Vacuum Gasification-Directional Condensation for Separation of Tellurium from Lead Anode Slime
Abstract
:1. Introduction
2. Experimental and Analysis
2.1. Experimental Materials
2.2. Theoretical Analysis
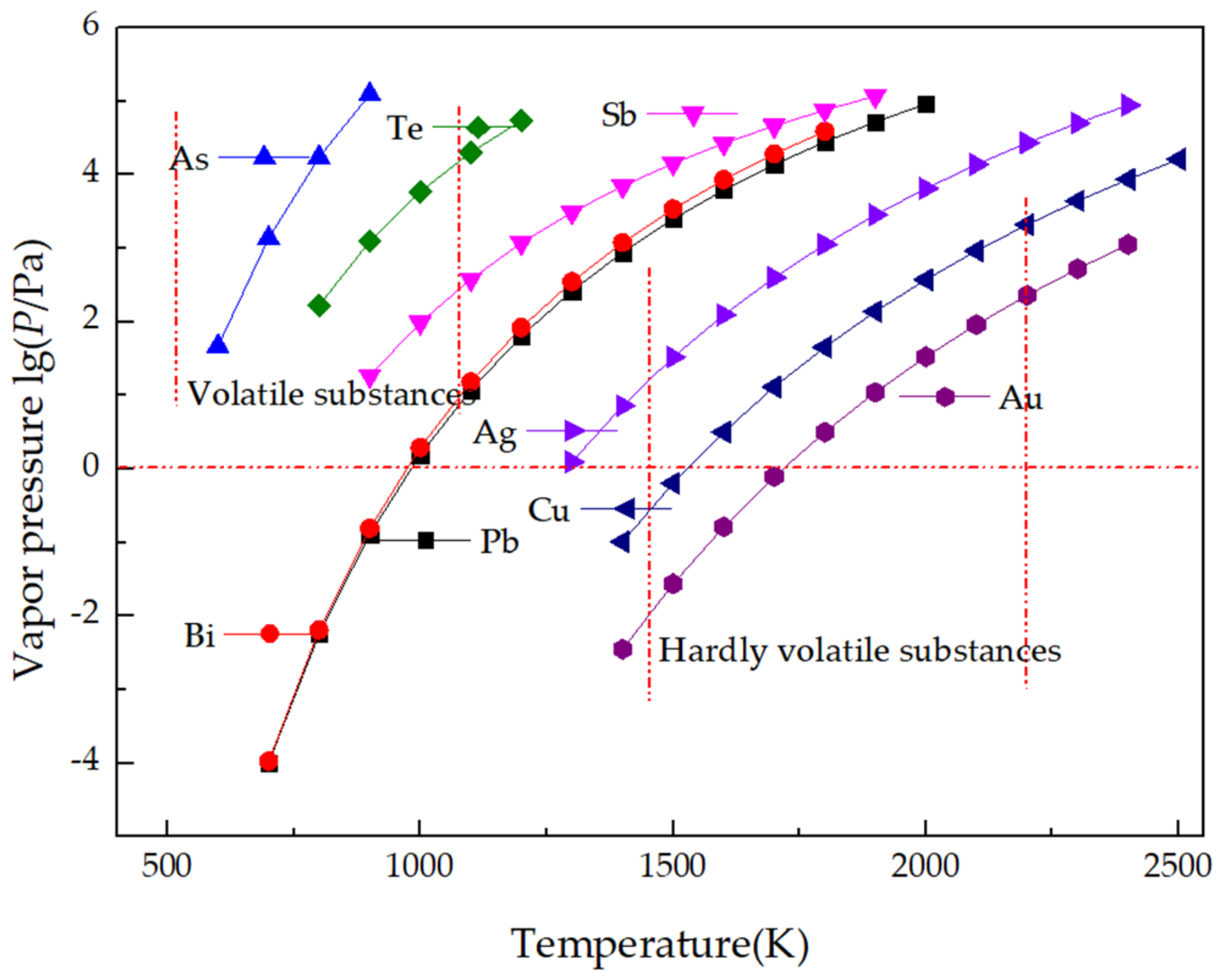
2.2.1. Criterion of Saturated Vapor Pressure of Pure Substance
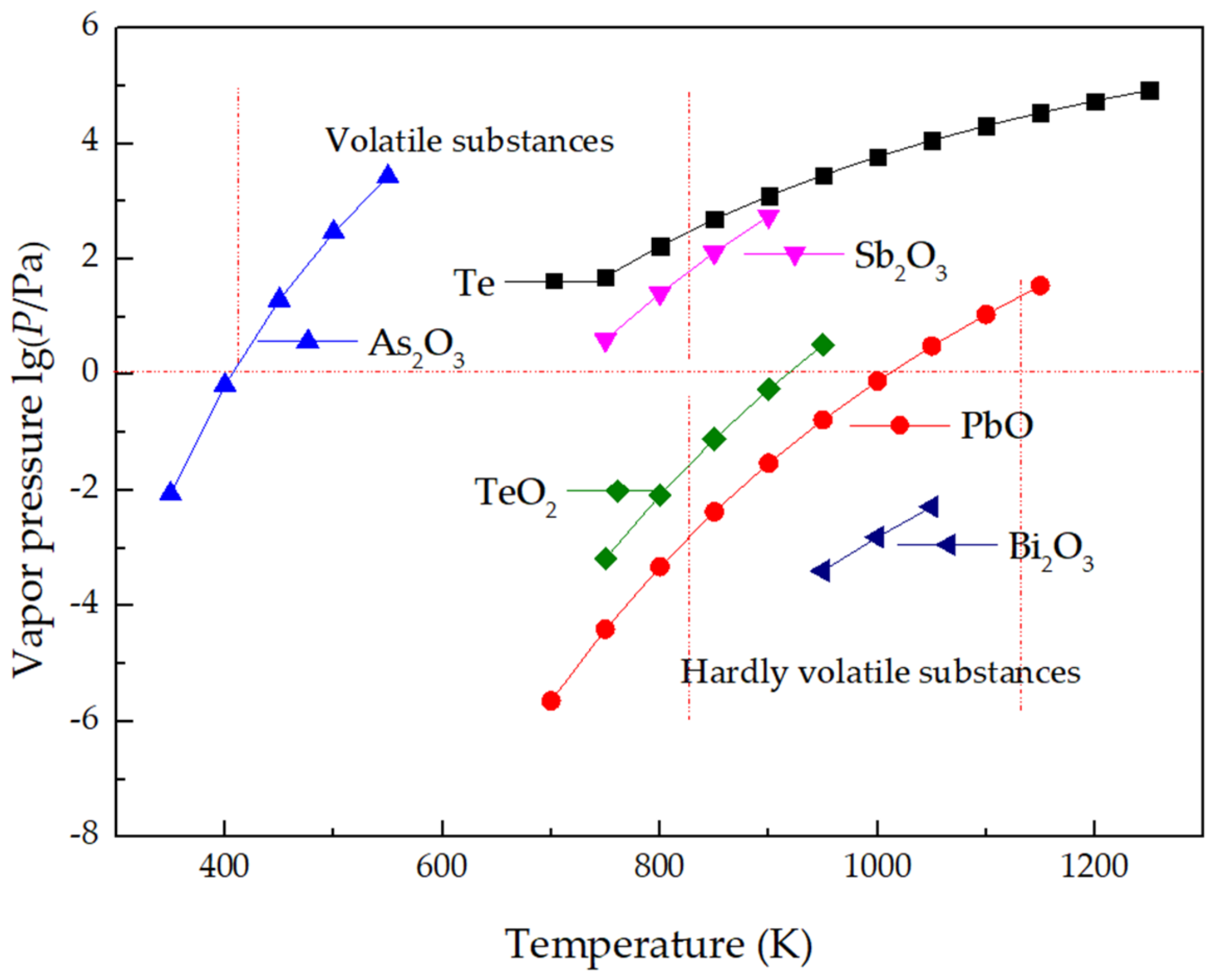
2.2.2. Saturated Vapor Pressure of Oxides in TLAS
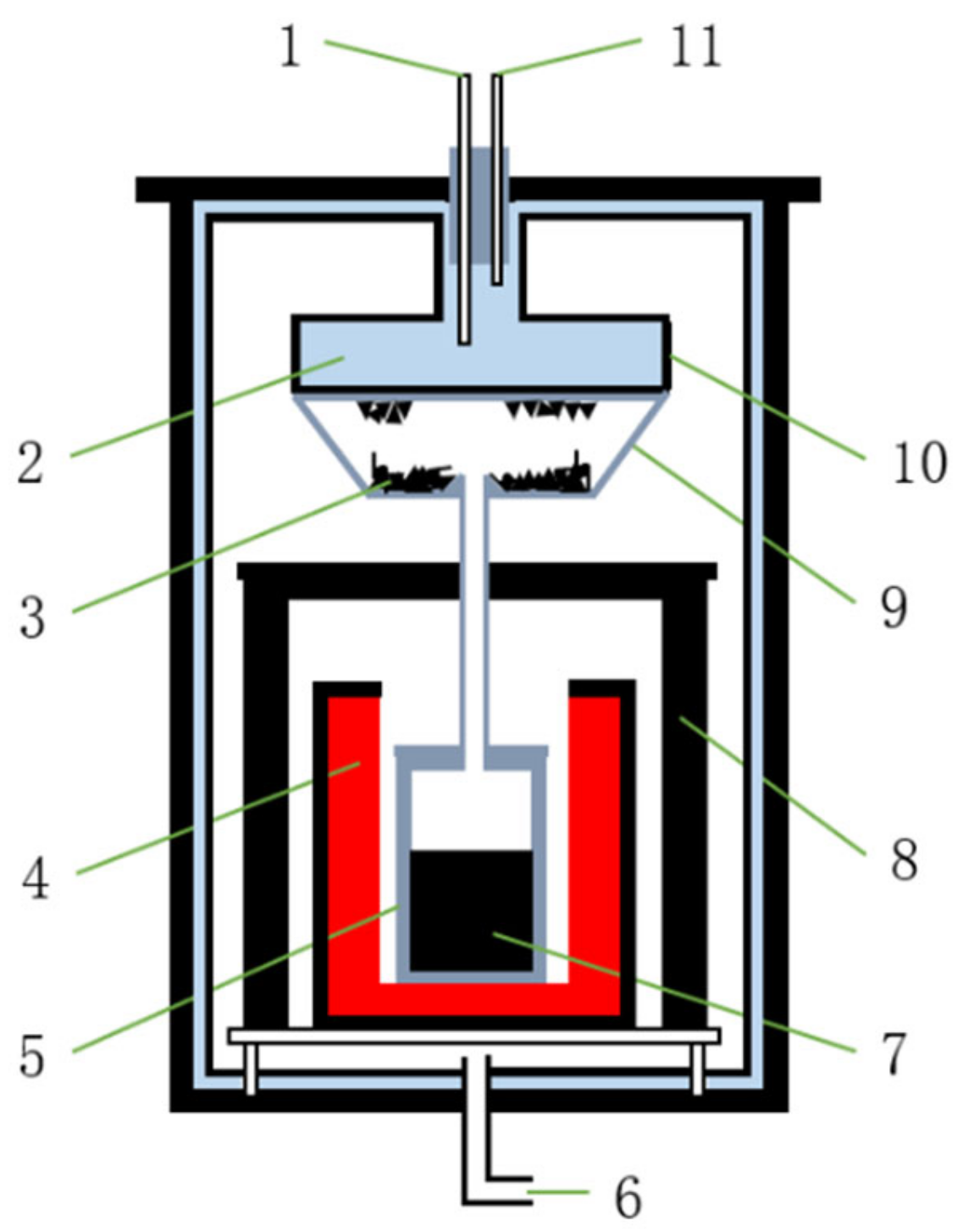
2.3. Experimental Equipment
3. Results and Discussion
3.1. Temperature Effects on Separation of Tellurium
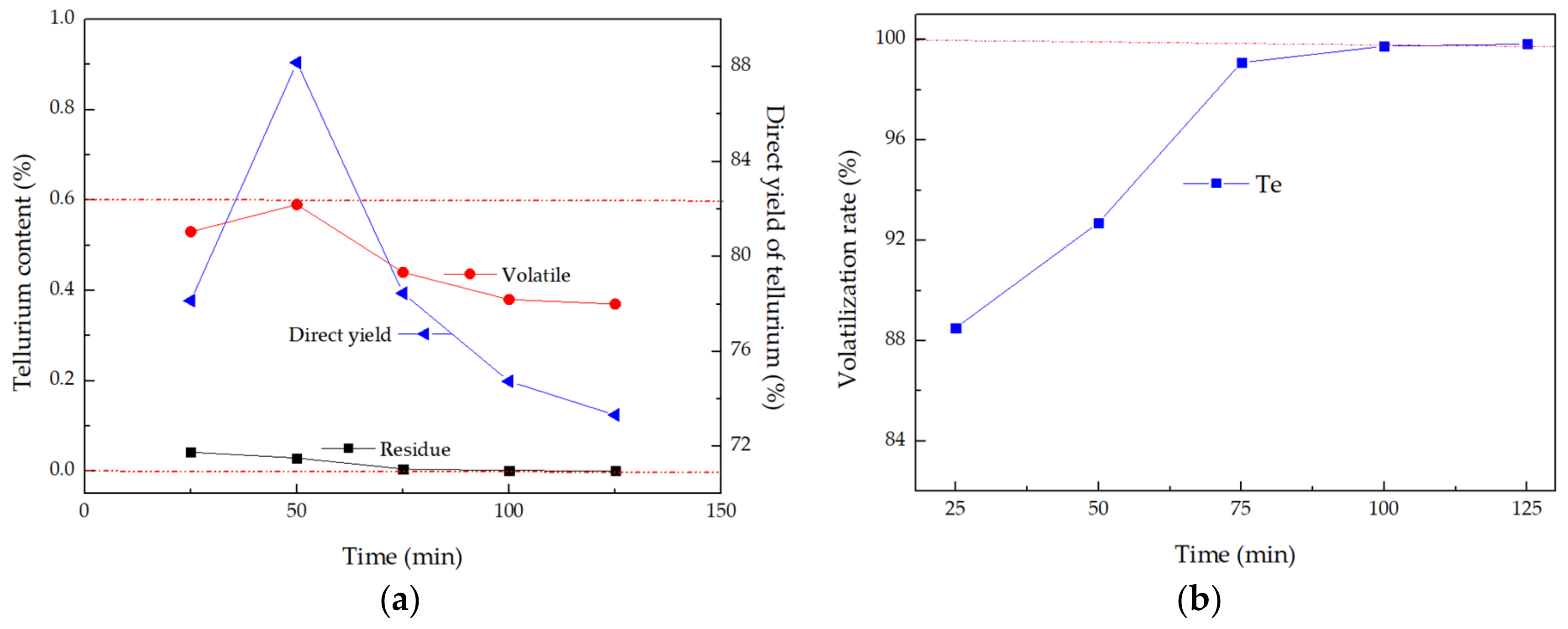
3.2. Time Effects on Separation of Tellurium
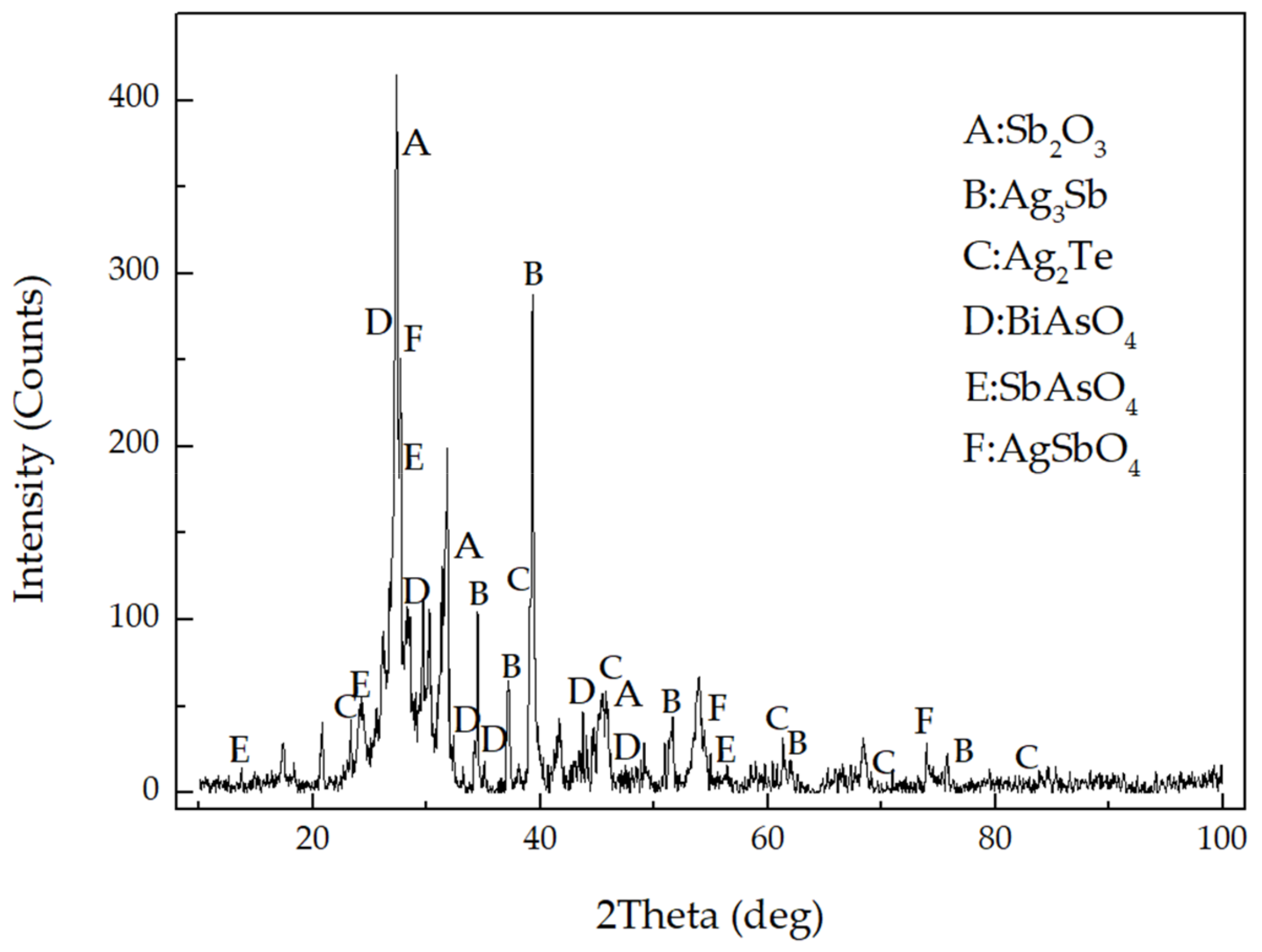
3.3. Chemical Composition of Tellurium Condensate
3.4. Environmental Implication for Separation
4. Conclusions
- (1)
- Vacuum gasification-directional condensation significantly enriched tellurium, and separated both heavy metal copper, and precious metal (gold and silver) into residuals;
- (2)
- Volatilization rate and direct yield of tellurium gradually increased with increasing temperature, and distillation temperature of 1173 K was the optimal separation temperature of tellurium from TLAS;
- (3)
- Volatilization and direct yield of tellurium were fluctuant with increasing constant time, and constant temperature time of 50 min was the optimal separation time of tellurium from TLAS;
- (4)
- Volatilization rate of 92% and direct yield rate of 88% elemental tellurium were achieved under the optimal conditions of distillation temperature 1173 K and constant temperature time 50 min;
- (5)
- Vacuum gasification-directional condensation was an important step in cleanly separating tellurium and reducing hazardous materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, P.; Wang, H.J.; Ye, F.C.; Yi, Y.; Ji, H.W.; Qiu, S.W. Current status and prospects of the recovery process of rare metal selenium and tellurium. Met. Mine 2020, 4, 52–60. (In Chinese) [Google Scholar]
- Makuei, M.M.; Senanayake, G. Extraction of tellurium from lead and copper bearing feed materials and interim metallurgical products—A short review. Miner. Eng. 2018, 115, 79–87. [Google Scholar] [CrossRef]
- Shao, L.X.; Diao, J.; Ji, C.Q.; Li, G. A novel and clean process for extracting tellurium and bismuth from Dashuigou tellurium ore by oxidizing leaching. Hydrometallurgy 2020, 191, 105205. [Google Scholar] [CrossRef]
- Kavlak, G.; Graedel, T.E. Global anthropogenic tellurium cycles for 1940–2010. Resour. Conserv. Recycl. 2013, 76, 21–26. [Google Scholar] [CrossRef]
- Allen, M.D.; Hutchings, G.J.; Bowker, M. Iron antimony oxide catalysts for the ammoxidation of propene to acrylonitrile: Comments on the method of preparation of tellurium promoted catalysts. Appl. Catal. A Gen. 2001, 217, 33–39. [Google Scholar] [CrossRef]
- Jin, W.; Su, J.L.; Chen, S.F.; Li, P.; Moats, M.S.; Maduraiveeran, G.; Lei, H. Efficient electrochemical recovery of fine tellurium powder from hydrochloric acid media via mass transfer enhancement. Sep. Purif. Technol. 2018, 203, 117–123. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, G.; Fan, J.L.; Li, Q.; Kianni, M.; Zhang, J.; Yang, H.W.; Chen, J.W.; Wang, R.L. Optimization of process on electrodeposition of 4N tellurium from alkaline leaching solutions. Hydrometallurgy 2018, 176, 17–25. [Google Scholar] [CrossRef]
- Missen, O.P.; Ram, R.; Mills, S.J.; Etschmann, B.; Reith, F.; Shuster, J.; Smith, D.J.; Brugger, J. Love is in the Earth: A review of tellurium (bio)geochemistry in surface environments. Earth-Sci. Rev. 2020, 204, 103150. [Google Scholar] [CrossRef]
- Fan, Y.Q.; Yang, Y.X.; Xiao, Y.P.; Zhao, Z.; Lei, Y. Recovery of tellurium from high tellurium-bearing materials by alkaline pressure leaching process: Thermodynamic evaluation and experimental study. Hydrometallurgy 2013, 139, 95–99. [Google Scholar] [CrossRef]
- Mal, J.; Nancharaiah, Y.V.; Maheshwari, N.; van Hullebusch, E.D.; Lens, P.N.L. Continuous removal and recovery of tellurium in an upflow anaerobic granular sludge bed reactor. J. Hazard. Mater. 2017, 327, 79–88. [Google Scholar] [CrossRef]
- Guo, X.Y.; Xu, Z.P.; Li, D.; Tian, Q.H.; Xu, R.Z.; Zhang, Z. Recovery of tellurium from high tellurium-bearing materials by alkaline sulfide leaching followed by sodium sulfite precipitation. Hydrometallurgy 2017, 171, 355–361. [Google Scholar] [CrossRef]
- Li, L.L. Study on recycling process of tellurium in copper lead anode slime. World Nonferrous Met. 2018, 12, 17–19. (In Chinese) [Google Scholar]
- Guo, X.Y.; Zhou, Y.; Zha, G.Z.; Jiang, W.L.; Yang, B.; Ma, W.H. A novel method for extracting metal Ag and Cu from high value-added secondary resources by vacuum distillation. Sep. Purif. Technol. 2020, 242, 116787. [Google Scholar] [CrossRef]
- Zhang, X.F.; Huang, D.X.; Jiang, W.L.; Zha, G.Z.; Deng, J.H.; Deng, P.; Kong, X.F.; Liu, D.C. Selective separation and recovery of rare metals by vulcanization-vacuum distillation of cadmium telluride waste. Sep. Purif. Technol. 2020, 230, 115864. [Google Scholar] [CrossRef]
- Zha, G.Z.; Yang, C.F.; Wang, Y.K.; Guo, X.Y.; Jiang, W.L.; Yang, B. New vacuum distillation technology for separating and recovering valuable metals from a high value-added waste. Sep. Purif. Technol. 2019, 209, 863–869. [Google Scholar] [CrossRef]
- Yang, B.; Zha, G.Z.; Hartley, W.; Kong, X.F.; Liu, D.C.; Xu, B.Q.; Jiang, W.L.; Guo, X.Y. Sustainable extraction of lead and re-use of valuable metals from lead-rich secondary materials. J. Clean. Prod. 2019, 219, 110–116. [Google Scholar] [CrossRef]
- Rao, S.; Liu, Z.Q.; Wang, D.X.; Cao, H.Y.; Zhu, W.; Zhang, K.F.; Tao, J.Z. Hydrometallurgical process for recovery of Zn, Pb, Ga and Ge from Zn refinery residues. Trans. Nonferrous Met. Soc. China 2021, 31, 555–564. [Google Scholar] [CrossRef]
- Che, Y.S.; Mai, G.P.; Li, S.L.; He, J.L.; Song, J.X.; Yi, J.H. Kinetic mechanism of magnesium production by silicothermic reduction of CaO·MgO in vacuum. Trans. Nonferrous Met. Soc. China 2020, 30, 2812–2822. [Google Scholar] [CrossRef]
- Wang, D.; Yang, B.; Xu, B.Q.; Yang, H.W. Prediction of azeotropy for binary alloys in vacuum distillation. Vacuum 2019, 166, 206–211. [Google Scholar] [CrossRef]
