status, and future prospects of using biotechnologies in the mineral extraction and metal recovery sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef]
- Liao, R.; Yu, S.-C.; Wu, B.-Q.; Zhao, C.-X.; Lin, H.; Hong, M.-X.; Wu, H.-Y.; Yang, C.-R.; Zhang, Y.-S.; Xie, J.-P.; et al. Sulfide mineral bioleaching: Understanding of microbe-chemistry assisted hydrometallurgy technology and acid mine drainage environment protection. J. Central South Univ. 2020, 27, 1367–1372. [Google Scholar] [CrossRef]
- Schippers, A. Microorganisms involved in bioleaching and nucleic acid-based molecular methods for their identification and quantification. In Microbial Processing of Metal Sulfides; Donati, E., Sand, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 3–33. [Google Scholar] [CrossRef]
- Fu, B.; Zhou, H.; Zhang, R.; Qiu, G. Bioleaching of chalcopyrite by pure and mixed cultures of Acidithiobacillus spp. and Leptospirillum ferriphilum. Int. Biodeterior. Biodegrad. 2008, 62, 109–115. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Rawlings, D.E. Heavy metal mining using microbes. Annu. Rev. Microbiol. 2002, 56, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef]
- Lizama, H.M.; Suzuki, I. Synergetic competitive inhibition of ferrous iron oxidation by Thiobacillus ferrooxidans by increasing concentration of ferric iron and cells. Appl. Environmen. Microbiol. 1989, 10, 2588–2591. [Google Scholar] [CrossRef]
- Dorofeyev, A.G.; Pivovarova, T.A.; Karavaiko, G.I. Kinetics of ferrous ion oxidation by Thiobacillus ferrooxidans: The effect of products of arsenopyrite oxidation on pH of the medium. Microbiology 1990, 59, 205–212. [Google Scholar]
- Rawlings, D.E. Characteristics and adaptability of iron– and sulfur–oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Factories 2005, 4, 13. [Google Scholar] [CrossRef]
- Vardanyan, A.; Stepanyan, S.; Vardanyan, N.; Markosyan, L.; Sand, W.; Vera, M.; Zhang, R. Study and assessment of microbial communities in natural and commercial bioleaching systems. Miner. Eng. 2015, 81, 167–172. [Google Scholar] [CrossRef]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Safety 2000, 45, 198–207. [Google Scholar] [CrossRef]
- Dopson, M.; Baker-Austin, C.; Koppineedi, P.R.; Bond, P.L. Growth in sulfidic mineral environments: Metal resistance mechanisms in acidophilic micro–organisms. Microbiology 2003, 149, 1959–1970. [Google Scholar] [CrossRef]
- Orell, A.; Navarro, C.A.; Arancibia, R.; Mobarec, J.C.; Jerez, C.A. Life in blue: Copper resistance mechanisms of bacteria and Archaea used in industrial biomining of minerals. Biotechnol. Adv. 2010, 28, 839–848. [Google Scholar] [CrossRef]
- Johnson, D.B.; Ghauri, M.A.; Said, M.F. Isolation and characterization of an acidophilic, heterotrophic bacterium capable of oxidizing ferrous iron. Appl. Environ. Microbiol. 1992, 58, 1423–1428. [Google Scholar] [CrossRef]
- Watling, H. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Rensing, C.; Grass, G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 2003, 27, 197–213. [Google Scholar] [CrossRef]
- Outten, F.W.; Huffman, D.L.; Hale, J.A.; O’Halloran, T.V. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 2001, 276, 30670–30677. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Thiele, D.J. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 2002, 6, 171–180. [Google Scholar] [CrossRef]
- Navarro, C.A.; Orellana, L.H.; Mauriaca, C.; Jerez, C.A. Transcriptional and functional studies of Acidithiobacillus ferrooxidans genes related to survival in the presence of copper. Appl. Environ. Microbiol. 2009, 75, 6102–6109. [Google Scholar] [CrossRef]
- Simmons, S.L.; DiBartolo, G.; Denef, V.J.; Goltsman, D.S.A.; Thelen, M.P.; Banfield, J.F. Population genomic analysis of strain variation in Leptospirillum group II bacteria involved in acid mine drainage formation. PLoS Biol. 2008, 6, 1427–1442. [Google Scholar] [CrossRef]
- Vartanyan, N.S.; Karavaiko, G.I.; Pivovarova, T.A.; Dorofeyev, A.G. The resistance of Sulfobacillus thermosulfidooxidans subsp. Asporogenes against copper, zinc and nickel ions. Microbiology 1990, 59, 587–594. [Google Scholar]
- Trevors, J.T.; Oddie, K.M.; Belliveau, B.H. Metal resistance in bacteria. FEMS Microbiol. Rev. 1985, 1, 39–54. [Google Scholar] [CrossRef]
- Kondratyeva, T.F.; Muntyan, L.N.; Karavaiko, G.I. Zinc– and arsenic-resistant strains of Thiobacillus ferrooxidans have increased copy numbers of chromosomal resistance genes. Microbiology 1995, 141, 1157–1162. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Tributsch, H.; Hansford, G.S. Reasons why ‘Leptospirillum’–like species rather than Thiobacillus ferrooxidans are the dominant iron–oxidizing bacteria in many commercial processes for the biooxidation of pyrite and related ores. Microbiology 1999, 145, 5–13. [Google Scholar] [CrossRef]
- Boon, M.; Ras, C.; Heijnen, J.J. The ferrous iron oxidation kinetics of Thiobacillus ferrooxidans in batch cultures. Appl. Microbiol. Biotechnol. 1999, 51, 813–819. [Google Scholar] [CrossRef]
- Dew, D.W.; Muhlbauer, R.; van Buuren, C. Bioleaching of copper sulfide concentrates with mesophiles and thermophiles. In Proceedings of the Alta Copper 99, Brisbane, Australia, 18–19 September 1999. [Google Scholar]
- Sampson, M.; Phillips, C. Influence of base metals on the oxidising ability of acidophilic bacteria during the oxidation of ferrous sulfate and mineral sulfide concentrates, using mesophiles and moderate thermophiles. Miner. Eng. 2001, 14, 317–340. [Google Scholar] [CrossRef]
