Influence of the Small Sc and Zr Additions on the As-Cast Microstructure of Al–Mg–Si Alloys with Excess Silicon
Abstract
:1. Introduction
2. Experimental Methods
2.1. Material Preparation
2.2. Material Characterization
2.3. Phase Diagram Calculation
3. Results
3.1. Grain Refinement
3.2. Microhardness Behavior
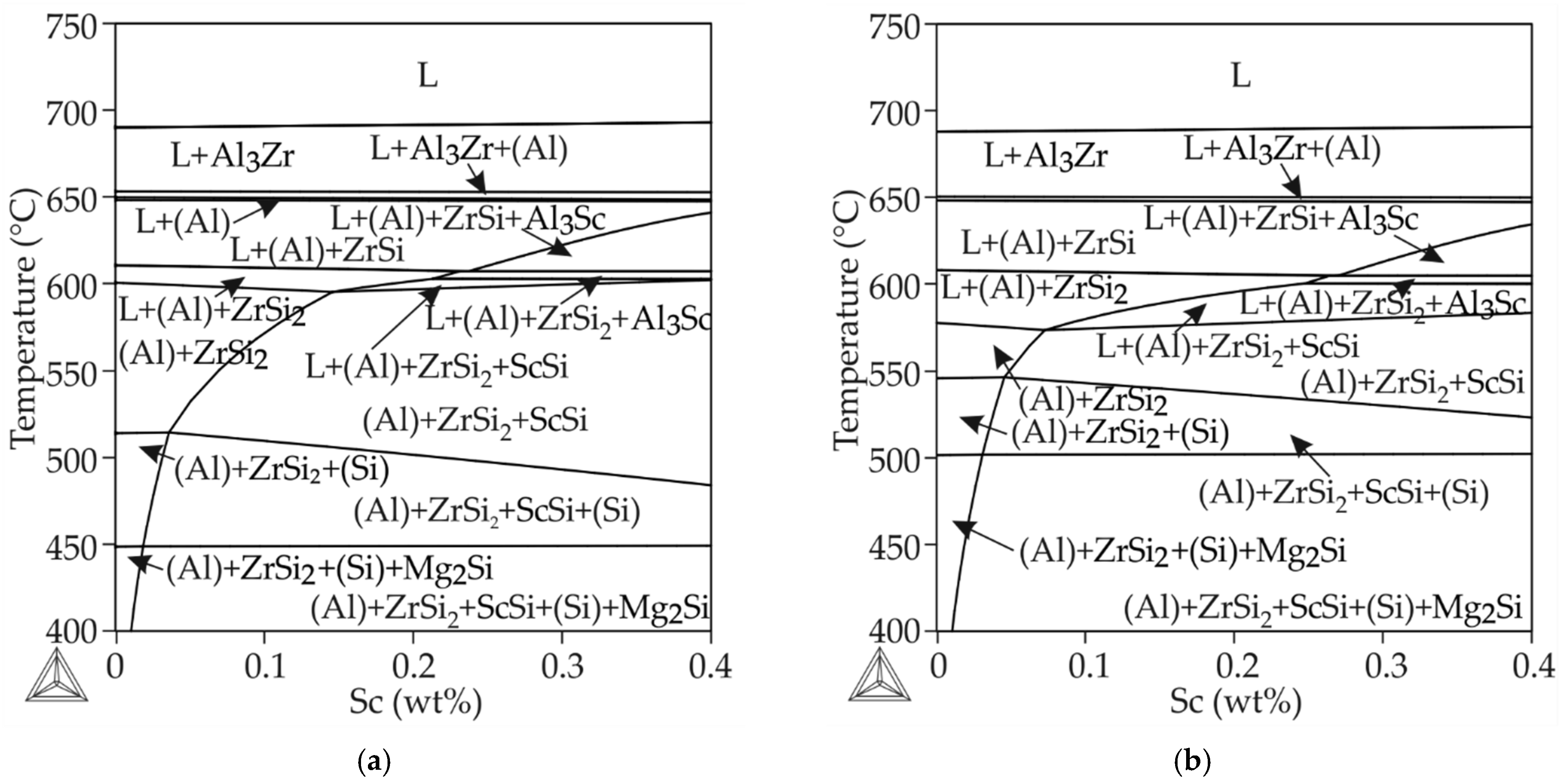
3.3. Phase Diagram Calculation Result
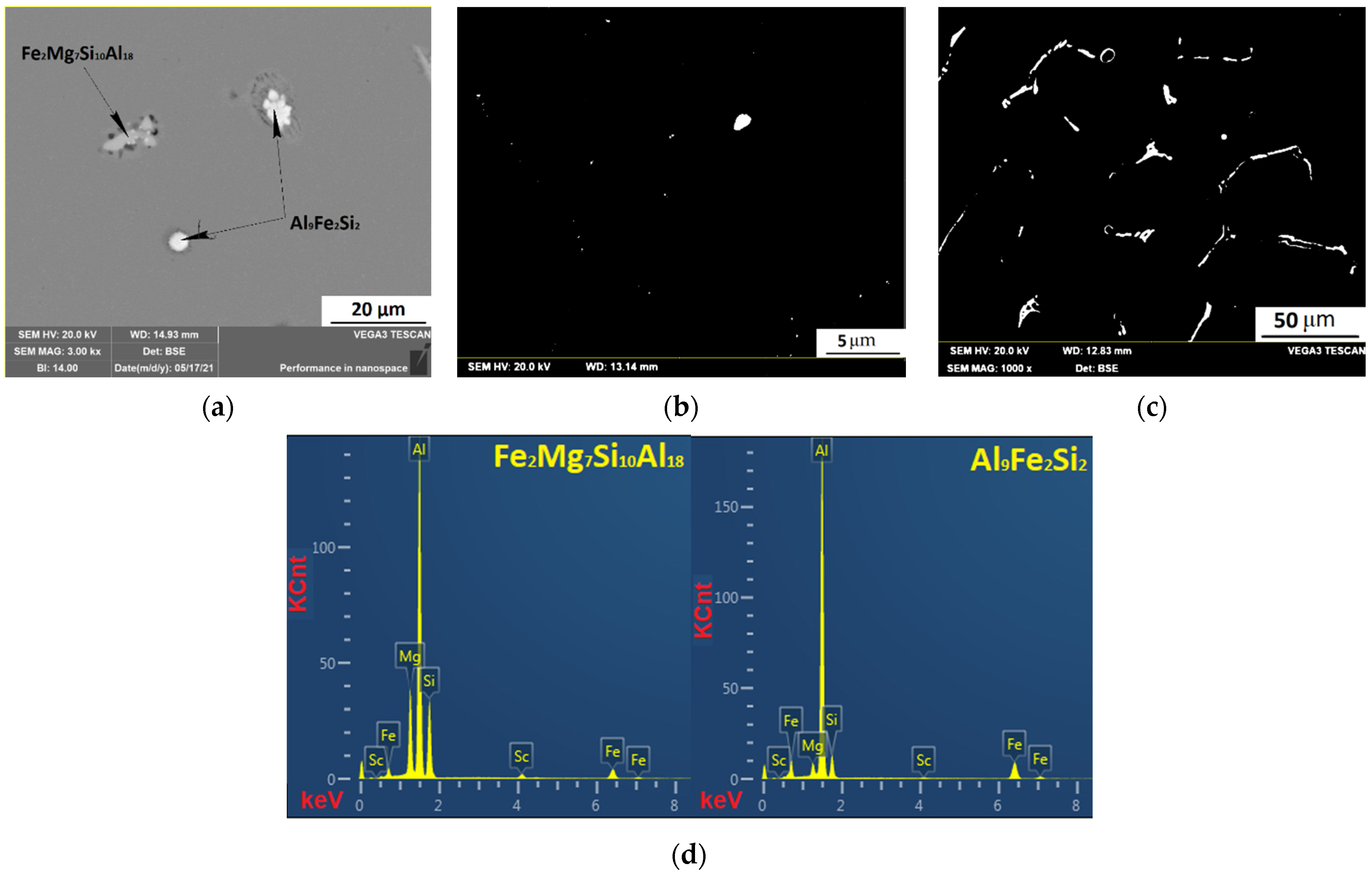
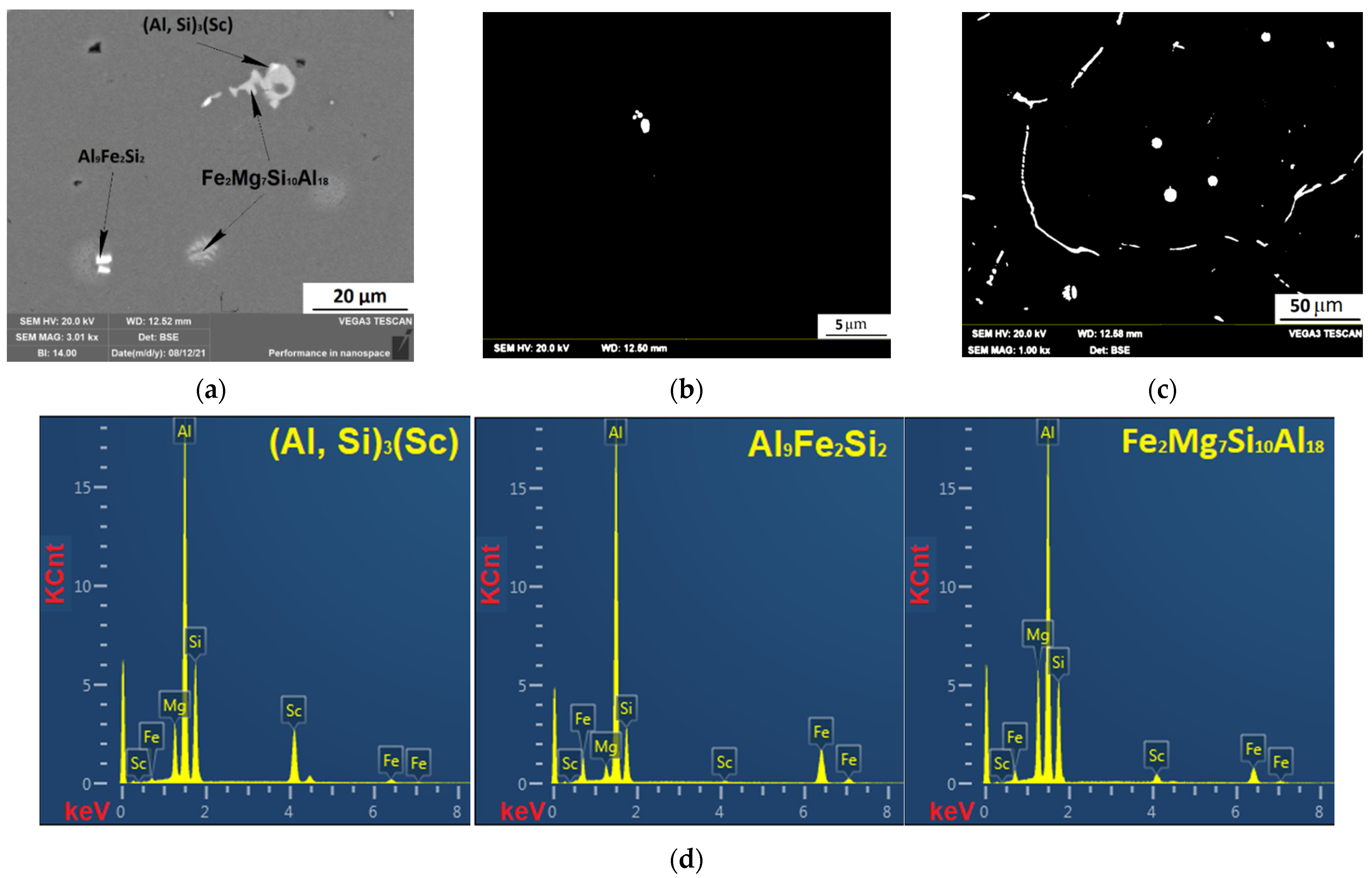
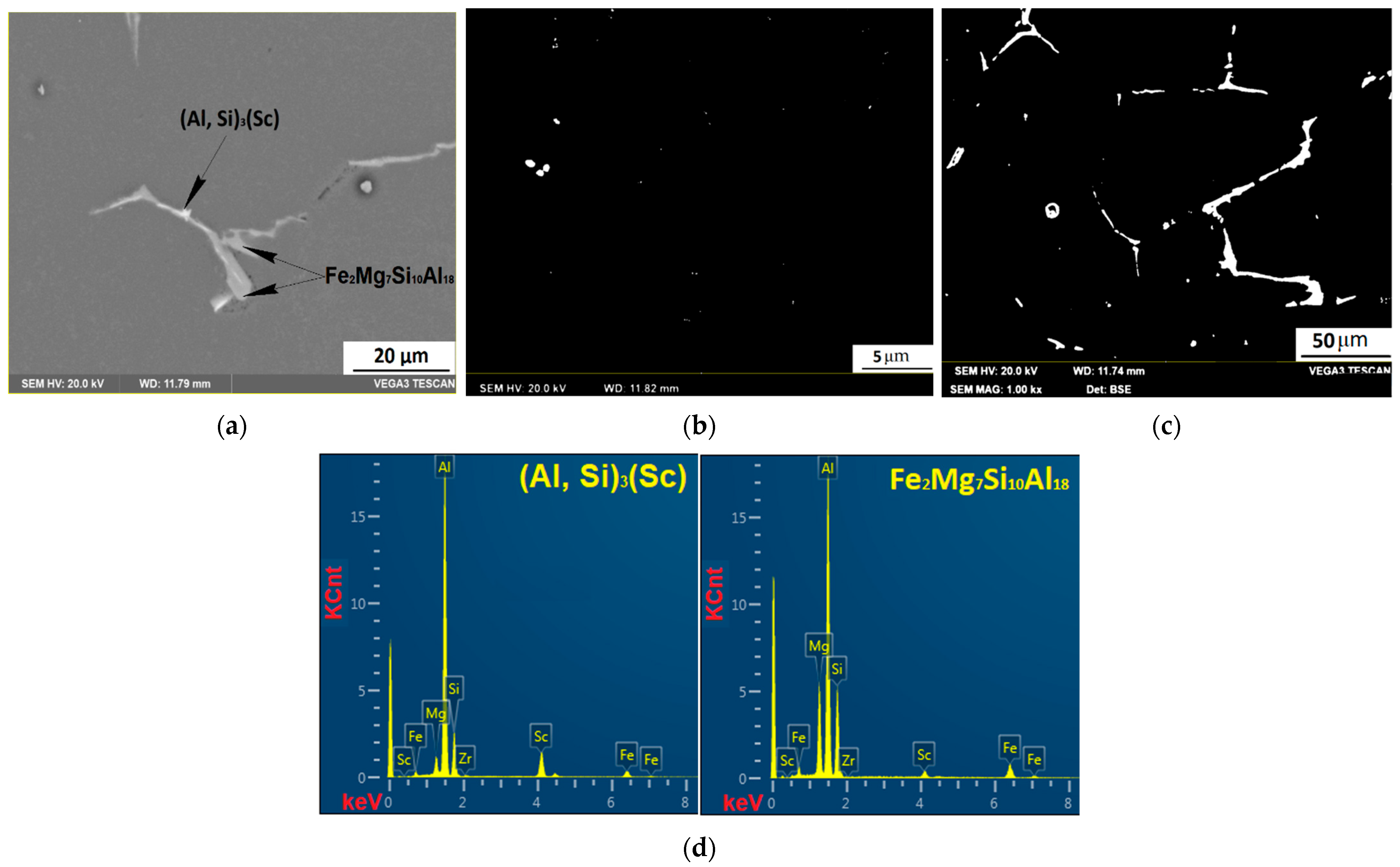
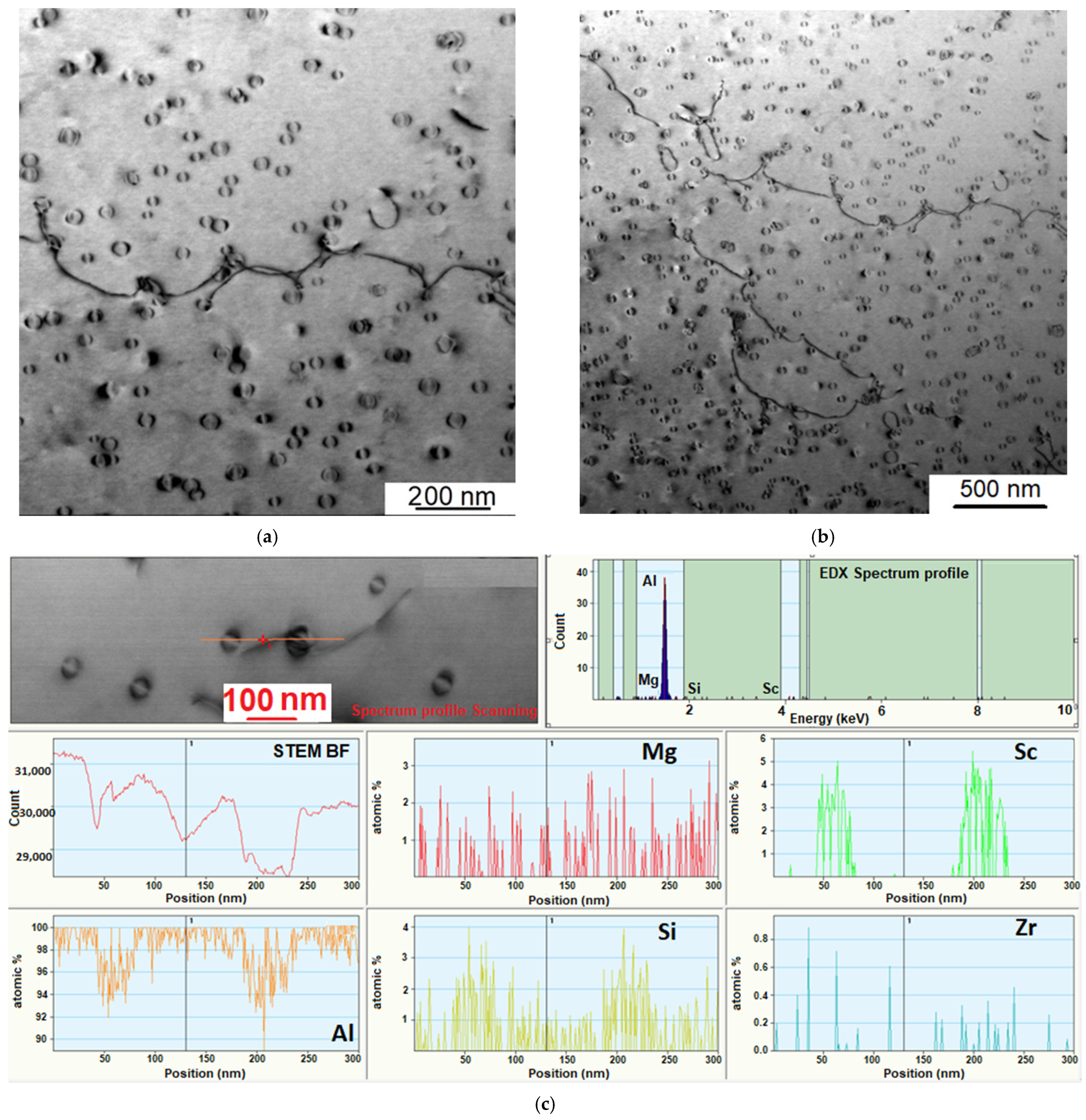
3.4. Investigation of the Intermetallic Participles by Scanning Electron Microscopy (SEM) Method
3.5. Investigation of the Despersoid by Transmission Electron Microscopy (TEM) Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fedorov, S.; Nanocomposites, V.B. Development of Mechanical Properties of Aluminum-Silicon Alloys. Smart Nanocompos. 2015, 6, 199–202. [Google Scholar]
- Bazhin, V.Y.; Gutema, E.M.; Savchenkov, S.A. Production Technology Features for Aluminum Matrix Alloys with a Silicon Carbide Framework. Metallurgist 2017, 60, 1267–1272. [Google Scholar] [CrossRef]
- Deev, V.B.; Degtyar, V.A.; Kutsenko, A.I.; Selyanin, I.F.; Voitkov, A.P. Resource-Saving Technology for the Production of Cast Aluminum Alloys. Steel Transl. 2007, 37, 991–994. [Google Scholar] [CrossRef]
- Akopyan, T.K.; Letyagin, N.V.; Belov, N.A.; Koshmin, A.N.; Gizatulin, D.S. Analysis of the Microstructure and Mechanical Properties of a New Wrought Alloy Based on the ((Al) + Al4(Ca,La)). Eutectic. Phys. Met. Metallogr. 2020, 121, 914–919. [Google Scholar] [CrossRef]
- Sizyakov, V.M.; Bazhin, V.Y.; Vlasov, A.A. Status and Prospects for Growth of the Aluminum Industry. Metallurgist 2010, 54, 409–414. [Google Scholar] [CrossRef]
