1. Introduction
It has been generally accepted that the reduction rate of iron oxide strongly depends on its chemical and physical characteristics during the reduction process. The chemical potential is typically defined by the temperature, oxygen potential, and activity of iron oxide in an ore. Many researchers [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10] have generally reported that the reduction rate of FeO increases as the temperature increases and the oxygen potential decreases. In addition, El-Geassy and many other researchers [
10,
11,
12,
13,
14,
15] have reported that when iron oxide reacts with other oxides to form different phases (magnesioferrite, dolomite, hercynite, SFCA, and jacobsite), the activity of the iron oxide decreases, and consequently, it has a negative effect on reduction. As such, the chemical potential for the reduction of iron oxide has been studied both qualitatively and quantitatively.
Many studies have been conducted on the effect of pores, a major physical property of iron oxide. Huang et al. [
16] and Turkdogan et al. [
17] studied the effect of porosity change on the reduction rate during the reduction process. The porosity of the pellets during the reduction process was increased owing to the cracks and morphology changes of iron oxide, and the pore size increased with increasing reduction temperature. Matthew et al. [
18] reported the morphology and pore shape of reduced iron according to the reduction condition.
There are many studies on the relationship between the reduction of iron oxide and the porosity of the initial state of iron oxide. Bahgat et al. [
19] and Kim et al. [
20] reported that SiO
2 reacts with FeO to form a dense phase (silicates, fayalite) at equilibrium, thereby reducing the porosity of FeO-SiO
2 pellets. Kim et al. [
20] and Takahashi et al. [
21] changed the initial state of porosity using CaO, and the corresponding reduction behavior was studied. When CaO is added, the porosity of the FeO-CaO pellet increases with its solubility. However, when the solubility increases (about 2.5 wt% CaO), the equilibrium phase forms calcium ferrite and the porosity of FeO–CaO decreases, as in the case of SiO
2 addition.
Kaneko [
22] proposed that the pores can be classified into intrinsic and extrinsic pores with respect to their origin and structure. Intrinsic pores are inherently possessed by the intraparticle arrangement. Extrinsic pores are created during the removal of foreign substances from the base material through various reactions. However, some studies [
16,
17,
18] only measured the change in intrinsic pores during the reduction process of iron oxide, and there is a limit to extrinsic pore control. In other studies [
19,
20,
21], pore control was performed through the addition of other substances; however, it is difficult to confirm the effect of pores because the activity of iron oxide is simultaneously changed.
According to Rouquerol et al. [
23], pores are classified into closed pores and open pores based their accessibility by to an external fluid. In detail, open pores are again divided into open only at one end (dead-end or saccafe) and open at two ends (through pores). Among them, closed pores and open only at one end (dead-end) adversely affect reduction because the gas flow is not smooth. Meanwhile, the pores that are open at two ends (through pores) help in the reduction process due to the permeability of the gas. The results of previous studies indicated the necessary for discussion of such pore quality.
Therefore, the present investigation was carried out to understand the effects of porosity on the reduction rate of FeO by introducing extrinsic pores and taking advantage of the sublimation property of FeCl2. FeCl2 has a high vapor pressure at high temperatures. The evaporation behavior of FeCl2 at high temperatures enables the formation of extrinsic pores in FeO without changing the activity of FeO. Through extrinsic pore control, the effect of the pore alone on reduction can be confirmed. In addition, for better understanding, the porosity and morphological changes (pore quality) are considered together using the labyrinth coefficient, which accounts for the porosity and tortuosity during the reduction process.
3. Results and Discussion
The apparent porosity of the initial state of the sample is shown in
Table 2. Standard FeO has a 23.9% (intrinsic pore) volumetric porosity; however, the extrinsic pore introduced by FeO using the evaporation of FeCl
2 has an overall volumetric porosity of up to 36.4%. As the amount of FeCl
2 removed by evaporation increases and the temperature increases, the amount of the introduced extrinsic pores increases. Herisau.
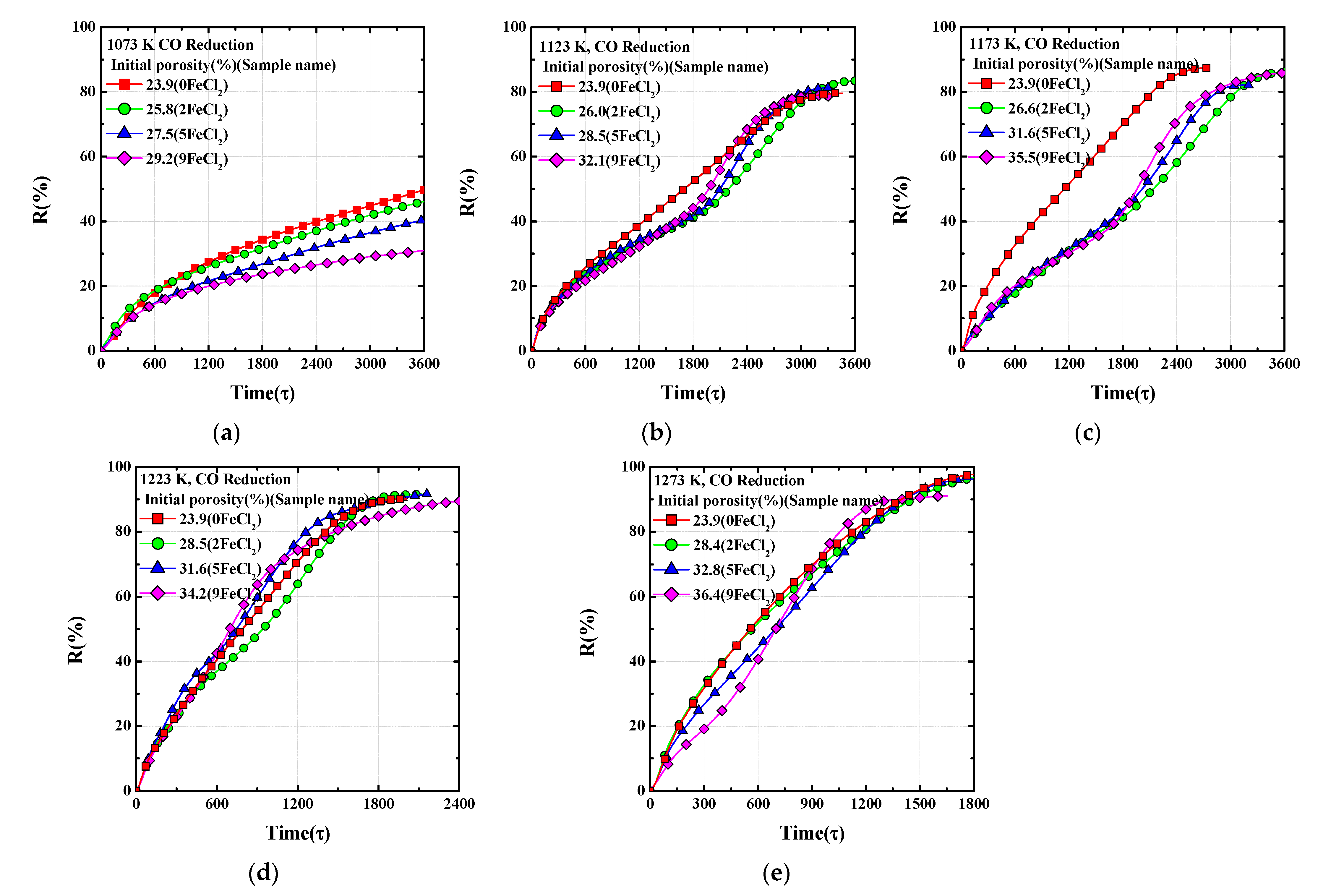
Figure 3 shows reduction behaviors of FeO for various temperatures ranging from 1073–1273 K. As the temperature increases, the reduction rate and the final reduction degree increase simultaneously in general. There are typical results based on the fundamental laws of diffusion theory and basic principles of chemical thermodynamics. El-Geassy [
25] has reported that the activation energy is 133.97 kJ/mole when pure FeO is reduced, and the activation energy based on the results of this experiment shows a similar value of 137.78 kJ/mole.
Figure 4 shows reduction behaviors of FeO with various porosities in the temperature range of 1073–1273 K. Despite the increase in overall porosity by introducing extrinsic pores, the initial reduction rate abnormally tends to decrease. The overall porosity and initial reduction rate followed an inverse relationship, and the trend shows an inflection point at which the reduction rate increases after approximately 40% of the degree of the reduction process. The tendency to increase the reduction rate after 40% reduction degree increased with the extrinsic pores. In addition, this abnormal behavior related to pores gradually decreased with increasing temperature. Bahgat [
19] and Kim [
20] reported that the initial porosity and reduction rate are proportional to each other. The positive effect of porosity on reduction rate is generally accepted. However, the results of this experiment show a behavior contrary to the general view of porosity and reduction rate.
The experimental results of this experiment are mathematically analyzed using the grain model proposed by Szekely [
26]. In the heterogeneous gas–solid reaction model of the cylindrical compact, the interfacial chemical reaction can be expressed as follows:
The gaseous mass transport through the product layer can be expressed as:
The mixed control can be expressed as follows:
where
and
are the conversion functions and
X is the fraction of reduction at a given time (τ).
kICR,
kGMT, and
kMixed are the apparent rate constants of the interfacial chemical reaction, gaseous mass transport through the product layer, and mixed control, respectively. If the correlation of time (τ) in Equations (2)–(4) shows a linear relationship, the rate constant can be derived from the slope of the straight line. The results of applying the formulas to the FeO reduction are presented in
Figure 5.
and
are the apparent rate constants in the mixed control of FeO introduced by the extrinsic pore and pure FeO. At the initial stage of reduction, the reduction rate decreases in proportion to the extrinsic pores. In addition, the dependence of the reduction rate on the extrinsic pores is relaxed by increasing the temperature. At a later stage of reduction (after the inflection point), the relationship between the reduction rate and extrinsic pores reverted to a normal behavior.
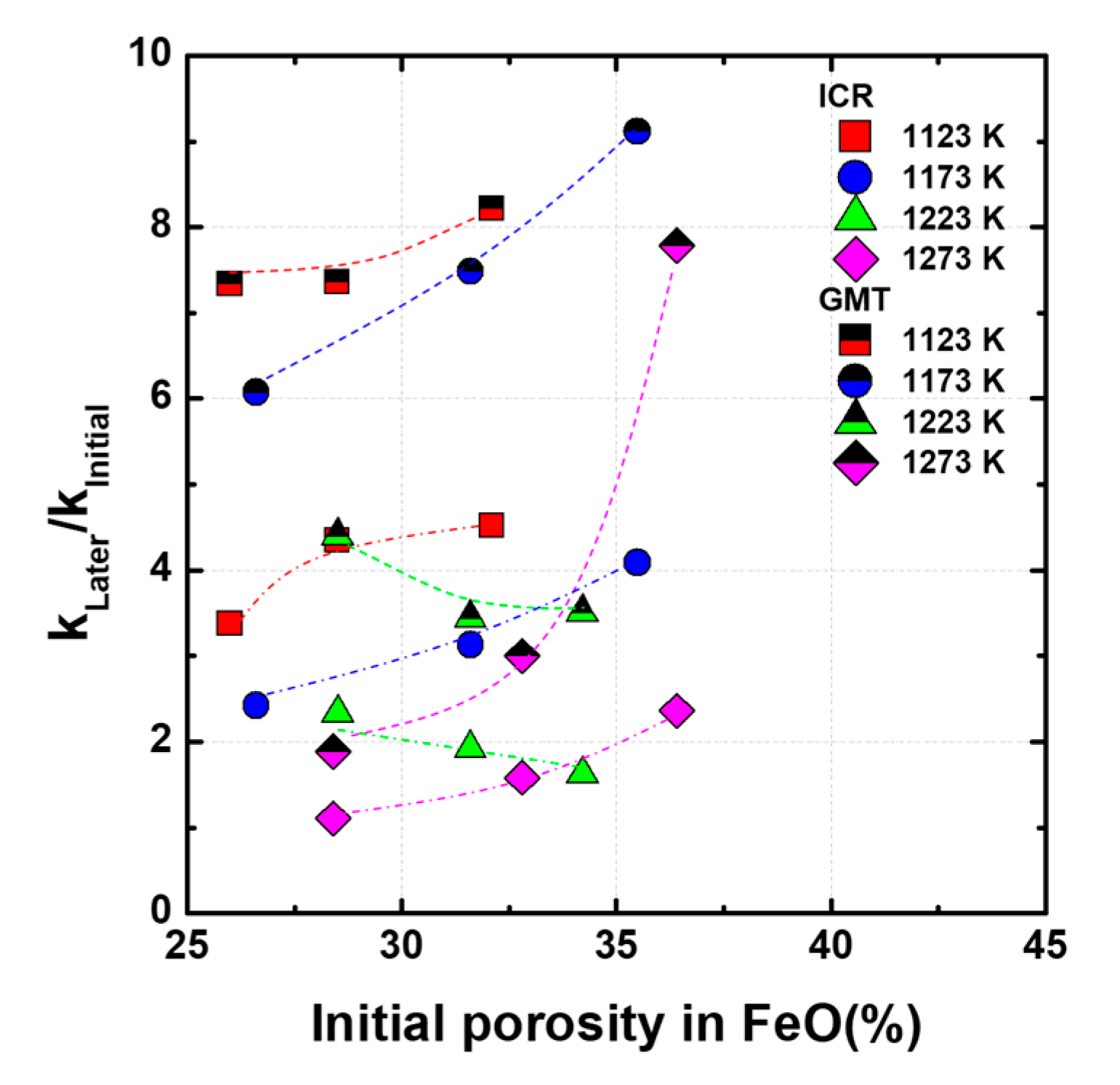
The dimensionless kinetic parameter, k
Later stage/k
Initial stage, is shown in
Figure 6, where k
Later stage and k
Initial stage represent the apparent rate constants in the initial and later stages, respectively. The rate constants of gaseous mass transport through the product layer against the interfacial chemical reaction significantly increased after half of the reduction is complete, compared to those observed for the initial reduction; further, the gaseous mass transport rate constants increased by 2–7 times after the later stage reduction. The abnormal reduction behavior is more affected by the diffusion of gas than by the chemical reaction. In addition, at 1173 K, where abnormal behavior is well confirmed,
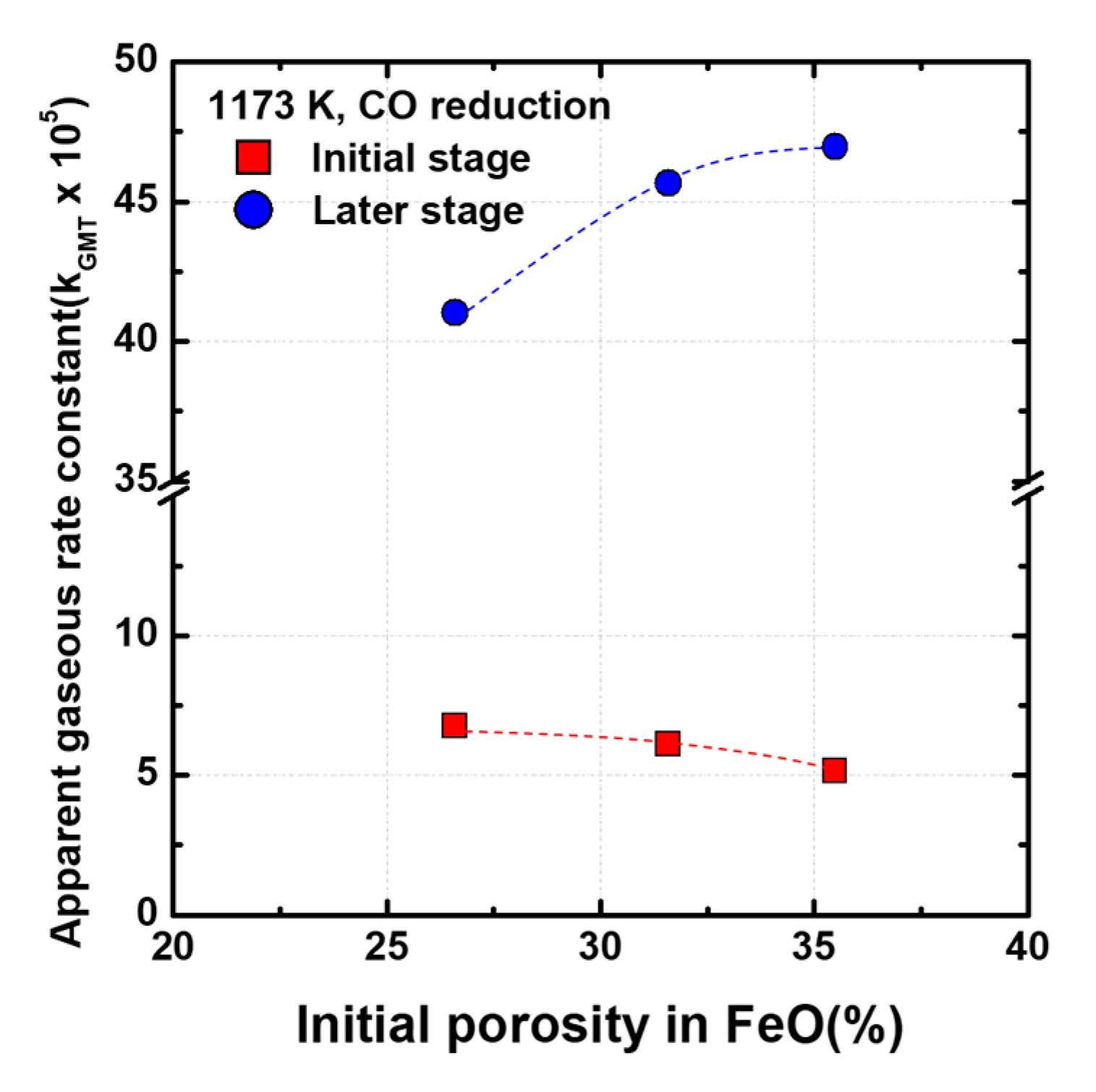
Figure 7 shows the apparent gaseous rate constant at the initial and later stages. In the initial stage of reduction, although the initial overall porosity in FeO increases, the apparent gaseous rate constant tends to decrease. However, in the later reduction stage, the apparent gaseous rate constant tends to increase in proportion to the initial overall porosity. It is believed that the diffusion behavior of the reducing gas changes because of the change in the morphology of FeO during the reduction process.
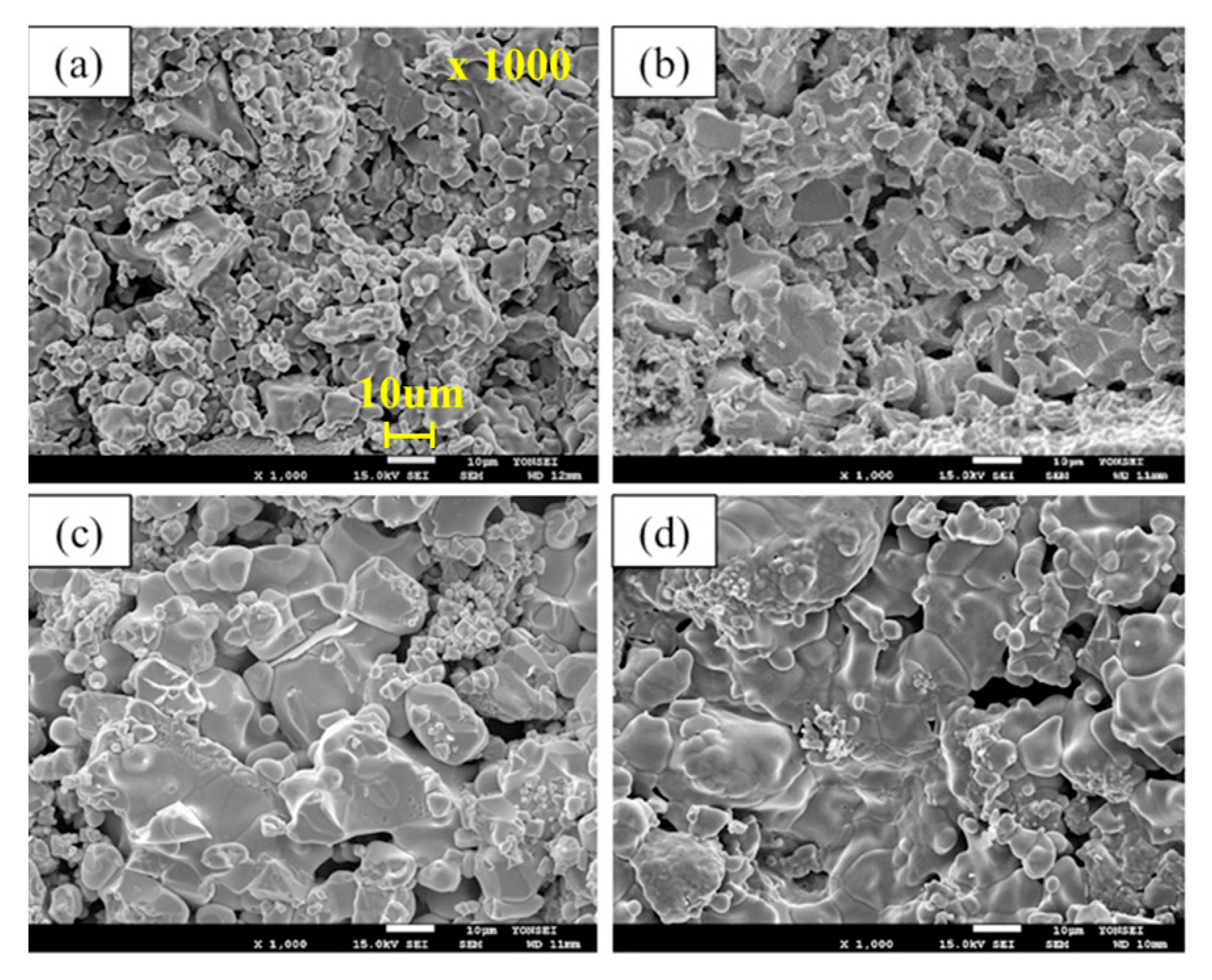
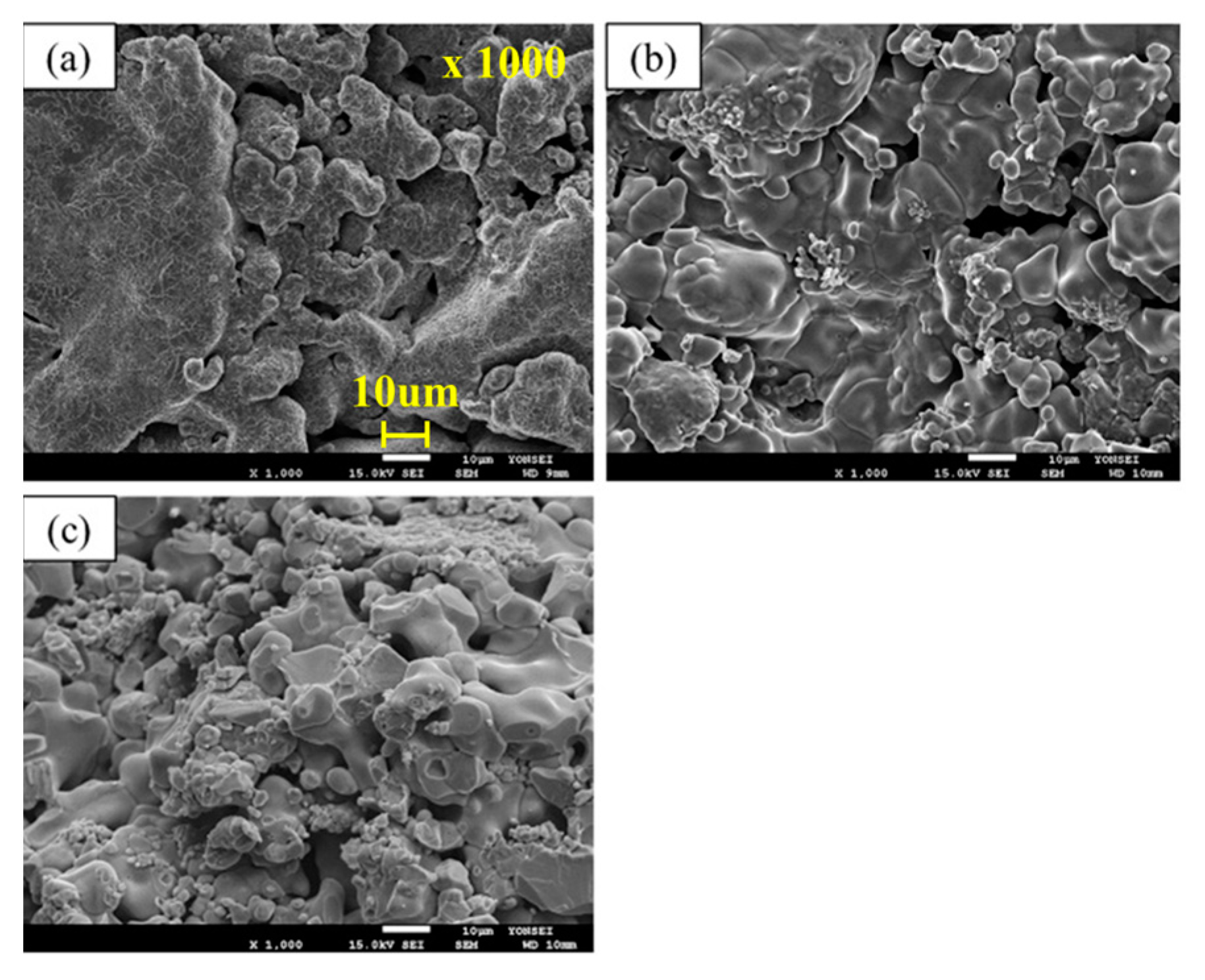
To confirm the morphology of the sample before reduction at 1173 K, the sample was analyzed using SEM and the results are shown in
Figure 1. In the case of pure FeO (
Figure 1a), it has spherical morphology. On the other hand, in the case of FeO with extrinsic pores (
Figure 1d), the spherical morphology mostly changed to a large plate-like morphology. In the case of increasing extrinsic pores in FeO, the plate-like morphology becomes pronounced. The experimental results indicate that the quality of the extrinsic pores plays an important role in controlling the initial reduction rate. Therefore, it is necessary to examine the dependency of not only the number of pores, but also other aspects of the relationship between the pores and the reduction rate.
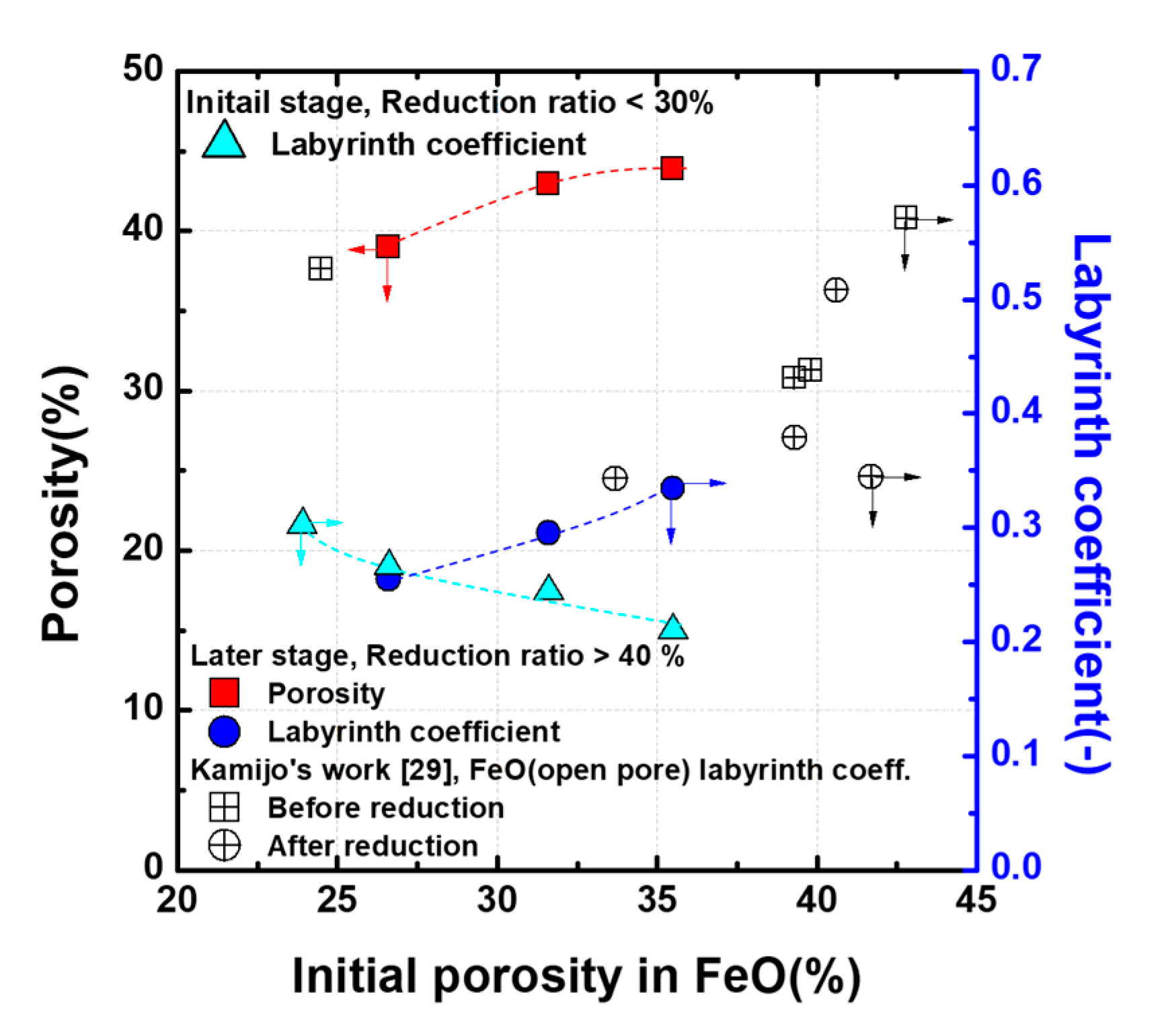
The relationship between the characteristics of pores and the reducing gas permeability can be evaluated using the labyrinth coefficient. The labyrinth coefficient represents the relationship between pore quantity and tortuosity, and it can be used to evaluate the gas permeability through the pores. The labyrinth coefficient can be expressed as follows [
27]:
where
is the labyrinth coefficient,
is the porosity of the FeO pellet, K is the equilibrium constant,
and
are the diffusion coefficients of CO and CO
2 in the pore. In addition, DS is an effective diffusion coefficient that can be calculated by Ishida-Wen’s model [
28]. A labyrinth coefficient value indicates the gas permeability in the FeO pellet. Applying this experimental data to Equation (5), it is shown in
Figure 8 as a function of the initial porosity in FeO. The initial reduction stage of porosity in FeO increases, and the labyrinth coefficient tends to decrease. This means that the extrinsic pores mainly generated in FeO by the evaporation of FeCl
2 are closed or open only at one end, which have a negative effect on reduction. Therefore, despite the increase in the overall pores, the initial reduction rate decreases because the formed pores are not suitable for reduction. However, as the reduction proceeds, the porosity in FeO increases and the labyrinth coefficient also tends to increase. During the reduction process, the structure of morphology changed in favor of gas diffusion, thereby increasing the labyrinth coefficient. These results are in good agreement with Kamijo’s [
29] research. Accordingly, it is thought that reduction behavior exhibits an abnormal behavior that accelerates the reduction rate after half of the reduction process is complete.
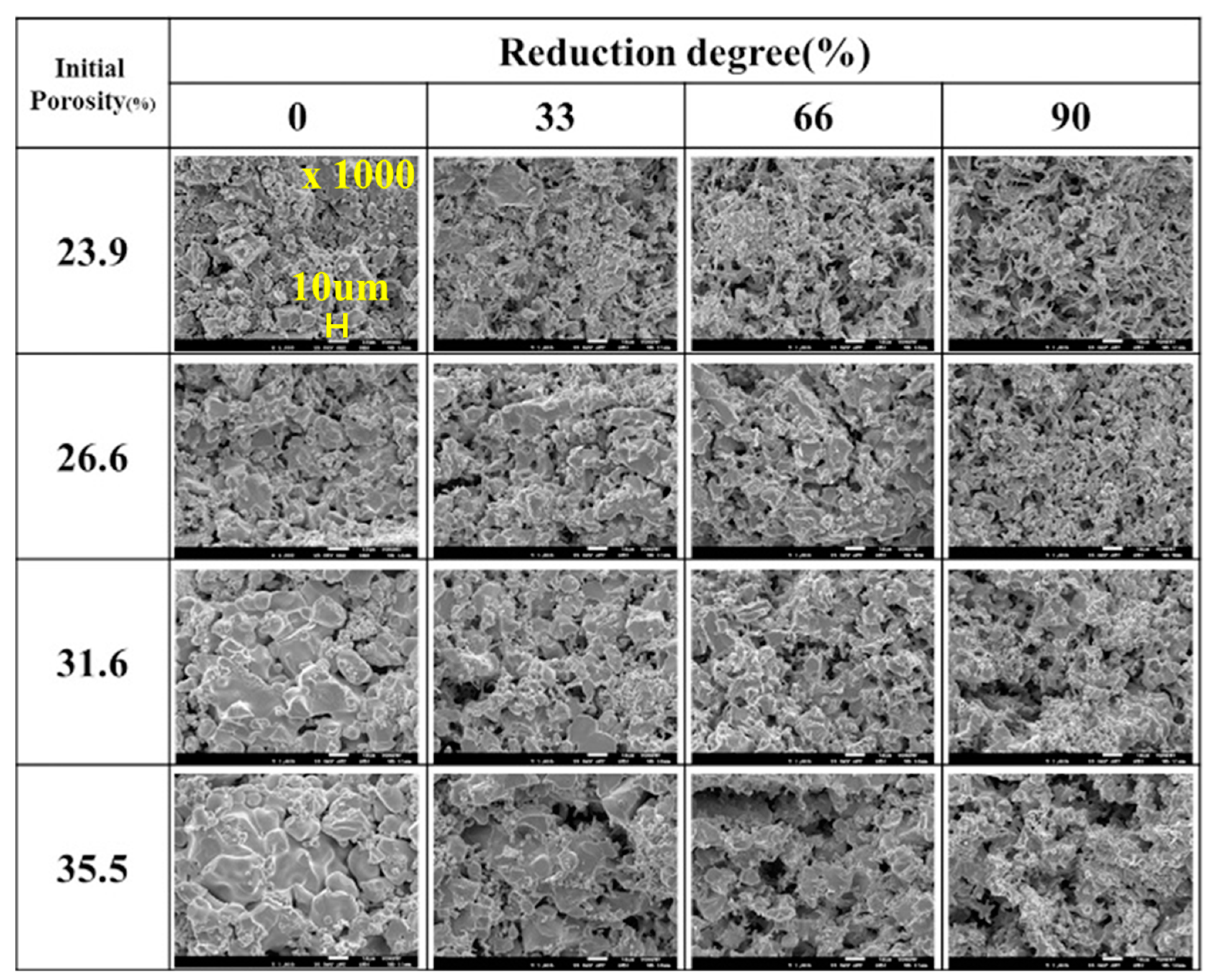
Figure 9 shows the effect of the initial overall porosity on the morphological change of reduced iron at 1173 K. For pure FeO with an initial porosity of 23.9%, spherical particles can be seen at the beginning of the reduction process, and the morphology changes into the porous state as the reduction progresses. With increasing initial overall porosity, a large plate-like morphology FeO can be seen at the beginning of the reduction process. In the initial stage of reduction, the diffusion of the reducing gas decreases due to the large plate-like morphology of FeO and the labyrinth coefficient decreases. Because of the difference in density between FeO and reduced iron, the large plate-like morphology FeO becomes smaller with the reduction procedure, and pores that are open only at one end evolve into those that are open at the two ends. Owing to the change in the pore shape, the labyrinth coefficient increases in proportion to the porosity in FeO after half the reduction process is complete. As a result, the flow of the reducing gas becomes smooth, and the reduction rate increases after half of the reduction process is carried out. After complete reduction, all the samples showed porous morphology, even though the initial porosity changed by the introduction of FeCl
2.
The effect of temperature on the morphology of FeO is shown in
Figure 10. At 1073 K, FeO showed large plate-like morphology. However, as the temperature increases, FeO has a morphology similar to that of pure FeO (spherical morphology). Therefore, as the temperature increases, the initial reduction rate of FeO that formed pores because of the introduction of FeCl
2 is similar to that of pure FeO. However, even at high temperatures, because of the formation of pores that are open only at one end having a large plate-like morphology, the initial reduction rate appears to decrease.
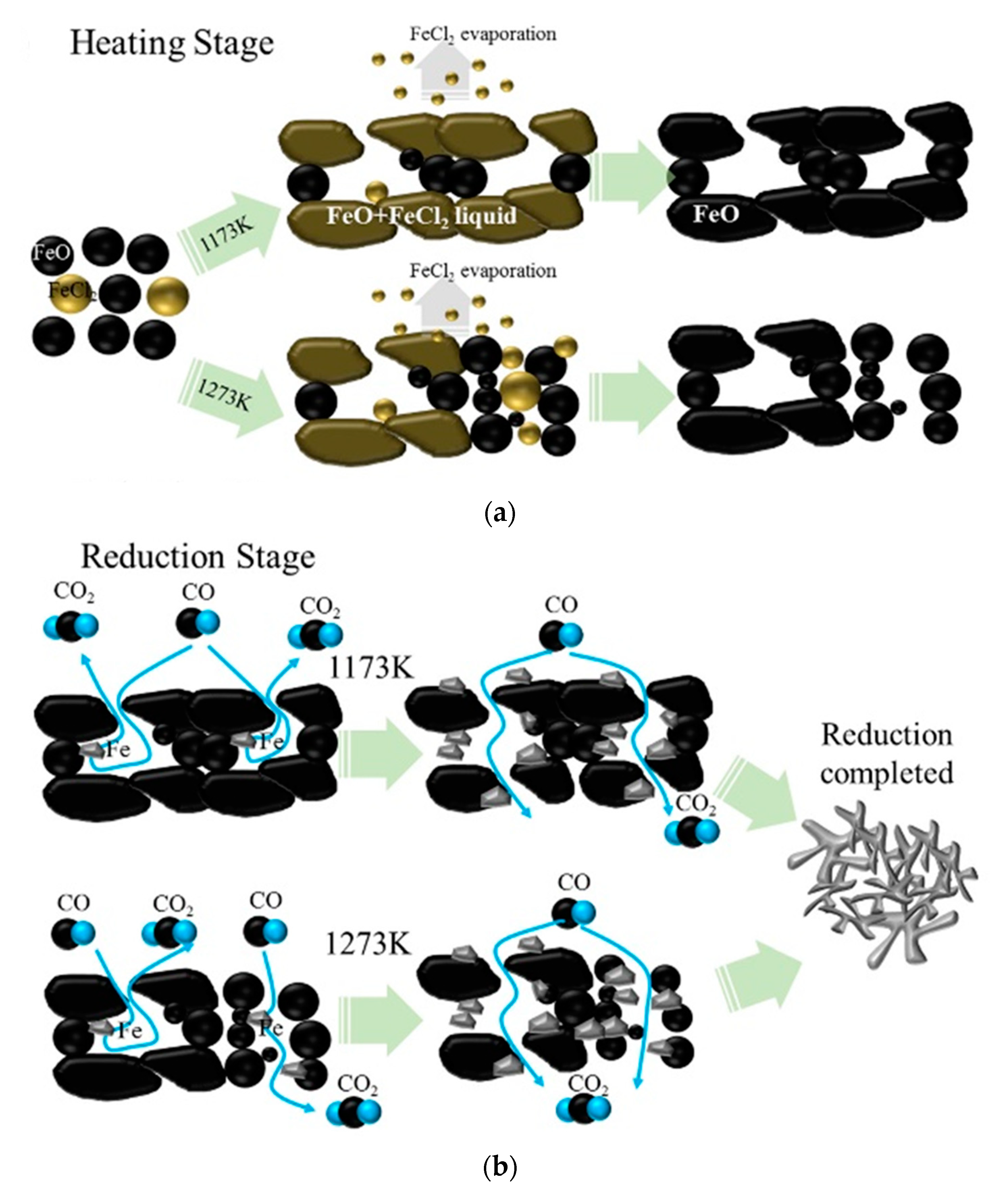
The formation of extrinsic pores of the open only at one end by FeCl
2 addition, and their behavior during reduction process is schematically represented in
Figure 11. In the heating stage (
Figure 11a), at a relatively low temperature (1173 K), FeCl
2 forms a liquid phase with FeO and then evaporates. As a result, several pores that are open only at one end are formed. However, at a relatively high temperature (1273 K), many fractions of FeCl
2 evaporate without reacting with FeO. As a result, fewer number of pores open only at one end are produced at lower temperatures. In the reduction stage (
Figure 11b), at relatively low temperatures (1173 K), the reduction rate decreases because of the formation of pores that are open only at one end, despite the increase in the overall porosity of the sample. During the reduction process, FeO with a large plate-like morphology is reduced to porous Fe, leading to the disappearance of pores open only at one end. As a result, the reduction rate increases after the middle stage of reduction. At a relatively high temperature (1273 K), the reduction behavior is similar to that at lower temperatures. However, since the number of open only at one end pore is less, the tendency of unusual behavior at low temperatures is low. After completion of the reduction process, porous iron with similar morphology is formed at both high and low temperatures.
It is generally accepted that the reduction rate of iron oxide is proportional to the overall porosity [
16,
17,
18,
19,
20,
21]. However, if the sample has pores open only at one end or closed pores, the reduction rate is not proportional to the overall porosity. Therefore, while considering the pores for the reduction rate, the number of pores as well as type and distribution of pores should be included.