- Ren, J.Q.; Xu, J.J.; Kong, L.X.; Yang, B.; Xu, B.Q. Model prediction of activity and vapor-liquid equilibrium of tin-based alloy system. Chin. J. Nonferrous Met. 2020, 30, 2399–2409. [Google Scholar]
- Zhang, Y.B.; Lin, H.G.; Gong, C.X. Technical Analysis of Non-ferrous Metal Vacuum Metallurgy. Metall. Mater. 2019, 39, 174–176. (In Chinese) [Google Scholar]
- Li, L.; Tian, Y.; Liu, D.C.; Zhou, H.J.; Dai, Y.N.; Yang, B. Pretreatment of lead anode slime with low silver by vacuum distillation for concentrating silver. J. Cent. South Univ. 2013, 20, 615–621. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Deng, J.H.; Jiang, W.L.; Mei, Q.S.; Liu, D.C. Application of vacuum distillation in refining crude lead. Vacuum 2018, 148, 140–148. [Google Scholar] [CrossRef]
- Odusote, Y.A.; Jabar, J.M.; Bolarinwa, H.S.; Akinbisehin, A.B. Application of molecular interaction volume model in separation of Ti-Al alloys in vacuum distillation. Vacuum 2019, 169, 108885. [Google Scholar] [CrossRef]
- He, Y.L.; