Experimental Determination of Phase Equilibria in the Na2O-SiO2-WO3 System
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. Determination of Equilibration Time
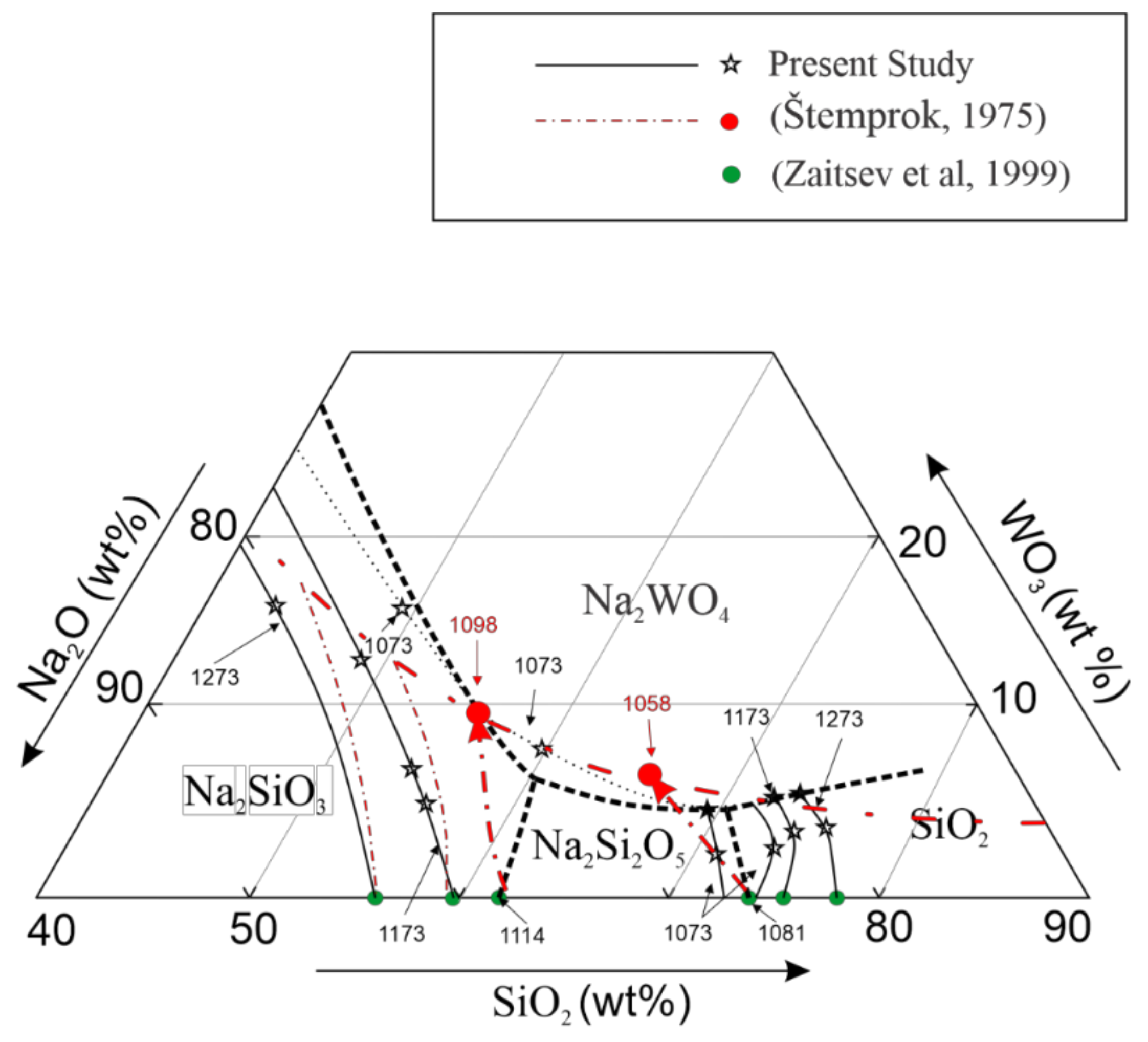
3.2. Description of the Ternary Phase Diagram
| Sample Number | Temperature (K) | Phase | Composition (wt%) | ||
|---|---|---|---|---|---|
| Na2O | SiO2 | WO3 | |||
| Sodium metasilicate primary phase field | |||||
| WE1 | 1073 | Na2SiO3 | 50.1 | 49.4 | 0.5 |
| Liquid | 37.5 | 48.8 | 13.7 | ||
| WE2 | 1173 | Na2SiO3 | 50.5 | 49.2 | 0.3 |
| Liquid | 38.7 | 56.1 | 5.2 | ||
| WE3 | 1173 | Na2SiO3 | 50.6 | 49.2 | 0.2 |
| Liquid | 38.2 | 54.4 | 7.4 | ||
| WE4 | 1173 | Na2SiO3 | 50.6 | 49.1 | 0.3 |
| Liquid | 37.9 | 48.9 | 13.2 | ||
| WE5 | 1273 | Na2SiO3 | 50.2 | 49.3 | 0.5 |
| Liquid | 38.9 | 43.0 | 18.1 | ||
| Sodium disilicate primary phase field | |||||
| WE6 | 1073 | Na2Si2O5 | 32.8 | 67 | 0.2 |
| Liquid | 26.4 | 71.1 | 2.5 | ||
| Tridymite primary phase field | |||||
| WE7 | 1073 | SiO2 | 0.1 | 99.9 | n.d |
| Liquid | 23.5 | 73.7 | 2.8 | ||
| WE8 | 1173 | SiO2 | 0.3 | 99.6 | 0.1 |
| Liquid | 22.1 | 74.2 | 3.7 | ||
| WE9 | 1273 | SiO2 | 0.3 | 99.6 | 0.1 |
| Liquid | 20.5 | 75.6 | 3.9 | ||
| Sodium tungstate two immiscible liquid region | |||||
| WE10 | 1073 | Liquid -Na2WO4 | 20.1 | n.d | 79.9 |
| Liquid -1 | 32.8 | 59.7 | 7.5 | ||
| Phase boundary | |||||
| WE11 | 1073 | Liquid -1 | 25.4 | 69.5 | 4.1 |
| Liquid -Na2WO4 | 20.2 | n.d | 79.8 | ||
| Na2Si2O5 | 32.9 | 67.1 | 0.2 | ||
| WE12 | 1173 | Liquid -1 | 22.1 | 72.3 | 5.6 |
| Liquid -Na2WO4 | 20.1 | n.d | 79.9 | ||
| SiO2 | 0.1 | 99.4 | 0.5 | ||
| WE13 | 1273 | Liquid -1 | 20.5 | 73.5 | 6.0 |
| Liquid -Na2WO4 | 20.4 | n.d | 79.6 | ||
| SiO2 | 0.2 | 99.5 | 0.3 | ||
| Only one liquid phase exists | |||||
| WE14 | 1073 | Liquid | 28.4 | 66.3 | 5.3 |
| WE15 | 1173 | Liquid | 36.8 | 53.8 | 9.4 |
| WE16 | 1173 | Liquid | 24.0 | 71.3 | 4.7 |
| WE17 | 1173 | Liquid | 26.3 | 68.5 | 5.2 |
| WE18 | 1273 | Liquid | 40.6 | 48.7 | 10.7 |
| Sample Number | Temperature (K) | Composition (wt%) | ||
|---|---|---|---|---|
| Na2O | SiO2 | WO3 | ||
| WE1 | 1073 | 25 | 70 | 5 |
| WE2 | 1173 | 40 | 55 | 5 |
| WE3 | 1173 | 38 | 54 | 8 |
| WE4 | 1173 | 42 | 49 | 9 |
| WE5 | 1273 | 42.5 | 42.5 | 5 |
| WE6 | 1073 | 27 | 70 | 3 |
| WE7 | 1073 | 17 | 80 | 3 |
| WE8 | 1173 | 17 | 80 | 3 |
| WE9 | 1273 | 17 | 80 | 3 |
| WE10 | 1073 | 33 | 57 | 10 |
| WE11 | 1073 | 27 | 65 | 8 |
| WE12 | 1173 | 15 | 79 | 6 |
| WE13 | 1273 | 15 | 79 | 6 |
| WE14 | 1073 | 30 | 65 | 5 |
| WE15 | 1173 | 35 | 50 | 15 |
| WE16 | 1173 | 25 | 70 | 5 |
| WE17 | 1173 | 30 | 65 | 5 |
| WE18 | 1273 | 42 | 49 | 9 |
| Crystalline Solid | Structure | Melting Temperature (K) | Ref. |
|---|---|---|---|
| Na2SiO3 | Tetragonal | 1361 | [26,27] |
| Na2Si2O5 | Tetragonal | 1143 | [28] |
| SiO2 | Tetragonal | 1984 | [29,30,31] |
| Na2WO4 | Spinel | 971 | [32,33] |

3.3. Comparisons with the Existing Ternary Phase Diagram
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Department of Defense. Strategic and Critical Materials 2013 Report on Stockpile Requirements; Office of the Under Secretary of Defense, US Department of Defense: Washington, DC, USA, 2013; p. 189.
- European Commission. Report on Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2014; pp. 1–37. [Google Scholar]
- Skirrow, R.G.; Huston, D.L.; Mernagh, T.P.; Thorne, J.P.; Duffer, H.; Senior, A. Critical Commodities for a High-Tech World: Australia’s Potential to Supply Global Demand; Geoscience Australia: Canberra, Australia, 2013. [Google Scholar]
- Fortier, S.M.; Nassar, N.T.; Lederer, G.W.; Brainard, J.; Gambogi, J.; McCullough., E.A. Draft Critical Mineral List—Summary of Methodology and Background Information—US Geological Survey Technical Input Document in Response to Secretarial Order No. 3359; U.S. Geological Survey: Washington, DC, USA, 2018; Volume 15. [CrossRef] [Green Version]
- Trasorras, J.R.L.; Wolfe, T.A.; Knabl, W.; Venezia, C.; Lemus, R.; Lassner, E.; Schubert, W.D.; Lüderitz, E.; Wolf, H.U. Tungsten, tungsten alloys, and tungsten compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: New York, NY, USA, 2016; pp. 1–53. [Google Scholar]
- Yih, S.; Wang, C. Tungsten: Sources, Metallurgy, Properties, and Applications; Springer: New York, NY, USA, 1979; p. 512. [Google Scholar]
- Shen, L.; Li, X.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Nie, C.; Zhang, J.; Steenari, B.M.; Ekberg, C. Comprehensive treatments of tungsten slags in China: A critical review. J. Environ. 2020, 270, 110927. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Uchida, K.; Baker, D.H. A High-Temperature, Two-Phase Extraction Technique for Tungsten Minerals; US Department of the Interior and Bureau of Mines: Washington, DC, USA, 1968; Volume 7106, pp. 1–11.
- Gomes, J.M.; Zadra, J.; Baker, D. Electrolytic Extraction of Tungsten from Western Scheelite; US Department of the Interior and Bureau of Mines: Washington, DC, USA, 1964; Volume 6512, pp. 1–12.
- James, P. Liquid-phase separation in glass-forming systems. J. Mater. Sci. 1975, 10, 1802–1825. [Google Scholar] [CrossRef]
- Yan, B.; Wang, X.; Yang, Z. Experimental study of phase equilibria in the MgO-SiO2-TiOx system. J. Alloys Compd. 2017, 695, 3476–3483. [Google Scholar] [CrossRef]
- Li, J.; Shu, Q.; Chou, K. Phase relations in CaO-SiO2-Al2O3-15 mass pct CaF2 System at 1523 K (1250 °C). Metall. Mater. Trans. B. 2014, 45, 1593–1599. [Google Scholar] [CrossRef]
- Shi, J.; Sun, L.; Qiu, J.; Zhang, B.; Jiang, M. Phase equilibria of CaO-SiO2-5wt% MgO-10wt% Al2O3-TiO2 system at 1300 °C and 1400 °C relevant to Ti-bearing furnace slag. J. Alloys Compd. 2017, 699, 193–199. [Google Scholar] [CrossRef]
- Wang, Z.; Sohn, I. Experimental determination of phase equilibria in the CaO-BaO-SiO2-12 mol pct. Al2O3-13 mol pct. MgO system at 1573 K and 1623 K. J. Am. Ceram. Soc. 2019, 102, 5632–5644. [Google Scholar] [CrossRef]
- Caillet, P. Composition of Alkali Tungstate-Tungstic Anhydride systems. CRH Acad. Sci. 1963, 256, 1986–1989. [Google Scholar]
- Sakka, S. Compound formation in Alkali Tungstate systems. Bull. Inst. Chem. Res. Kyoto Univ. 1969, 46, 300–308. [Google Scholar]
- Chang, L.L.Y.; Sachdev, S. Alkali Tungstates: Stability relations in the systems A2OWO3-WO3. J. Am. Ceram. Soc. 1975, 58, 267–270. [Google Scholar] [CrossRef]
- Štemprok, M. Geological significance of immiscibility in fused silicate systems containing tungsten and molybdenum. Int. Geol. Rev. 1975, 17, 1306–1316. [Google Scholar] [CrossRef]
- Jak, E.; Hayes, P.C. Phase equilibria determination in complex slag systems. Miner. Process. Extract. Metall. Rev. 2008, 117, 1, 1–17. [Google Scholar] [CrossRef]
- Santoso, I.; Taskinen, P. Phase equilibria of the Na2O–SiO2 system between 1173 and 1873 K. Can. Metall. Q. 2016, 55, 243–250. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, J.; Li, M.; Chen, M.; Zhao, B. Phase equilibria of the SiO2–V2O5 system. Ceram. Int. 2020, 46, 24053–24059. [Google Scholar] [CrossRef]
- Wang, D.; Chen, M.; Jiang, Y.; Wang, S.; Zhao, Z.; Evans, T.; Zhao, B. Phase equilibria studies in the CaO–SiO2–Al2O3–MgO system with CaO/SiO2 ratio of 0.9. J. Am. Ceram. Soc. 2020, 103, 7299–7309. [Google Scholar] [CrossRef]
- Ritchie, N.; Newbury, D.; Leigh, S. Breaking the 1% accuracy barrier in EPMA. Microsc. Microanal. 2012, 18, 1006–1007. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Shelkova, N.E.; Lyakishev, N.P.; Mogutnov, B.M. Thermodynamic properties and phase equilibria in the Na2O-SiO2 system. Phys. Chem. Chem. Phys. 1999, 1, 1899–1907. [Google Scholar] [CrossRef]
- Anis, M.; Shirsat, M.D.; Hussaini, S.S.; Joshi, B.; Muley, G.G. Effect of sodium metasilicate on structural, optical, dielectric and mechanical properties of ADP crystal. J. Mater. Sci Technol. 2016, 32, 62–67. [Google Scholar] [CrossRef]
- Morey, G.W.; Bowen, N.L. The Binary System Sodium Metasilicate-silica. J. Phys. Chem. 1924, 29, 1167–1179. [Google Scholar] [CrossRef]
- You, J.; Jiang, G.; Xu, K. High temperature Raman spectra of sodium disilicate crystal, glass and its liquid. J. Non Cryst Solids 2001, 282, 125–131. [Google Scholar] [CrossRef]
- Ringdalen, E. Changes in quartz during heating and the possible effects on Si production. J. Oper. Manag. 2015, 67, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Welche, P.; Heine, V.; Dove, M. Negative thermal expansion in beta-quartz. Phys. Chem. Miner. 1998, 26, 63–77. [Google Scholar] [CrossRef]
- Konnert, J.H.; Appleman, D.E. The crystal structure of low tridymite. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1978, 34, 391–403. [Google Scholar] [CrossRef]
- Dkhilalli, F.; Megdiche, B.S.; Rasheed, M.; Barille, R.; Shihab, S.; Guidara, K.; Megdiche, M. Characterizations and morphology of sodium tungstate particles. R. Soc. Open Sci. 2018, 5, 172214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaste, R.C.; Suby, S.M.S.; Hodman, C.D. Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1979; p. B-129. [Google Scholar]

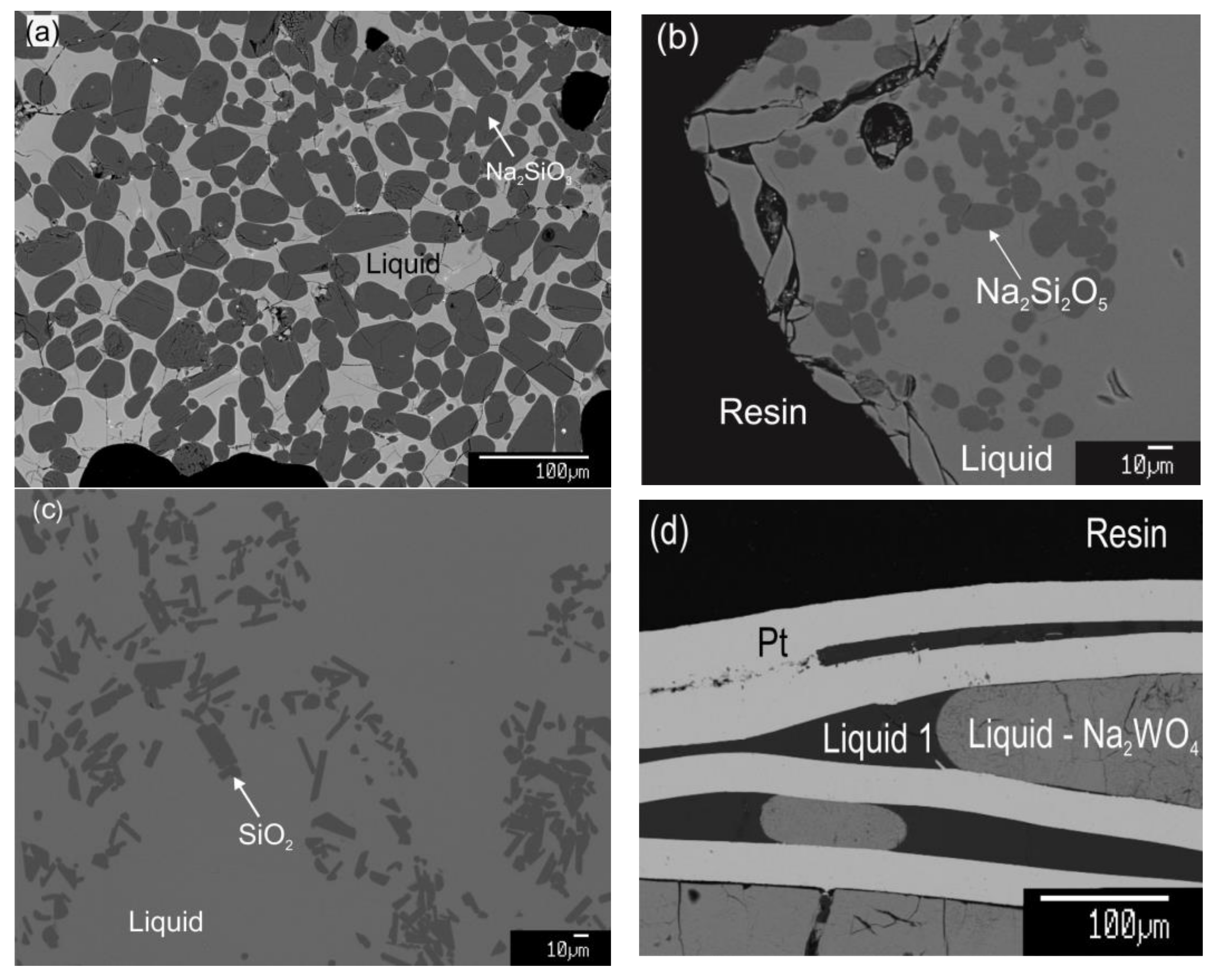
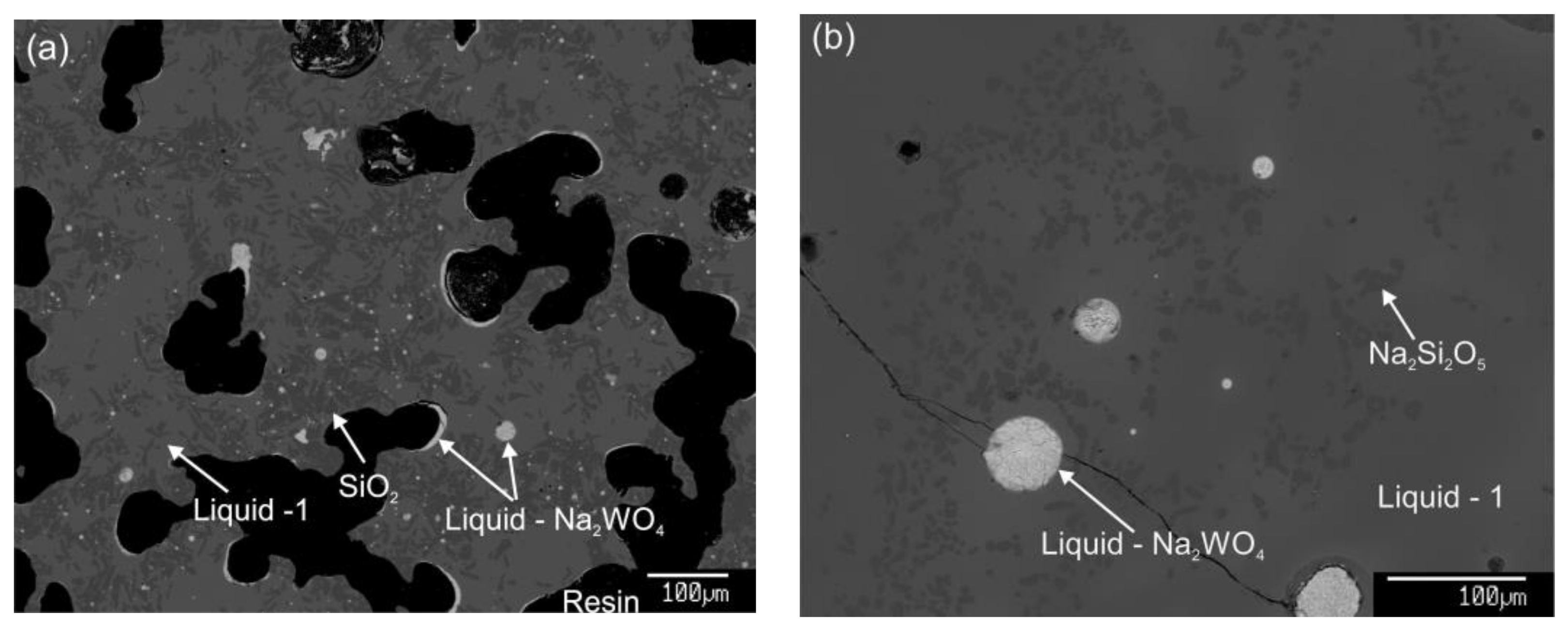
| Temperature (K) | Holding Time (h) | Phase | Composition (wt%) | ||
|---|---|---|---|---|---|
| Na2O | SiO2 | WO3 | |||
| 1073 | 72 168 | Liquid | 23.5 | 73.7 | 2.8 |
| Tridymite | 0.1 | 99.9 | n.d | ||
| Liquid | 23.4 | 73.6 | 3.0 | ||
| Tridymite | 0.2 | 99.8 | n.d | ||
| 1173 | 24 72 | Liquid | 22.1 | 74.2 | 3.7 |
| Tridymite | 0.3 | 99.6 | 0.1 | ||
| Liquid | 22.2 | 74.3 | 3.5 | ||
| Tridymite | 0.2 | 99.8 | n.d | ||
| 1273 | 24 72 | Liquid | 20.5 | 75.6 | 3.9 |
| Tridymite | 0.3 | 99.6 | 0.1 | ||
| Liquid | 20.4 | 75.8 | 3.8 | ||
| Tridymite | 0.2 | 99.7 | 0.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Su, K.; Ma, X. Experimental Determination of Phase Equilibria in the Na2O-SiO2-WO3 System. Metals 2021, 11, 2014. https://doi.org/10.3390/met11122014
Zhao B, Su K, Ma X. Experimental Determination of Phase Equilibria in the Na2O-SiO2-WO3 System. Metals. 2021; 11(12):2014. https://doi.org/10.3390/met11122014
Chicago/Turabian StyleZhao, Baojun, Kun Su, and Xiaodong Ma. 2021. "Experimental Determination of Phase Equilibria in the Na2O-SiO2-WO3 System" Metals 11, no. 12: 2014. https://doi.org/10.3390/met11122014
APA StyleZhao, B., Su, K., & Ma, X. (2021). Experimental Determination of Phase Equilibria in the Na2O-SiO2-WO3 System. Metals, 11(12), 2014. https://doi.org/10.3390/met11122014








