The Influence of La and Ce on Microstructure and Mechanical Properties of an Al-Si-Cu-Mg-Fe Alloy at High Temperature
Abstract
1. Introduction
| Al Alloys | UTS T > 100 °C | UTS T > 200 °C | UTS T > 250 °C | UTS T > 300 °C | UTS T > 350 °C | Ref. |
|---|---|---|---|---|---|---|
| A319 | 225 MPa/200 °C | 90.8 MPa/300 °C | 25.6 MPa/400 °C | [19] | ||
| 360 | 150 MPa/205 °C | 50 MPa/315 °C | 30 MPa/370 °C | [20] | ||
| A360 | 145 MPa/205 °C | 45 MPa/315 °C | 30 MPa/370 °C | [20] | ||
| 359 | 125 MPa/260 °C | 50 MPa/315 °C | 30 MPa/370 °C | [21] | ||
| 319 | 140 MPa/200 °C | 66 MPa/315 °C | 48 MPa/370 °C | [22] | ||
| 332 | 165 MPa/205 °C | 110 MPa/260 °C | 90 MPa/315 °C | [23] | ||
| 354 | 325 MPa/150 °C | 290 MPa/205 °C | 90 MPa/315 °C | [24] | ||
| 355/T5 | 160 MPa/150 °C | 103 MPa/205 °C | 25 MPa/371 °C | [25] | ||
| 356/T6 | 83 MPa/205 °C | 28 MPa/315 °C | 17 MPa/375 °C | [26] | ||