- Belov, N.; Akopyan, T.; Korotkova, N.; Murashkin, M.; Timofeev, V.; Fortuna, A. Structure and Properties of Ca and Zr Containing Heat Resistant Wire Aluminum Alloy Manufactured by Electromagnetic Casting. Metals 2021, 11, 236. [Google Scholar] [CrossRef]
- Hirsch, J. Aluminium in Innovative Light-Weight Car Design. Mater. Trans. 2011, 52, 818–824. [Google Scholar] [CrossRef] [Green Version]
- Lamberti, M.; Escher, F. Aluminium Foil as a Food Packaging Material in Comparison with Other Materials. Food Rev. Int. 2007, 23, 407–433. [Google Scholar] [CrossRef]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.J.; De Smet, P.; Haszler, A.; Vieregge, A. Recent Development in Aluminium Alloys for the Automotive Industry. Mater. Sci. Eng. 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Rambabu, P.; Eswara Prasad, N.; Kutumbarao, V.V.; Wanhill, R.J.H. Aluminium Alloys for Aerospace Applications. In Aerospace Materials and Material Technologies; Prasad, N., Wanhill, R., Eds.; Indian Institute of Metals Series; Springer: Singapore, 2017; pp. 29–52. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Werinos, M.; Moszner, F.; Gerstl, S.S.A.; Francis, M.F.; Curtin, W.A.; Löffler, J.F.; Uggowitze, P.J. Diffusion on demand to control precipitation aging: Application to Al-Mg-Si alloys. Phys. Rev. Lett. 2014, 22, 225701. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Tang, J.; Liu, X.; Liang, C. Effect of copper on precipitation and baking hardening behavior of Al-Mg-Si alloys. Trans. Nonferrous Met. Soc. China 2014, 7, 2289–2294. [Google Scholar] [CrossRef]
- Kim, J.H.; Kobayashi, E.; Sato, T. Effects of Cu addition on behavior of nanoclusters during multi-step aging in Al-Mg-Si alloy. Mater. Trans. 2011, 5, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.I.U.; Gang, Z.; Liu, C.M.; Liang, Z. Effects of different tempers on precipitation hardening of 6000 series aluminium alloys. Trans. Nonferrous Met. Soc. China 2007, 1, 122–127. [Google Scholar]