- Harrison, J.J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Genet. 2007, 5, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, P.; Özkaya, B.; Kaksonen, A.; Tuovinen, O.; Puhakka, J. Inhibition kinetics of iron oxidation by Leptospirillum ferriphilum in the presence of ferric, nickel and zinc ions. Hydrometallurgy 2009, 97, 137–145. [Google Scholar] [CrossRef]
- Vardanyan, A.; Vardanyan, N.; Khachatryan, A.; Zhang, R.; Sand, W. Adhesion to mineral surfaces by cells of Leptospirillum, Acidithiobacillus and Sulfobacillus from Armenian sulfide ores. Miner. 2019, 9, 69. [Google Scholar] [CrossRef]
- Vardanyan, N.; Badalyan, H.; Markosyan, L.; Vardanyan, A.; Zhang, R.; Sand, W. Newly isolated Acidithiobacillus sp. Ksh from Kashen copper ore: Peculiarities of EPS and colloidal exopolysaccharide. Front. Microbiol. 2020, 11, 1802. [Google Scholar] [CrossRef]
- Mackintosh, M.E. Nitrogen fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 1978, 105, 215–218. [Google Scholar] [CrossRef]
- Castro, L.; Zhang, R.; Muñoz, J.A.; González, F.; Blázquez, M.L.; Sand, W.; Ballester, A. Characterization of exopolymeric substances (EPS) produced by Aeromonas hydrophila under reducing conditions. Biofouling 2014, 30, 501–511. [Google Scholar] [CrossRef]
- Karavaiko, G.I.; Rossi, G.; Agate, A.D.; Groudev, S.N.; Avakyan, Z.A. Biotechnology of metals. Manual/Eds. Centre for International Projects GKNT. 1988; 350. [Google Scholar]
- Pirt, S.J. Principles of Microbe and Cell Cultivation; Blackwell Scientific Publications: Oxford, UK; London, UK, 1975. [Google Scholar]
- Vardanyan, N.S.; Akopyan, V.P. Leptospirillum–like bacteria and evaluation of their role in pyrite oxidation. Microbiology 2003, 72, 438–442. [Google Scholar] [CrossRef]
- Kovalenko, T.V.; Karavaiko, G.I.; Piskunov, V.P. Effect of Fe3+ ions on Thiobacillus ferrooxidans oxidation of ferrous oxide at various temperatures. Microbiology 1982, 51, 156–160. [Google Scholar]
- Norris, P.R.; Barry, D.W.; Hinson, D. Iron and mineral oxidation by acidophilic bacteria: Affinities for iron and attachment to pyrite. In Biohydrometallurgy, Proceedings of International Symposium, Science and Technology Letters.; Norris, P.R., Kelly, D.P., Eds.; Kew: Surrey, UK, 1988; pp. 43–59. [Google Scholar]
- Penev, K.; Karamanev, D. Batch kinetics of ferrous iron oxidation by Leptospirillum ferriphilum at moderate to high total iron concentration. Biochem. Eng. J. 2010, 50, 54–62. [Google Scholar] [CrossRef]
- Curutchet, G.; Pogliani, C.; Donati, E.; Tedesco, P. Effect of iron (III) and its hydrolysis products (jarosites) on Thiobacillus ferrooxidans growth and on bacterial leaching. Biotechnol. Lett. 1992, 14, 329–334. [Google Scholar] [CrossRef]
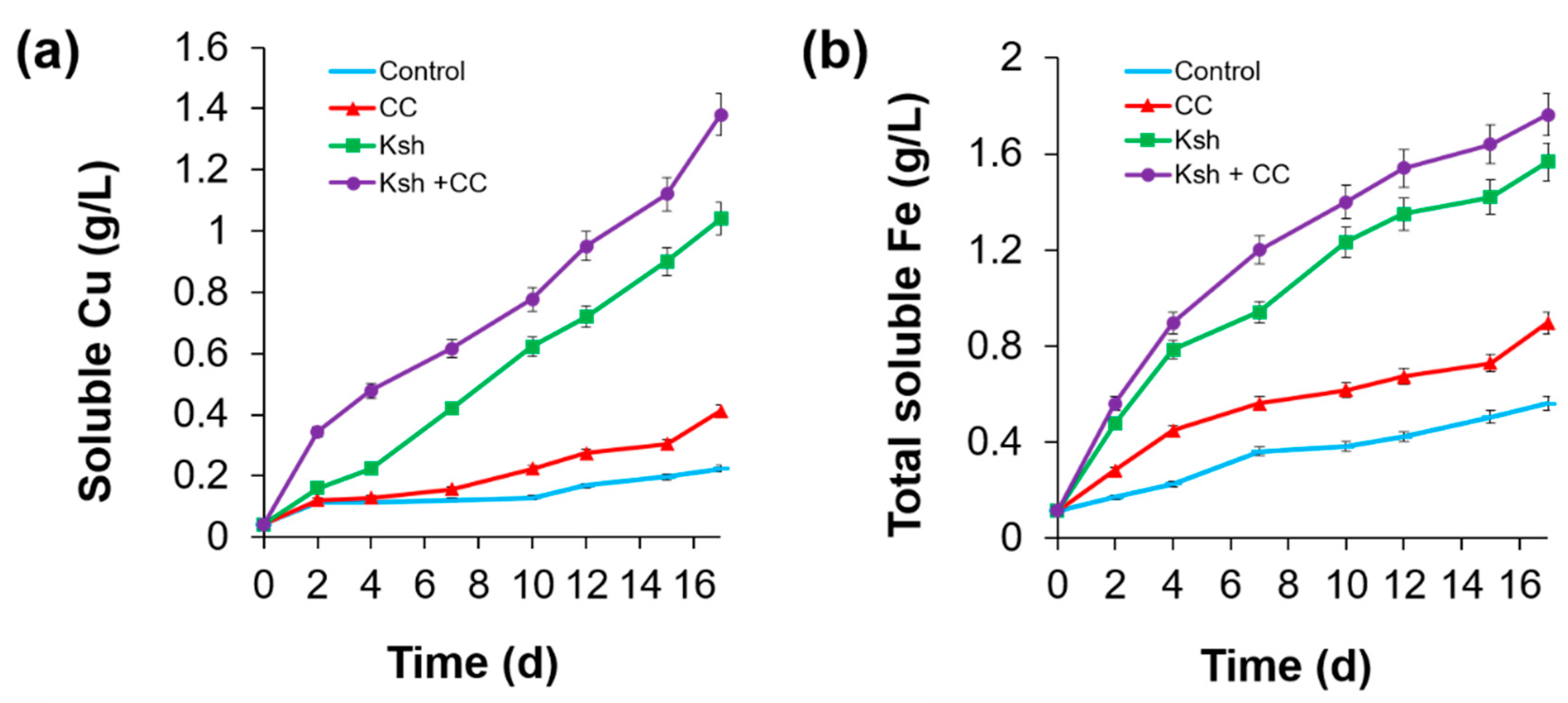

| Bacteria | Extracted Fe for 20 Days | pH, Initial/Final | Final ORP mV | |||
|---|---|---|---|---|---|---|
| mg/L | % | |||||
| Fe3+ | Fe2+ | Fe Total | ||||
| Control | 0 | 840 | 840 | 4.8 | 1.7/1.6 | 600 |
| L. ferriphilum | 784 | 1456 | 2240 | 12.8 | 1.7/1.3 | 625 |
| At. ferrooxidans Ksh | 1624 | 1792 | 3416 | 19.5 | 1.7/1.2 | 635 |
| At. ferrooxidans Ksh + L. ferriphilum CC | 5824 | 616 | 6440 | 36.8 | 1.7/1.3 | 775 |
| Fe2+ Concentration (mM) | µmax (1/h) | Vmax (mM/h) |
|---|---|---|
| 50 | 0.31 ± 0.024 | 1.7 |
| 100 | 0.48 ± 0.018 | 6.2 |
| 200 | 0.41 ± 0.017 | 6.0 |
| 300 | 0.28 ± 0.026 | 4.6 |
| 400 | 0.090 ± 0.00082 | 3.7 |
| Fe3+ Concentration (mM) | µmax (1/h) | Vmax (mM/h) |
|---|---|---|
| 2.0 | 0.35 ± 0.025 | 6.5 |
| 20.0 | 0.32 ± 0.026 | 6.1 |
| 50.0 | 0.26 ± 0.014 | 5.5 |
| 75.0 | 0.19 ± 0.010 | 3.2 |
| 100.0 | 0.16 ± 0.011 | 1.9 |
| Metal ions (mM) | Fe, Oxidized in 46–48 h | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu2+ | Zn2+ | Ni2+ | Co2+ | |||||
| g/L | % | g/L | % | g/L | % | g/L | % | |
| 0 | 3.2 ± 0.19 | 100 | 1.6 × 0.023 | 100 | 3.4 ± 0.028 | 100 | 3.8 ± 0.024 | 100 |
| 10 | – | – | 1.0 ± 0.036 | 64.3 | – | – | 1.5 ± 0.0048 | 38.8 |
| 20 | 1.9 ± 0.049 | 58.4 | 0.67 ± 0.015 | 42.8 | 1.8 ± 0.0036 | 53.3 | 1.2 ± 0.0017 | 31.3 |
| 50 | 1.6 ± 0.014 | 50.0 | 0.56 ± 0.013 | 35.7 | 1.5 ± 0.018 | 43.3 | 0.85 ± 0.013 | 22.6 |
| 100 | 1.3 ± 0.0049 | 39.6 | 0.50 ± 0.0050 | 17.6 | 1.1 ± 0.049 | 31.7 | 0.78 ± 0.0086 | 20.9 |
| 150 | 1.2 ± 0.0080 | 36.9 | – | – | 0.84 ± 0.011 | 25.0 | 0.74 ± 0.0029 | 19.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khachatryan, A.; Vardanyan, N.; Vardanyan, A.; Zhang, R.; Castro, L. The Effect of Metal Ions on the Growth and Ferrous IronOxidation by Leptospirillum ferriphilum CC Isolated from Armenia Mine Sites. Metals 2021, 11, 425. https://doi.org/10.3390/met11030425
Khachatryan A, Vardanyan N, Vardanyan A, Zhang R, Castro L. The Effect of Metal Ions on the Growth and Ferrous IronOxidation by Leptospirillum ferriphilum CC Isolated from Armenia Mine Sites. Metals. 2021; 11(3):425. https://doi.org/10.3390/met11030425
Chicago/Turabian StyleKhachatryan, Anna, Narine Vardanyan, Arevik Vardanyan, Ruiyong Zhang, and Laura Castro. 2021. "The Effect of Metal Ions on the Growth and Ferrous IronOxidation by Leptospirillum ferriphilum CC Isolated from Armenia Mine Sites" Metals 11, no. 3: 425. https://doi.org/10.3390/met11030425
APA StyleKhachatryan, A., Vardanyan, N., Vardanyan, A., Zhang, R., & Castro, L. (2021). The Effect of Metal Ions on the Growth and Ferrous IronOxidation by Leptospirillum ferriphilum CC Isolated from Armenia Mine Sites. Metals, 11(3), 425. https://doi.org/10.3390/met11030425