| A357/T62 | 270 MPa/150 °C | 250 MPa/205 °C | 70 MPa/315 °C | [27] | ||
| EN-AC46000 | 215 MPa/200 °C | 110 MPa/300 °C | 20 MPa/400 °C | [28] |
2. Experimental
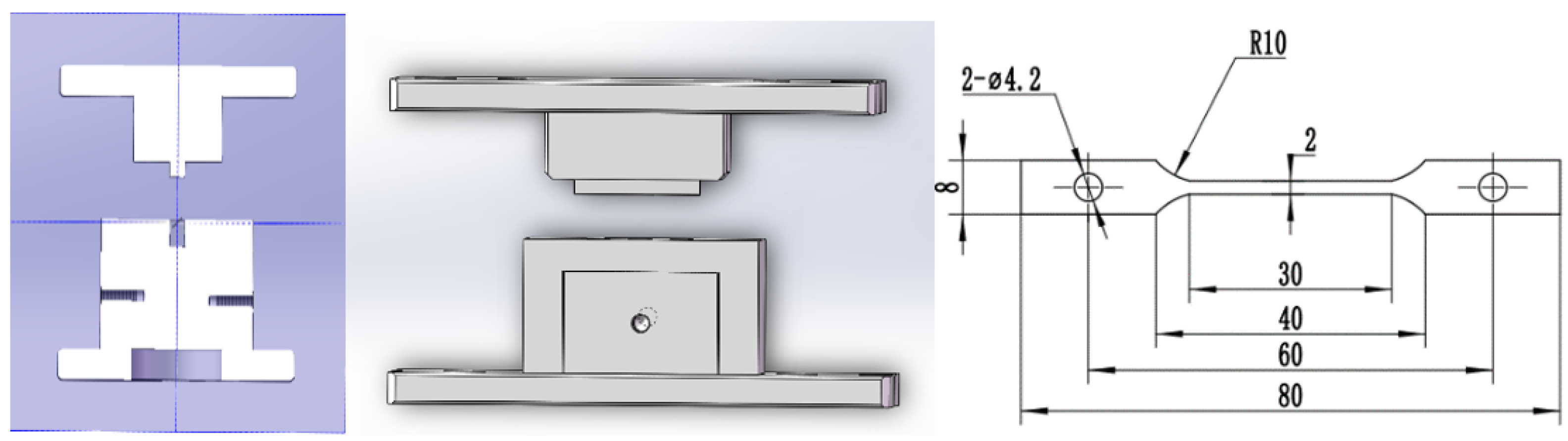
2.1. Sample Manufacturing
2.1.1. Alloy Fabrication
2.1.2. Sample Casting
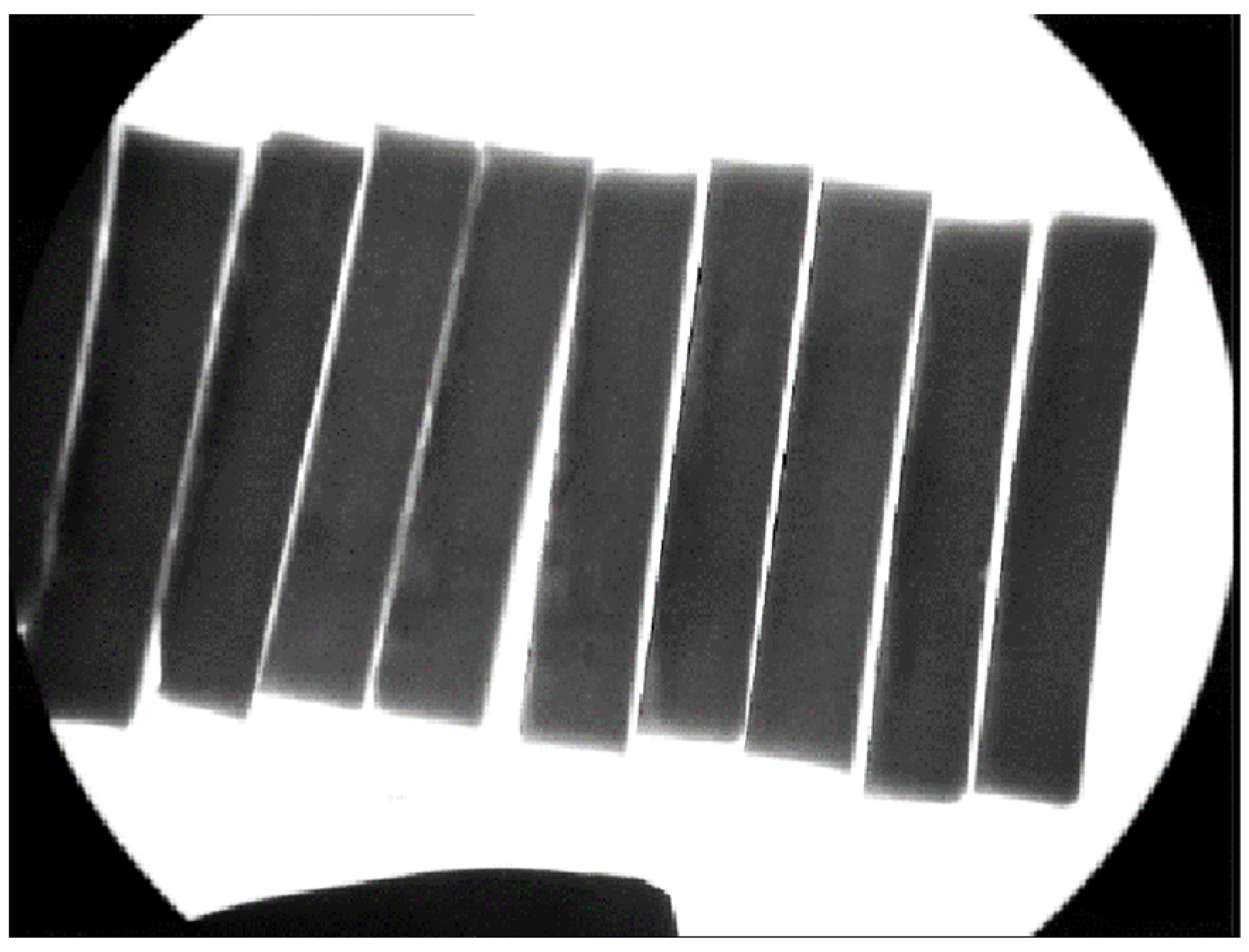
2.1.3. Sample Quality Assurance
2.2. Material Characterization
2.2.1. X-ray Diffraction Analysis
2.2.2. Scanning Electron Microscopy
2.2.3. Hardness
2.2.4. Tensile Tests at Room and Elevated Temperatures
3. Results and Discussion
3.1. Internal Soundness of the Test Material
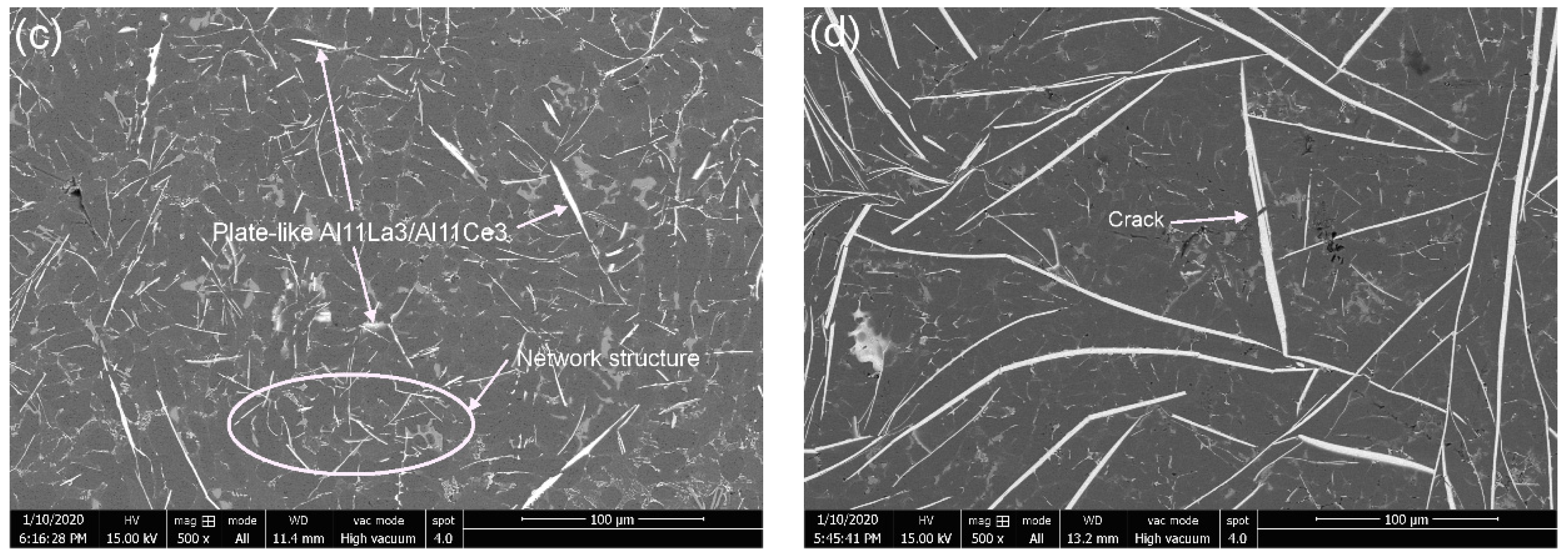
3.2. Microstructural Characteristics
3.3. Mechanical Properties Characteristics
3.3.1. Hardness
3.3.2. Tensile Properties
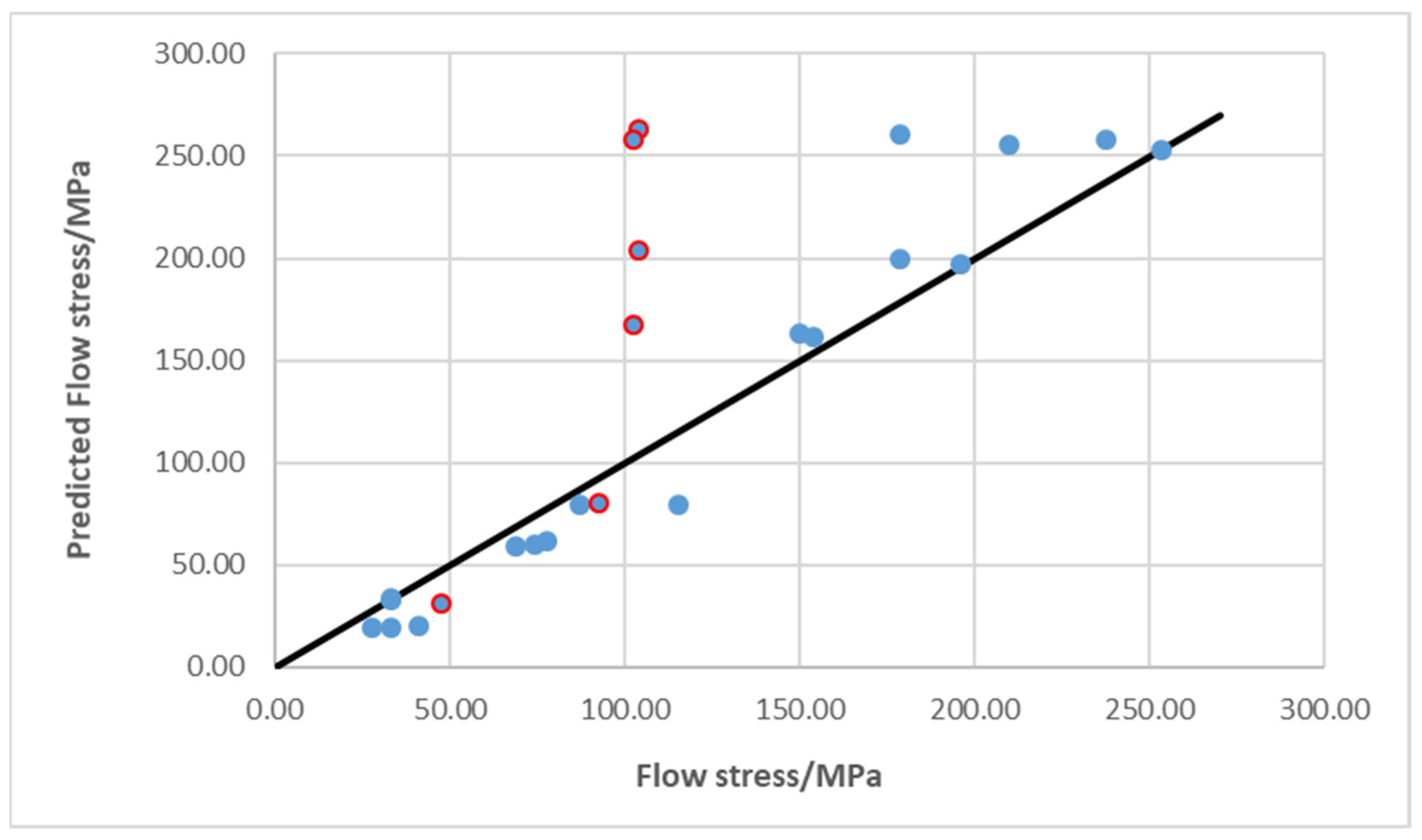
3.4. Modeling Particle Contribution to the Strength
4. Conclusions
- Squeeze-casting could be used to produce a sound material for all compositions
- (Ce and La) addition primarily resulted in the precipitation of Al11(Ce, La)3 and a possible modification of the α-Al15(Fe, Mn)3Si2, as Chinese script precipitates were absent.
- Al11(Ce, La)3 precipitated, as a primary precipitate, in small amounts at 2% (Ce and La) additions. At 4% (Ce and La) addition, a fully developed coarse primary precipitated plate-like Al11(Ce, La)3 formed.
- (Ce and La) additions did affect the mechanical properties due to the precipitation of Al11(Ce, La)3 phase.
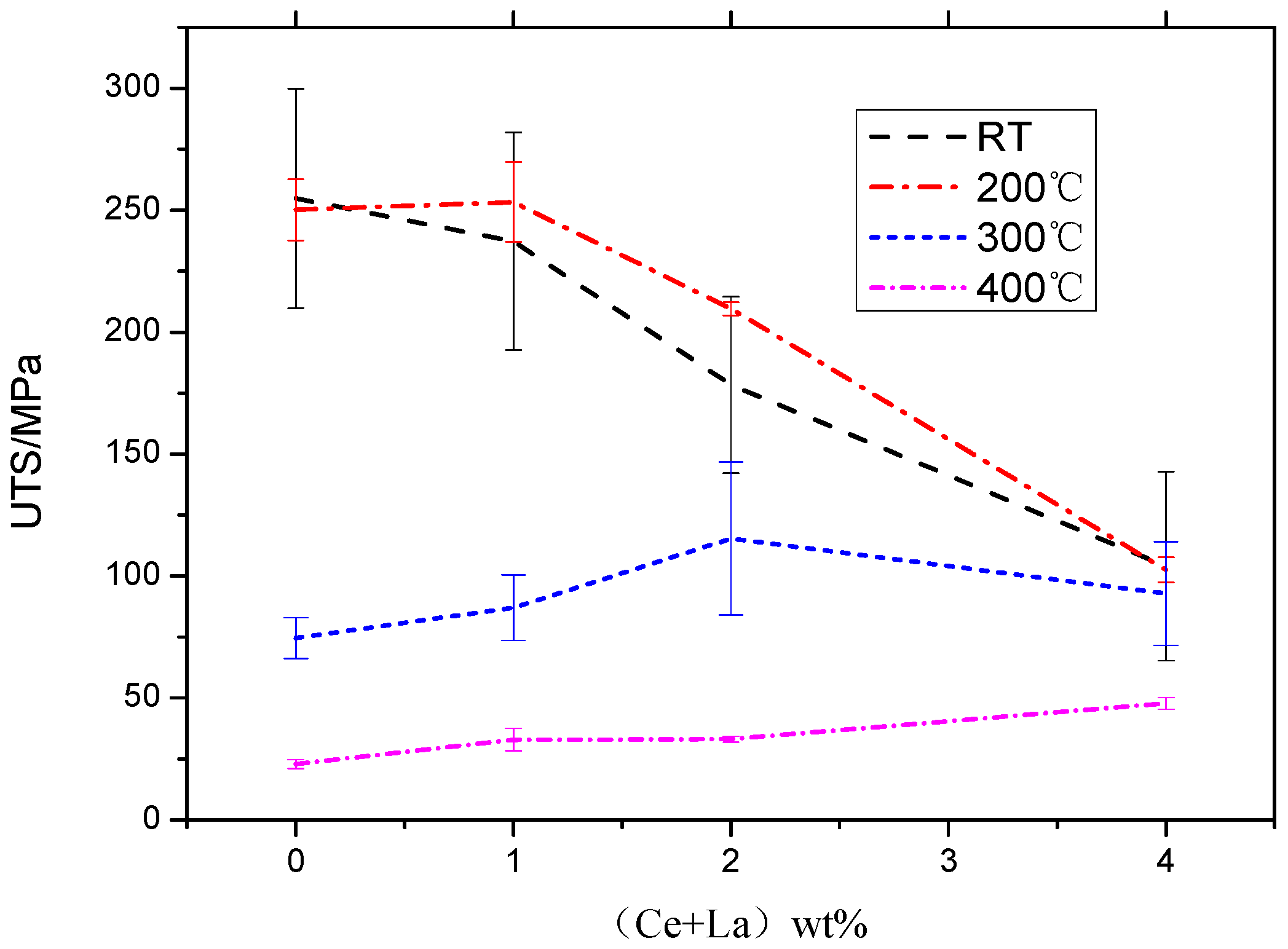
- At room temperature, the UTS significantly drop with the additions of (Ce and La). The 4% RE addition showed the biggest drop of 59% strength compared with the material without RE addition. This drop was due to the cracks formed in the coarse primary Al11(Ce, La)3 phases; the stress concentration was arising from the high aspect ratio plate-like phases;