- Liang, Z. Clustering and Precipitation in Al-Mg-Si Alloys. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 28 November 2012. [Google Scholar]
- Werinos, M.; Antrekowitsch, H.; Ebner, T.; Prillhofer, R.; Curtin, W.A.; Uggowitzer, P.J.; Pogatscher, S. Design strategy for controlled natural aging in Al–Mg–Si alloys. Acta Mater. 2016, 118, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Zhen, L.; FEI, W.D.; Kang, S.B.; Kim, H.W. Precipitation behaviour of Al-Mg-Si alloys with high silicon content. J. Mater. Sci. 1997, 7, 1895–1902. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Leitner, H.; Ebner, T.; Uggowitzer, P.J. Mechanisms controlling the artificial aging of Al–Mg–Si Alloys. Acta Mater. 2011, 9, 3352–3363. [Google Scholar] [CrossRef]
- Zakharov, V.V.; Fisenko, I.A. Some Principles of Alloying of Aluminum Alloys with Scandium and Zirconium in Ingot Production of Deformed Semiproducts. Metal Sci. Heat Treat. 2019, 3, 217–221. [Google Scholar] [CrossRef]
- Zakharov, V.V. Combined alloying of aluminum alloys with scandium and zirconium. Metal Sci. Heat Treat. 2014, 3, 281–286. [Google Scholar] [CrossRef]
- Röyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 1, 19–44. [Google Scholar] [CrossRef]
- Shvechkov, E.I.; Filatov, Y.A.; Zakharov, V.V. Mechanical and Life Properties of Sheets from Alloys of the Al–Mg–Sc System. Metal Sci. Heat Treat. 2017, 59, 454–462. [Google Scholar] [CrossRef]
- Aryshenskii, E.V.; Hirsch, J.; Konovalov, S.V.; Prahl, U. Specific features of microstructural evolution during hot rolling of the as-cast magnesium-rich aluminum alloys with added transition metal elements. Metall. Mater. Trans. 2019, 12, 5782–5799. [Google Scholar] [CrossRef]