- At 200 °C, the 1% (Ce and La) additions showed the highest UTS. The improvement at 200 °C was 6.7% compared to the material without RE addition. The reason for the improvement was the smaller size precipitates carry part of the load without causing detrimental stress concentrations;
- At 300 °C, the 2% (Ce and La) additions showed the highest UTS (115.36 MPa), which is a 54.63% strength improvement compared to the RE-free material. A network structure of Al11(Ce, La)3 appears, that through a distributed load-bearing effect improved the high-temperature strength;
- At 400 °C, the 4% (Ce and La) additions showed the highest UTS 47.82 MPa, which is a 108.91% strength-improvement compared with RE-free addition. Although the large plate-like morphology of the primary precipitated Al11(Ce, La)3 phase significantly hampers the room temperature UTS, the high-temperature stable phases could carry the load and improve the high-temperature strength.
- Through the modeling analysis, the nature of the strengthening mechanisms revealed that the dominant contribution to yield strength was the load-bearing mechanism.
- Similarly, elastic modulus mismatch contributed to hardening and thus also kept the tensile strength high (UTS).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kenworthy, J.R. Transport energy use and greenhouse gases in urban passenger transport systems: A study of 84 global cities. In Proceedings of the International Sustainability Conference, Fremantle, Australia, 17–19 September 2003; pp. 17–19. Available online: http://researchrepository.murdoch.edu.au/21463/ (accessed on 19 September 2003).
- Serrenho, A.C.; Norman, J.B.; Allwood, J.M. The impact of reducing car weight on global emissions: The future fleet in Great Britain. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A. Developing Material Requirements for Automotive Brake Disc. Mod. Concepts Mater. Sci. 2019, 2. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, D.; Kalsi, N.S.; Sharma, S.; Pruncu, C.I.; Pimenov, D.Y.; Rao, K.V.; Kapłonek, W. Comparative study on the mechanical, tribological, morphological and structural properties of vortex casting processed, Al–SiC–Cr hybrid metal matrix composites for high strength wear-resistant applications: Fabrication and characterisations. J. Mater. Res. Technol. 2020, 9, 13607–13615. [Google Scholar] [CrossRef]
- Samuel, A.; Ab, C.F. Simulation Study on the Thermomechanical Behaviour of Al-Mc Automotive Brake Discs; Eurobrake: Dresden, Germany, 2019; pp. 1–12. [Google Scholar]
- Dieter, G.E.; Bacon, D.J. Mechanical Metallurgy; McGraw-Hill: New York, NY, USA, 1986; Volume 3, pp. 184–241. [Google Scholar]
- Ceschini, L.; Dahle, A.; Gupta, M.; Jarfors, A.E.W.; Jayalakshmi, S.; Morri, A.; Rotundo, F.; Toschi, S.; Singh, R.A. Chaper 1, Metal Matrix Nanocomposites: An Overview. In Aluminum and Magnesium Metal Matrix Nanocomposites; Springer: Berlin, Germany, 2017; pp. 1–17. [Google Scholar]
- Shaha, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Ageing characteristics and high-temperature tensile properties of Al–Si–Cu–Mg alloys with micro-additions of Mo and Mn. Mater. Sci. Eng. A 2017, 684, 726–736. [Google Scholar] [CrossRef]
- Farkoosh, A.R.; Chen, X.G.; Pekguleryuz, M. Dispersoid strengthening of a high temperature Al-Si-Cu-Mg alloy via Mo addition. Mater. Sci. Eng. A 2015, 620, 181–189. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Bjurenstedt, A.; Seifeddine, S.; Jarfors, A.E.W. The effects of fe-particles on the tensile properties of Al-Si-Cu alloys. Metals 2016, 6, 314. [Google Scholar] [CrossRef]
- Stadler, F.; Antrekowitsch, H.; Fragner, W.; Kaufmann, H.; Uggowitzer, P.J. Effect of main alloying elements on strength of Al-Si foundry alloys at elevated temperatures. Int. J. Cast Met. Res. 2012, 25, 215–224. [Google Scholar] [CrossRef]
- Lee, J.A. Cast aluminum alloy for high temperature applications. In Proceedings of the 132nd TMS Annual Meeting & Exhibition, San Diego, CA, USA, 2–6 March 2003; Volume 1, pp. 145–151. [Google Scholar]
- Bogdanoff, T.; Dahle, A.K.; Seifeddine, S. Effect of Co and Ni Addition on the Microstructure and Mechanical Properties at Room and Elevated Temperature of an Al–7%Si Alloy. Int. J. Met. 2017, 12, 434–440. [Google Scholar] [CrossRef]
- Stadler, F.; Antrekowitsch, H.; Fragner, W.; Kaufmann, H.; Uggowitzer, P.J. The effect of Ni on the high-temperature strength of Al-Si cast alloys. In Materials Science Forum; Trans tech Publications: Switzerland, 2011; Volume 690, pp. 274–277. [Google Scholar] [CrossRef]
- Feng, J.; Ye, B.; Zuo, L.; Qi, R.; Wang, Q.; Jiang, H.; Huang, R.; Ding, W. Effects of Ni content on low cycle fatigue and mechanical properties of Al-12Si-0.9Cu-0.8Mg-xNi at 350 °C. Mater. Sci. Eng. A 2017, 706, 27–37. [Google Scholar] [CrossRef]
- Song, M.; Xiao, D.; Zhang, F. Effect of Ce on the thermal stability of the Ω phase in an Al-Cu-Mg-Ag alloy. Rare Met. 2009, 28, 156–159. [Google Scholar] [CrossRef]
- Liao, H.C.; Xu, H.T.; Hu, Y.Y. Effect of RE addition on solidification process and high-temperature strength of Al−12%Si−4%Cu−1.6%Mn heat-resistant alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 1117–1126. [Google Scholar] [CrossRef]
- SAwe, A.; Seifeddine, S.; Jarfors, A.E.W.; Lee, Y.C.; Dahle, A.K. Development of new Al-Cu-Si alloys for high temperature performance. Adv. Mater. Lett. 2017, 8, 695–701. [Google Scholar] [CrossRef]
- Anderson, K.; Weritz, J.; Kaufman, J.G. 360.0 and A360.0*. In Properties and Selection of Aluminum Alloys; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 558–559. [Google Scholar]
- Anderson, K.; Weritz, J.; Kaufman, J.G. 359.0*. In Properties and Selection of Aluminum Alloys; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 556–557. [Google Scholar]
- Anderson, K.; Weritz, J.; Kaufman, J.G. 319.0, A319.0, B319.0, and 320.0*. In Properties and Selection of Aluminum Alloys; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 533–535. [Google Scholar]
- Anderson, K.; Weritz, J.; Kaufman, J.G. 332.0. In Properties and Selection of Aluminum Alloys; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 536–537. [Google Scholar]
- Anderson, K.; Weritz, J.; Kaufman, J.G. 354.0. In Properties and Selection of Aluminum Alloys; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 542–543. [Google Scholar]
- Anderson, K.; Weritz, J.; Kaufman, J.G. 355.0 and C355.0*. In Properties and Selection of Aluminum Alloys; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 544–547. [Google Scholar]
- Anderson, K.; Weritz, J.; Kaufman, J.G. 356.0 and A356.08*. In Properties and Selection of Aluminum Alloys; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 548–552. [Google Scholar]
- Anderson, K.; Weritz, J.; Kaufman, J.G. 357.0 and Variations A357.0 to F357.0*. In Properties and Selection of Aluminum Alloys; ASM International: Almere, The Netherlands, 2019; Volume 2B, pp. 553–555. [Google Scholar]
- Gariboldi, E.; Lemke, J.N.; Ozhoga-Maslovskaja, O.; Timelli, G.; Bonollo, F. High temperature behaviour of 46000, 46100, 47100 Al die cast parts. Metall. Ital. 2016, 108, 13–16. [Google Scholar]
- Chang, J.; Moon, I. Refinement of cast microstructure of hypereutectic Al-Si alloys through the addition of rare earth metals. J. Mater. Sci. 1998, 3, 5015–5023. [Google Scholar] [CrossRef]
- Ghassemali, E.; Riestra, M.; Bogdanoff, T.; Kumar, B.S.; Seifeddine, S. Hall-Petch equation in a hypoeutectic Al-Si cast alloy: Grain size vs. secondary dendrite arm spacing. Procedia Eng. 2017, 207, 19–24. [Google Scholar] [CrossRef]
- Kai, W. Introduction of 2002 Edition National Standard of Metallic Materials—Brinell Hardness Test. Heat Treat. Met. China 2006, 2002, 101–103. [Google Scholar]
- Du, Y.; He, C.; Schuster, J.C.; Liu, S.; Xu, H. Thermodynamic description of the Ni-Si-Ti ternary system. Int. J. Mater. Res. 2006, 97, 543–555. [Google Scholar] [CrossRef]
- Fan, C.; Long, S.Y.; de Yang, H.; Wang, X.J.; Zhang, J.C. Influence of Ce and Mn addition on α-Fe morphology in recycled Al-Si alloy ingots. Int. J. Miner. Metall. Mater. 2013, 20, 890–895. [Google Scholar] [CrossRef]
- Baldan, R.; Malavazi, J.; Couto, A.A. Microstructure and mechanical behavior of Al9Si0.8Fe alloy with different Mn contents. Mater. Sci. Technol. 2017, 33, 1192–1199. [Google Scholar] [CrossRef]
- Elgallad, E.M.; Ibrahim, M.F.; Doty, H.W.; Samuel, F.H. Microstructural characterisation of Al–Si cast alloys containing rare earth additions. Philos. Mag. 2018, 98, 1337–1359. [Google Scholar] [CrossRef]
- Aghaie, E. Effect of Cerium Addition on Improvement of Mechanical Properties of B319 Powertrain Aluminum Alloy. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2019; p. 130. [Google Scholar]




| Alloy No. | EN AC 46000 | A1 | A2 | A3 | A4 | Comment |
|---|---|---|---|---|---|---|
| Element | (wt)% | (wt)% | (wt)% | (wt)% | (wt)% | |
| Copper (Cu) | 2–4 | 1.84 | 1.82 | 1.92 | 1.84 | Ecotoxicity |
| Nickel (Ni) | <0.55 | 1.74 | 1.76 | 1.87 | 1.73 | Elevated temperature performance |
| Iron (Fe) | 0.6–1.1 | 0.15 | 0.14 | 0.14 | 0.10 | Kept low for ductility |
| Manganese (Mn) | <0.55 | 0.86 | 0.89 | 0.76 | 0.74 | Solution hardening |
| Titanium (Ti) | 0.2 | 0.14 | 0.19 | 0.14 | 0.25 | Grain refinement |
| Magnesium (Mg) | 0.15–0.55 | 0.80 | 0.88 | 0.86 | 0.91 | Wetting agent for SiC additions |
| Silicon (Si) | 8–11 | 10.00 | 9.88 | 10.20 | 9.50 | Castability and hardness |
| Cerium (Ce) | 0 | 0 | 0.5 | 1 | 2 | Target for study |
| Lanthanum (La) | - | 0 | 0.5 | 1 | 2 | Target for study |
| Zinc (Zn) | <1.2 | - | - | - | - | |
| Chromium (Cr) | <0.15 | - | - | - | - | |
| Aluminum (Al) | Bal. | Bal. | Bal. | Bal. | Bal. |
| Alloy | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| SDAS (µm) | 27.31 ± 0.72 | 28.80 ± 3.10 | 32.00 ± 5.71 | 26.28 ± 4.09 |
| Particle Feret diameter (µm) | - | 14.35 ± 7.13 | 19.70 ± 12.37 | 40.62 ± 31.38 |
| Particle fraction (%) | 0 | 0.65 | 3.12 | 7.23 |
| Ce weight percentage in particles (%) | 0 | 0.09 | 0.48 | 1.15 |
| La weight percentage in particles (%) | 0 | 0.10 | 0.50 | 1.15 |
| Alloys | Yield Strength/YS (MPa) | Hardness | |||
| RT | 200 °C | 300 °C | 400 °C | HB | |
| A1 | 196 | 161 | 59 | 19. | 70 |
| A2 | 196. | 154 | 69 | 28 | 70 |
| A3 | 178 | 150 | 74 | 33 | 66 |
| A4 | 104 | 103 | 78 | 41 | 68 |
| Alloys | Tensile Properties/UTS (MPa) | ||||
| RT | 200 °C | 300 °C | 400 °C | ||
| EN AC 46000 | 280 | 215 | 110 | 20 | [27] |
| A1 | 255 | 250 | 75 | 23 | |
| A2 | 237 | 253 | 87 | 33 | |
| A3 | 178 | 210 | 115 | 33 | |
| A4 | 104 | 103 | 93 | 48 | |
| Alloys | Elongations/ε (m/m) | ||||
| RT | 200 °C | 300 °C | 400 °C | ||
| A1 | 0.00940 | 0.02830 | 0.07890 | 0.13690 | |
| A2 | 0.01090 | 0.01580 | 0.05120 | 0.22820 | |
| A3 | 0.00630 | 0.01140 | 0.02010 | 0.14500 | |
| A4 | 0.01500 | 0.00130 | 0.00830 | 0.04650 | |
| Parameter | Yield Strength/YS (MPa) | |||
|---|---|---|---|---|
| RT | 200 °C | 300 °C | 400 °C | |
| 196.39 | 161 | 59.2 | 19.19 | |
| 0 | ||||
| 477 | ||||
| 1.47 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, A.; Jarfors, A.E.W.; Zheng, J.; Wang, K.; Yu, G. The Influence of La and Ce on Microstructure and Mechanical Properties of an Al-Si-Cu-Mg-Fe Alloy at High Temperature. Metals 2021, 11, 384. https://doi.org/10.3390/met11030384
Du A, Jarfors AEW, Zheng J, Wang K, Yu G. The Influence of La and Ce on Microstructure and Mechanical Properties of an Al-Si-Cu-Mg-Fe Alloy at High Temperature. Metals. 2021; 11(3):384. https://doi.org/10.3390/met11030384
Chicago/Turabian StyleDu, Andong, Anders E. W. Jarfors, Jinchuan Zheng, Kaikun Wang, and Gegang Yu. 2021. "The Influence of La and Ce on Microstructure and Mechanical Properties of an Al-Si-Cu-Mg-Fe Alloy at High Temperature" Metals 11, no. 3: 384. https://doi.org/10.3390/met11030384
APA StyleDu, A., Jarfors, A. E. W., Zheng, J., Wang, K., & Yu, G. (2021). The Influence of La and Ce on Microstructure and Mechanical Properties of an Al-Si-Cu-Mg-Fe Alloy at High Temperature. Metals, 11(3), 384. https://doi.org/10.3390/met11030384