- Peng, Z.W.; Li, J.F.; Sang, F.J.; Chen, Y.L.; Zhang, X.H.; Zheng, Z.Q.; Pan, Q.L. Structures and tensile properties of Sc-containing 1445 Al-Li alloy sheet. J. Alloys Compd. 2018, 747, 471–483. [Google Scholar] [CrossRef]
- Rokhlin, L.L.; Bochvar, N.R.; Leonova, N.P.; Sukhanov, A.V. Effect of additional doping with scandium and scandium with zirconium on strength properties of the alloys of Al–Mg 2 Si system. Inorg. Mater. 2016, 15, 1467–1471. [Google Scholar] [CrossRef]
- Babaniaris, S.; Ramajayam, M.; Jiang, L.; Langan, T.; Dorin, T. Tailored precipitation route for the effective utilisation of Sc and Zr in an Al-Mg-Si alloy. Materialia 2020, 10, 100656. [Google Scholar] [CrossRef]
- Zupanič, F.; Steinacher, M.; Žist, S.; Bončina, T. Microstructure and Properties of a Novel Al-Mg-Si Alloy AA 6086. Metals 2021, 11, 368. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Babaniaris, S.; Jiang, L.; Langan, T.J. Precipitation sequence in Al–Mg–Si–Sc–Zr alloys during isochronal aging. Materialia 2019, 8, 100437. [Google Scholar] [CrossRef]
- Kwon, E.P.; Do Woo, K.; Kim, S.H.; Kang, D.S.; Lee, K.J.; Jeon, J.Y. The effect of an addition of Sc and Zr on the precipitation behavior of AA6061 alloy. Met. Mater. Int. 2010, 5, 701–707. [Google Scholar] [CrossRef]
- Babaniaris, S.; Ramajayam, M.; Jiang, L.; Langan, T.; Dorin, T. Developing an optimized homogenization process for Sc and Zr containing Al-Mg-Si alloys. Light Met. 2019, 1, 1445–1453. [Google Scholar]
- Lityńska-Dobrzyńska, L. Precipitation of phases in Al-Mg-Si-Cu alloy with Sc and Zr additions during heat treatment. Solid State Phenom.—Trans. Tech. Publ. Ltd. 2007, 130, 163–166. [Google Scholar] [CrossRef]
- Vlach, M.; Smola, B.; Stulíková, I.; Očenášek, V. Microstructure and mechanical properties of the AA6082 aluminium alloy with small additions of Sc and Zr. Int. J. Mater. Res. 2009, 3, 420–423. [Google Scholar] [CrossRef]
- GOST 9450–76. Measurements Microhardness by Diamond Instruments Indentation; Academy of Sciences of the USSR: Moscow, Russia, 1993. [Google Scholar]
- Thermo-Calc Software. TCAL4 Al-Based Alloy Database, Version 4. Available online: https://www.engineering-eye.com/THERMOCALC/details/db/pdf/thermo-calc/02/tcal40_extended_info.pdf (accessed on 1 May 2021).
- Aryshenskii, E.; Hirsch, J.; Konovalov, S.; Aryshenskii, V.; Drits, A. Influence of mg content on texture development during hot plain-strain deformation of aluminum alloys. Metals 2021, 11, 865. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, D.; Liu, Z.L.; Taylor, J.A.; Easton, M.A.; Zhang, M.X. The grain refinement mechanism of cast aluminium by zirconium. Acta Mater. 2013, 61, 5636–5645. [Google Scholar] [CrossRef]
- Yu, A.W.; Yang, C.G.; Wang, S.L.; Liu, F.C.; Zheng, Q. Effect of Sc, Zr grain refiner on the microstructure and mechanical properties of pure aluminum. Appl. Mech. Mater.—Trans. Tech. Publ. Ltd. 2014, 508, 16–21. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, R. Grain size-dependent Mg/Si ratio effect on the microstructure and mechanical/electrical properties of Al-Mg-Si-Sc alloys. J. Mater. Sci. Technol. 2019, 7, 1354–1363. [Google Scholar] [CrossRef]
- Yong, D.; Shuhong, L.; Baiyun, H.; Chang, Y.A.; Xie, F.Y.; Ying, Y.; Chen, S.L. Thermodynamic description of the Al-Fe-Mg-Mn-Si system and investigation of microstructure and microsegregation during directional solidification of an Al-Fe-Mg-Mn-Si alloy. Z. Für Met. 2005, 96, 1351–1362. [Google Scholar]
- Litnska-Dobrzynska, L.; Dutkiewicz, J.; Maziarz, W.; Ochin, P. Structure and properties of Al-Mg-Si alloys with Zr and Sc additions produced by melt spinning and twin rolling casting techniques. Kov. Mater. 2010, 48, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Dorin, T.; Ramajayam, M.; Langan, T.J. Effects of Mg, Si, and Cu on the formation of the Al3Sc/Al3Zr dispersoids. In Proceedings of the International Conference on Aluminium Alloys, Montréal, QC, Canada, 17–20 June 2018; Volume 16, pp. 1–11. Available online: http://www.icaa-conference.net/ICAA16/Papers/Plenary%20and%20Early%20Career/404566%20Dorin_final.pdf (accessed on 1 May 2021).
- Booth-Morrison, C.; Mao, Z.; Diaz, M.; Dunand, D.C.; Wolverton, C.; Seidman, D.N. Role of silicon in accelerating the nucleation of Al3(Sc,Zr) precipitates in dilute Al–Sc–Zr alloys. Acta Mater. 2012, 16, 4740–4752. [Google Scholar] [CrossRef]
- Norman, A.F.; Prangnell, P.B.; McEwen, R.S. The solidification behaviour of dilute aluminium–scandium alloys. Acta Mater. 1998, 16, 5715–5732. [Google Scholar] [CrossRef]
- Yashin, V.V.; Aryshensky, E.V.; Latushkin, I.A.; Stozharov, D.A. Study of kinetics of the supersaturated solid solution decomposition in alloys of the Al-Mg system with transition elements addition. Tsvetnye Met. 2020, 11, 77–84. [Google Scholar]
- Nes, E.; Vatne, H.E. The 40 (111) Orientation Relationship in Recrystallisation/Die 40 (111) Orientierungsbeziehung bei der Rekristallisation. Z. Für Met. 1996, 87, 448–453. [Google Scholar]
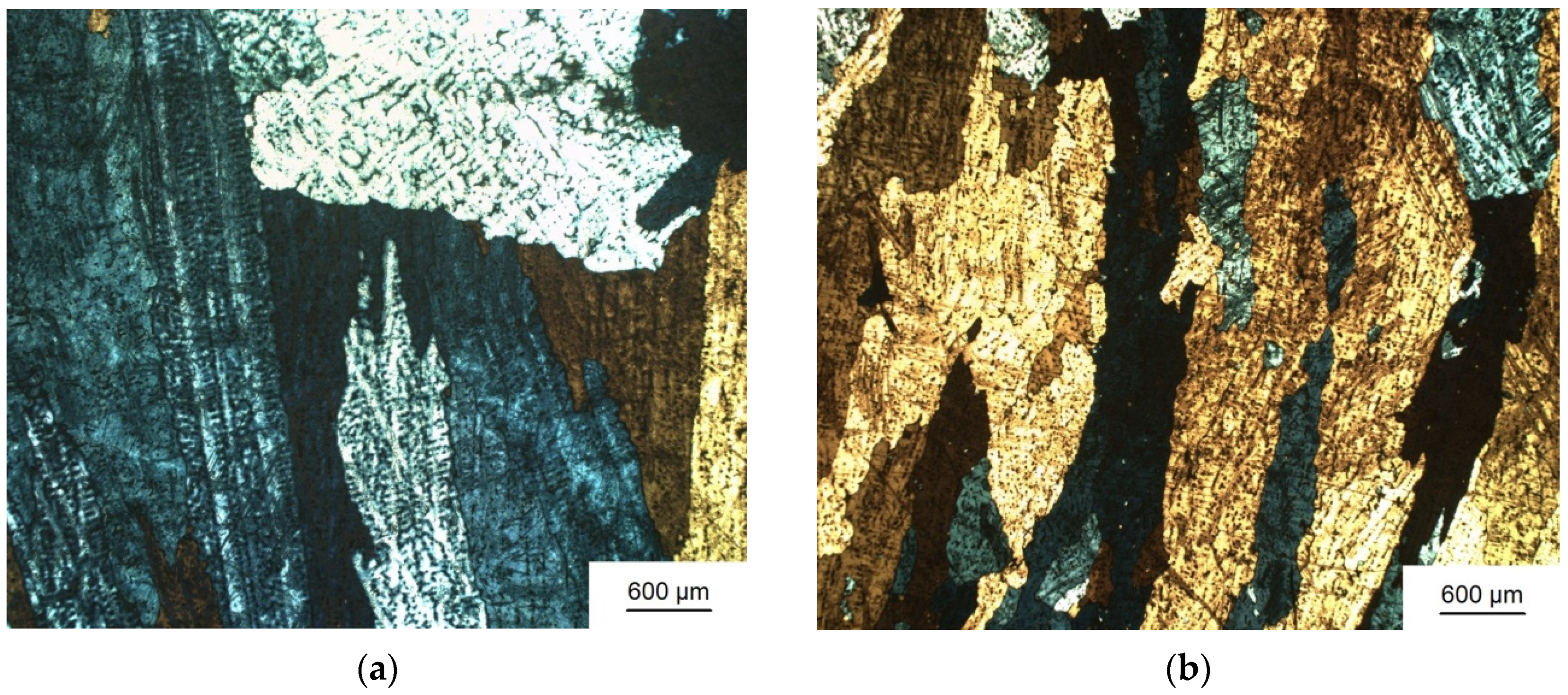
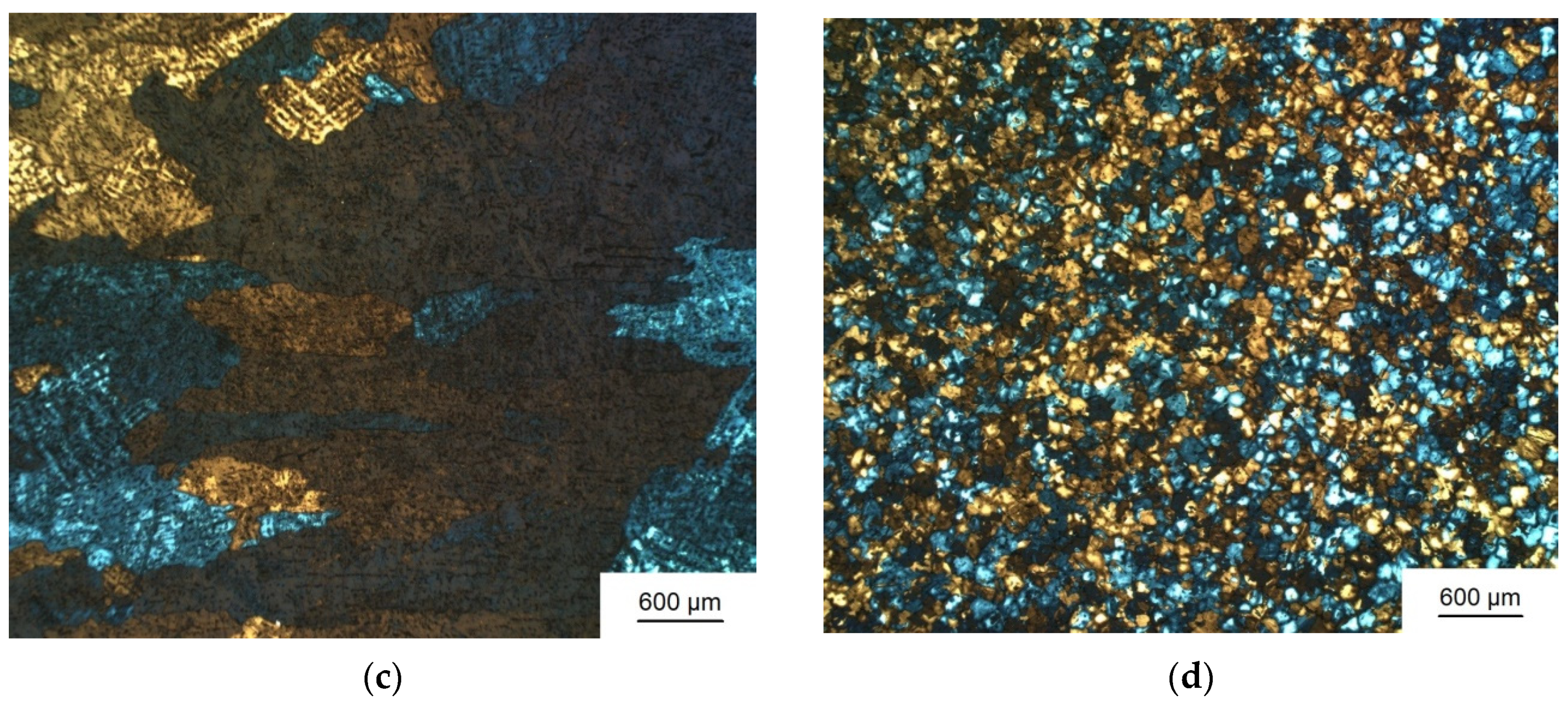
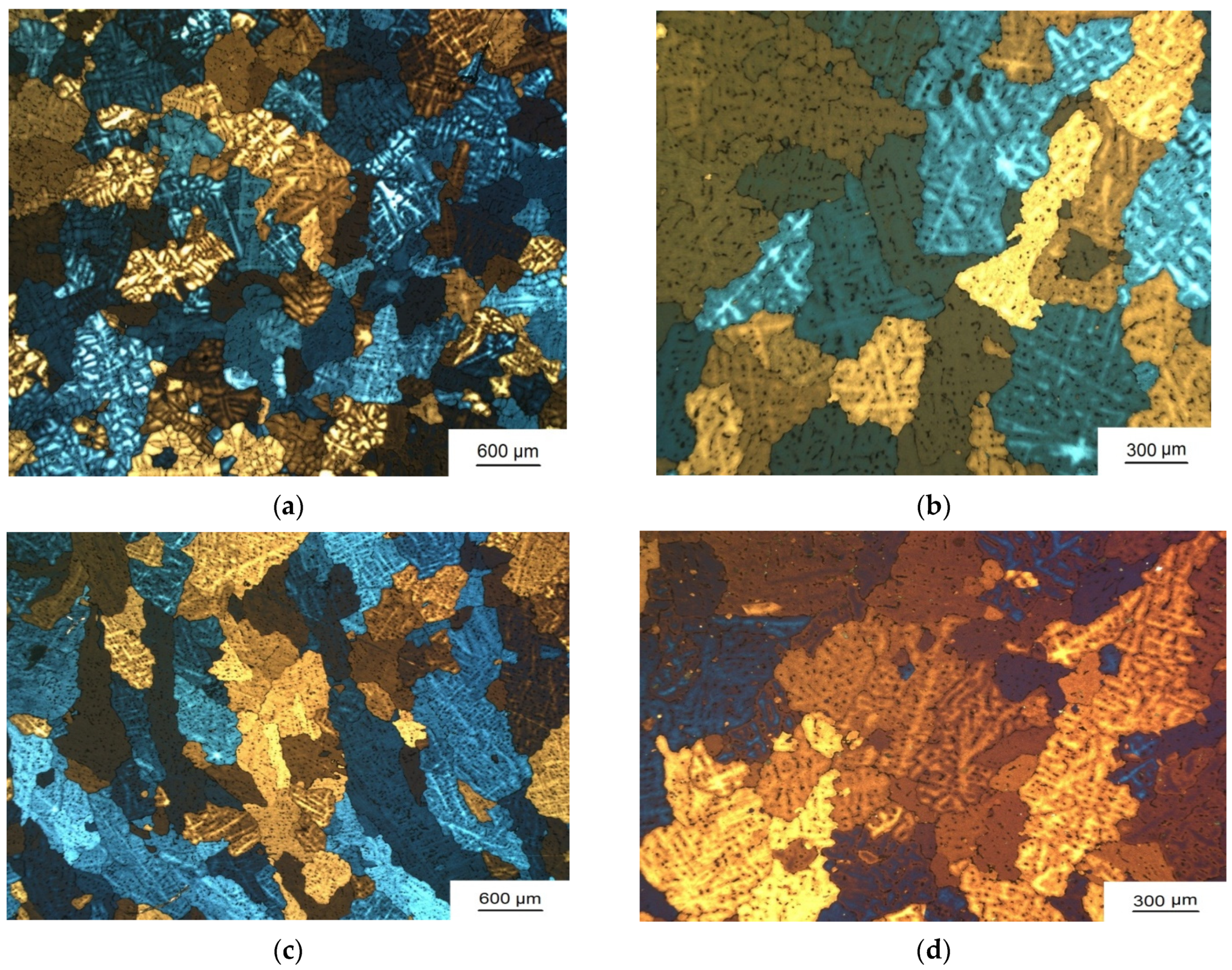
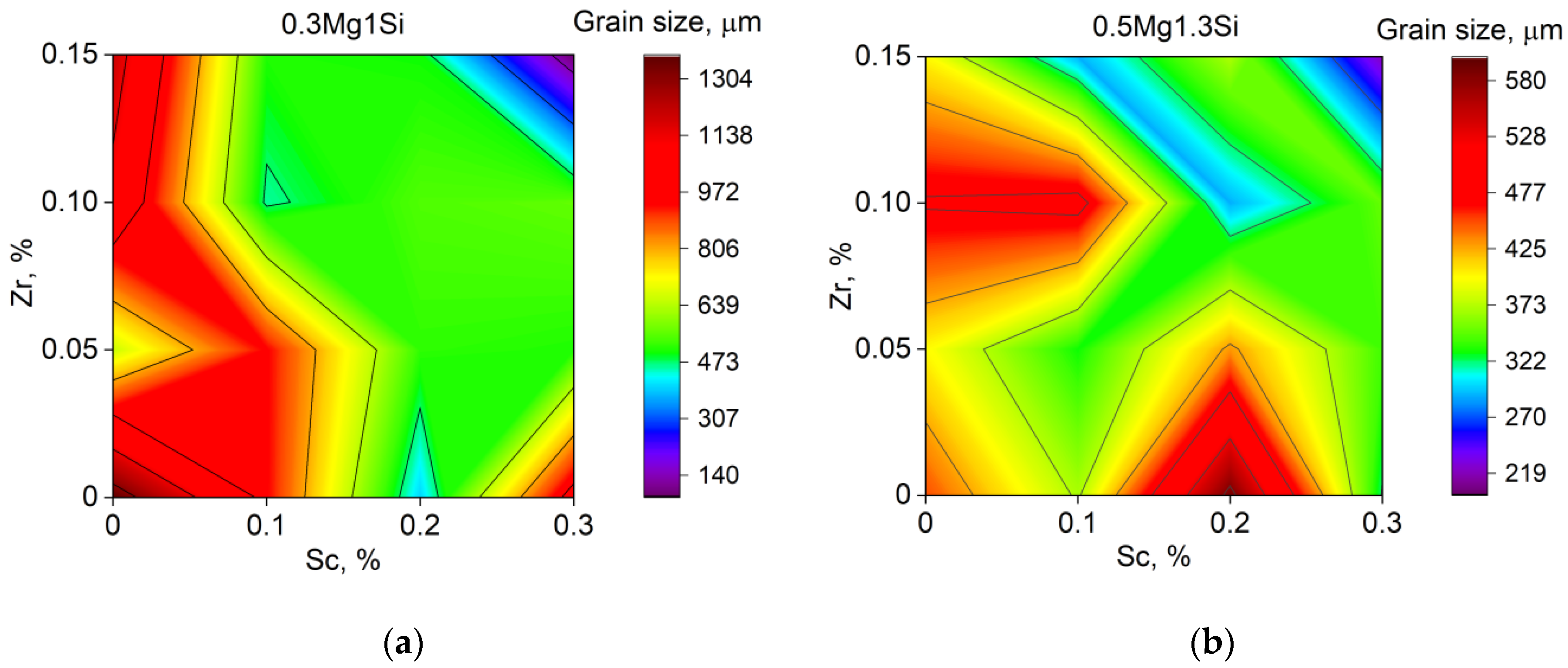


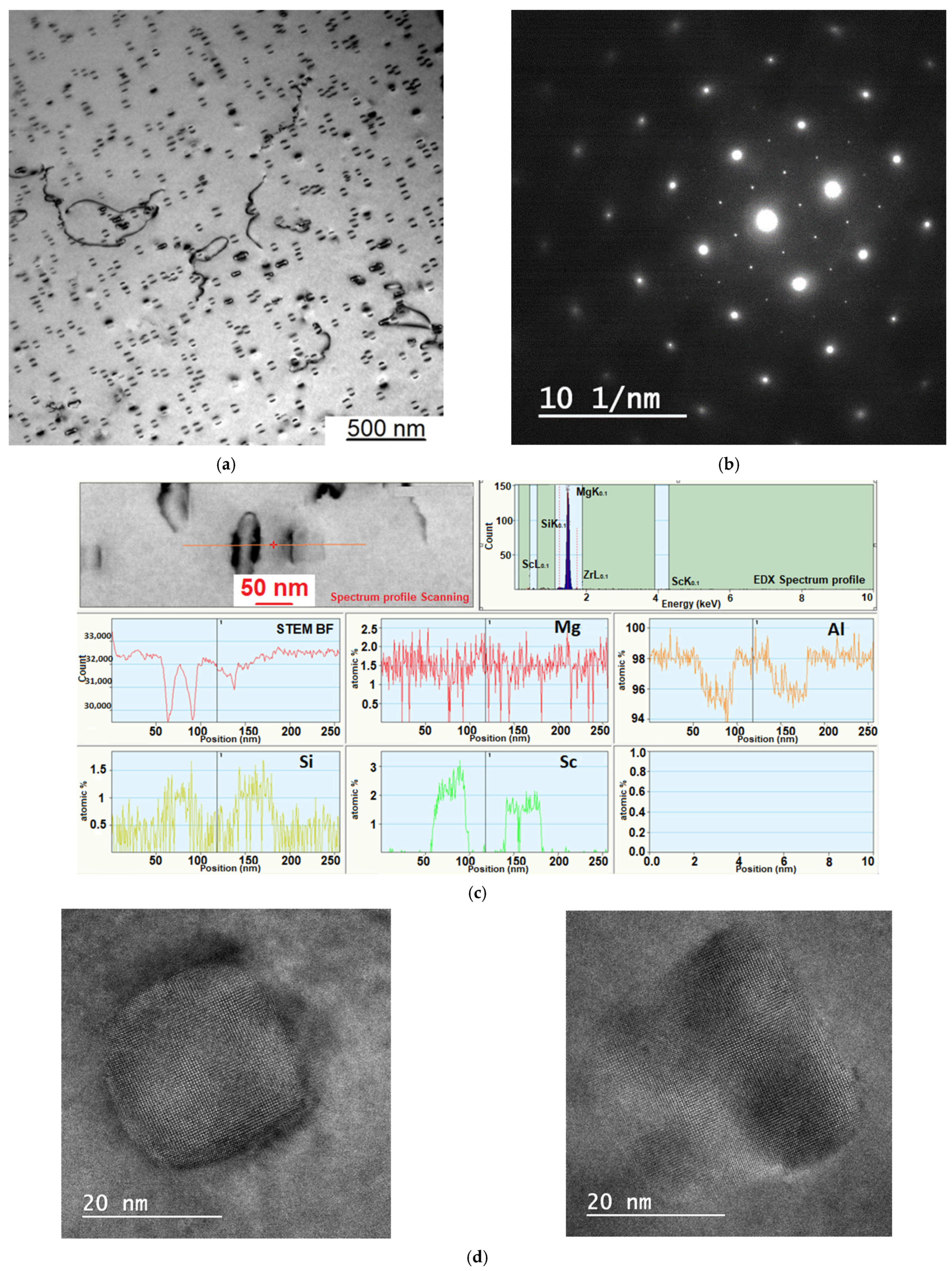
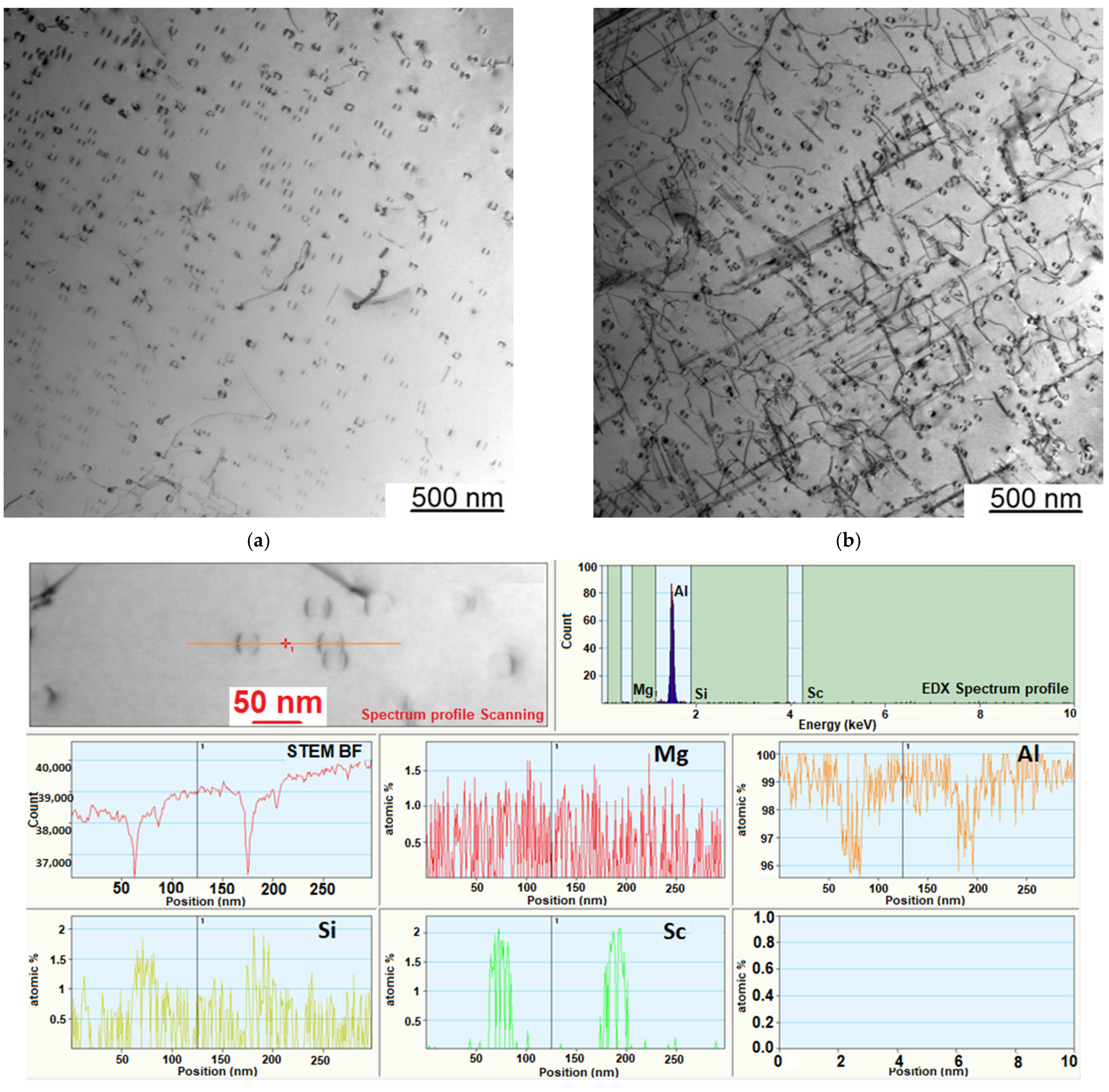
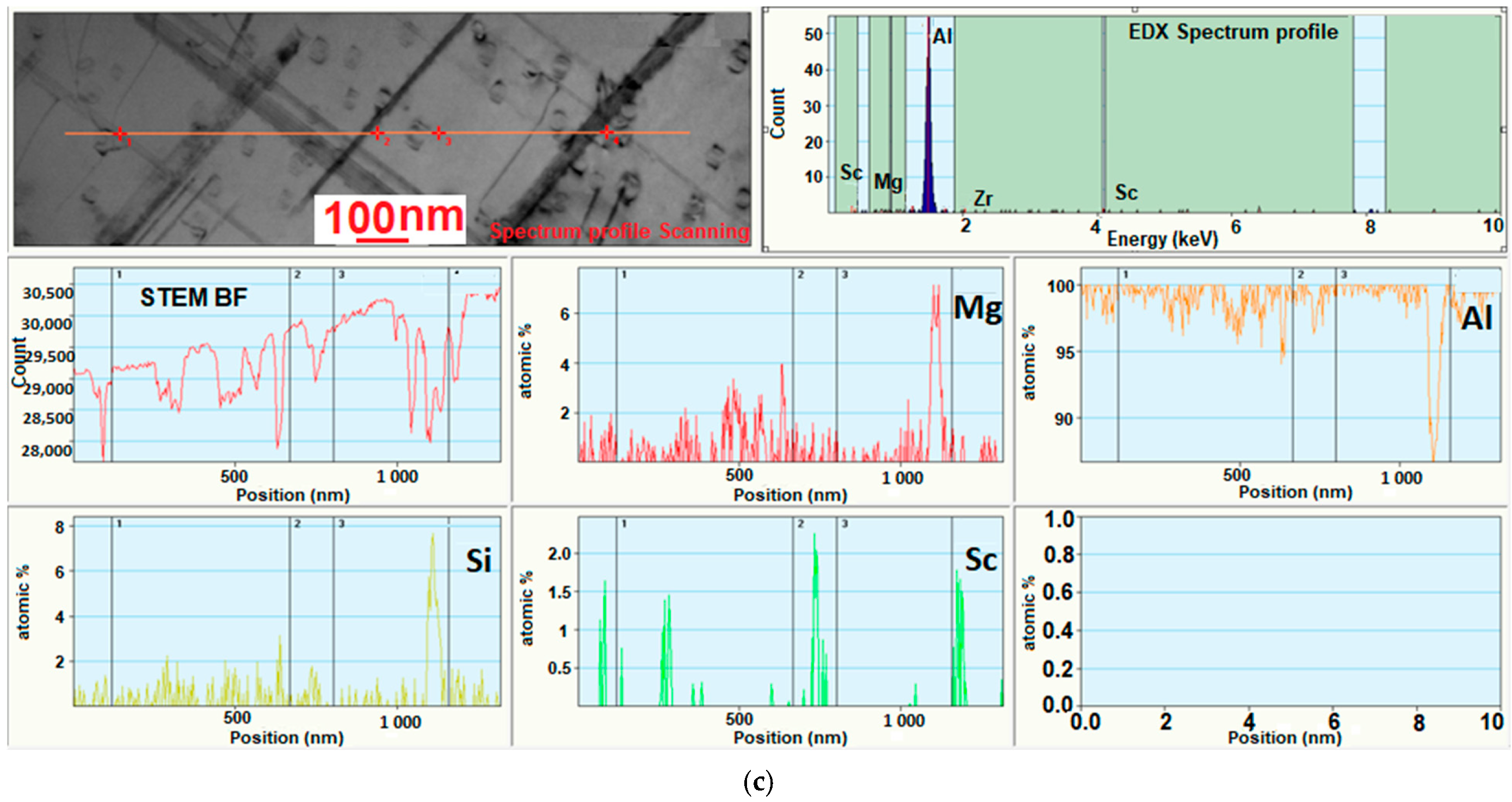

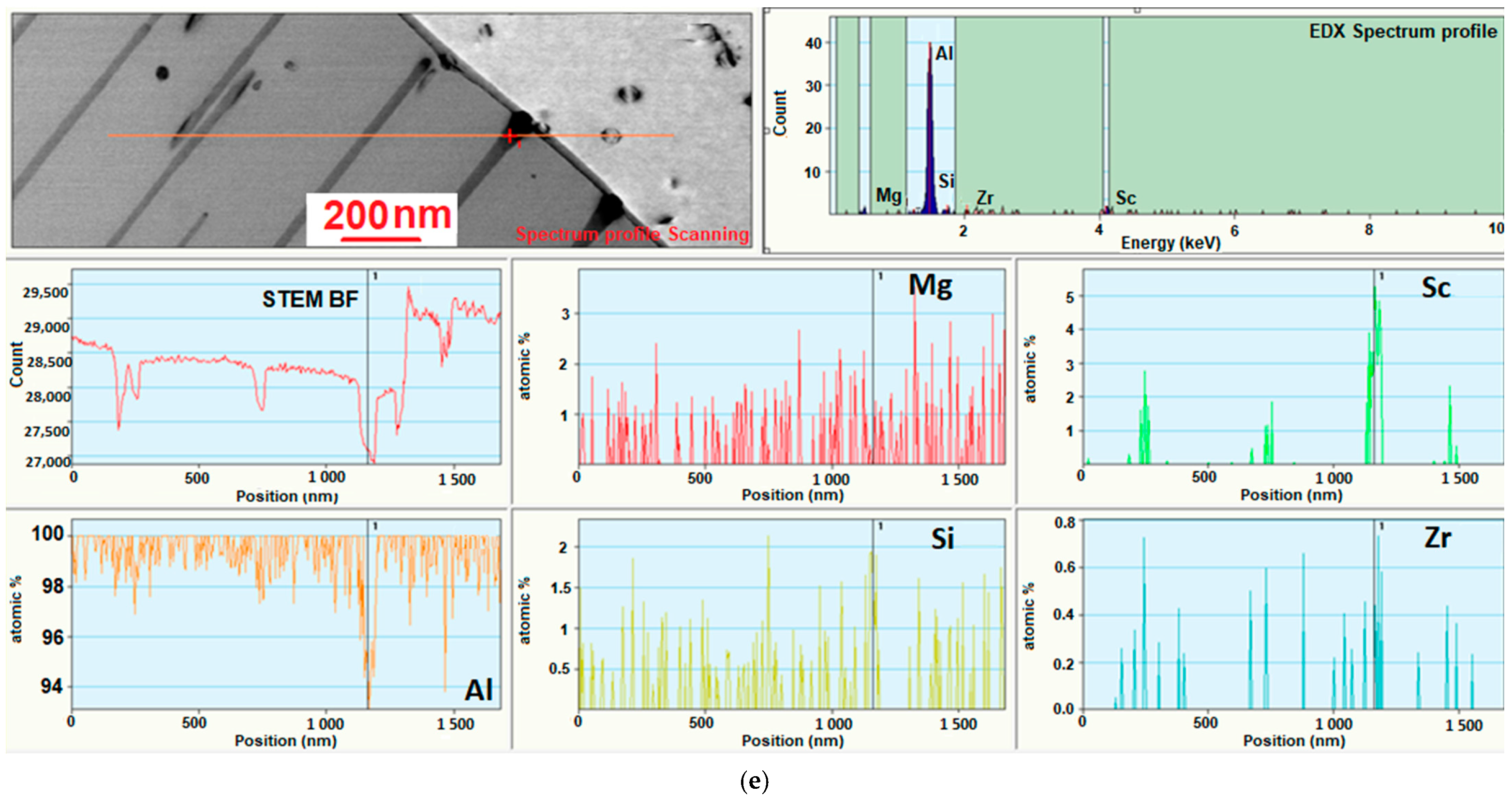
| Sample Number № 1 | Chemical Composition | Sample Number № 2 | Chemical Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Mg | Si | Sc | Zr | Al | Mg | Si | Sc | Zr | ||
| 1 | base | 0.3 | 1.0 | 0 | 0 | 1 | base | 0.5 | 1.3 | 0 | 0 |
| 2 | base | 0.3 | 1.0 | 0 | 0.05 | 2 | base | 0.5 | 1.3 | 0 | 0.05 |
| 3 | base | 0.3 | 1.0 | 0 | 0.1 | 3 | base | 0.5 | 1.3 | 0 | 0.1 |
| 4 | base | 0.3 | 1.0 | 0 | 0.15 | 4 | base | 0.5 | 1.3 | 0 | 0.15 |
| 5 | base | 0.3 | 1.0 | 0.1 | 0 | 5 | base | 0.5 | 1.3 | 0 | 0 |
| 6 | base | 0.3 | 1.0 | 0.1 | 0.05 | 6 | base | 0.5 | 1.3 | 0.1 | 0 |
| 7 | base | 0.3 | 1.0 | 0.1 | 0.1 | 7 | base | 0.5 | 1.3 | 0.1 | 0.05 |
| 8 | base | 0.3 | 1.0 | 0.1 | 0.15 | 8 | base | 0.5 | 1.3 | 0.1 | 0.1 |
| 9 | base | 0.3 | 1.0 | 0.2 | 0 | 9 | base | 0.5 | 1.3 | 0.1 | 0.15 |
| 10 | base | 0.3 | 1.0 | 0.2 | 0.05 | 10 | base | 0.5 | 1.3 | 0.2 | 0.05 |
| 11 | base | 0.3 | 1.0 | 0.2 | 0.1 | 11 | base | 0.5 | 1.3 | 0.2 | 0.1 |
| 12 | base | 0.3 | 1.0 | 0.2 | 0.15 | 12 | base | 0.5 | 1.3 | 0.2 | 0.15 |
| 13 | base | 0.3 | 1.0 | 0.3 | 0 | 13 | base | 0.5 | 1.3 | 0.3 | 0 |
| 14 | base | 0.3 | 1.0 | 0.3 | 0.05 | 14 | base | 0.5 | 1.3 | 0.3 | 0.05 |
| 15 | base | 0.3 | 1.0 | 0.3 | 0.1 | 15 | base | 0.5 | 1.3 | 0.3 | 0.1 |
| 16 | base | 0.3 | 1.0 | 0.3 | 0.15 | 16 | base | 0.5 | 1.3 | 0.3 | 0.15 |
| Alloy | Area Share, % | Average Amount per 500 Microns2 | Average Size, μm |
|---|---|---|---|
| Al0.3Mg1Si0.3Sc | 0.14 | 21 | 0.05 |
| Al0.5Mg1.3Si0.3Sc | 0.87 | 3 | 0.59 |
| Al0.3Mg1Si0.3Sc0.15Zr | 0.2 | 16 | 0.08 |
| Al0.5Mg1.3Si0.3Sc0.15Zr | 0.11 | 21 | 0.03 |
| Alloy | Area Share, % | Average Amount per 500 Microns2 | Average Size, μm |
|---|---|---|---|
| Al0.3Mg1Si0.3Sc | 2.15 | 90 | 12.4 |
| Al0.5Mg1.3Si0.3Sc | 1.5 | 71 | 10.6 |
| Al0.3Mg1Si0.3Sc0.15Zr | 1.8 | 78 | 11.6 |
| Al0.5Mg1.3Si0.3Sc0.15Zr | 1.7 | 69 | 12.8 |
| Alloy | Phase | At. % | |||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Si | Mg | Sc | Zr | ||
| Al0.3Mg1Si0.3Sc | Fe2Mg7Si10Al18 | 58.54 | 4.1 | 13.91 | 22.44 | ||
| Al9Fe2Si2 | 76.69 | 9.7 | 10.01 | ||||
| Solid solution | 98.23 | 0.48 | 0.27 | 0.11 | |||
| Al0.5Mg1.3Si0.3Sc | Al9Fe2Si2 | 66.17 | 14.46 | 16.02 | |||
| Fe2Mg7Si10Al18 | 53.99 | 5.35 | 15.43 | 23.6 | |||
| (Al, Si)3(Sc) | 51.65 | 27.02 | 11.93 | ||||
| Solid solution | 98.7 | 0.79 | 0.4 | 0.12 | |||
| Al0.3Mg1Si0.3Sc0.15Zr | Fe2Mg7Si10Al18 | 66.04 | 3.14 | 9.94 | 19.95 | ||
| Al9Fe2Si2 | 60.11 | 7.08 | 22.66 | ||||
| (Al, Si)3(Sc) | 61.83 | 24 | 7.06 | ||||
| Al3(Sc0.6Zr0.4) | 82.88 | 11.44 | 4.61 | ||||
| Solid solution | 98.72 | 0.82 | 0.28 | 0.14 | 0.04 | ||
| Al0.5Mg1.3Si0.3Sc0.15Zr | Fe2Mg7Si10Al18 | 54.08 | 5.3 | 15 | 24.11 | ||
| (Al, Si)3(Sc) | 66.9 | 16.94 | 8.33 | ||||
| Solid solution | 99.32 | 0.21 | 0.32 | 0.1 | 0.05 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aryshenskii, E.; Lapshov, M.; Hirsch, J.; Konovalov, S.; Bazhenov, V.; Drits, A.; Zaitsev, D. Influence of the Small Sc and Zr Additions on the As-Cast Microstructure of Al–Mg–Si Alloys with Excess Silicon. Metals 2021, 11, 1797. https://doi.org/10.3390/met11111797
Aryshenskii E, Lapshov M, Hirsch J, Konovalov S, Bazhenov V, Drits A, Zaitsev D. Influence of the Small Sc and Zr Additions on the As-Cast Microstructure of Al–Mg–Si Alloys with Excess Silicon. Metals. 2021; 11(11):1797. https://doi.org/10.3390/met11111797
Chicago/Turabian StyleAryshenskii, Evgenii, Maksim Lapshov, Jurgen Hirsch, Sergey Konovalov, Viacheslav Bazhenov, Alexander Drits, and Denis Zaitsev. 2021. "Influence of the Small Sc and Zr Additions on the As-Cast Microstructure of Al–Mg–Si Alloys with Excess Silicon" Metals 11, no. 11: 1797. https://doi.org/10.3390/met11111797
APA StyleAryshenskii, E., Lapshov, M., Hirsch, J., Konovalov, S., Bazhenov, V., Drits, A., & Zaitsev, D. (2021). Influence of the Small Sc and Zr Additions on the As-Cast Microstructure of Al–Mg–Si Alloys with Excess Silicon. Metals, 11(11), 1797. https://doi.org/10.3390/met11111797







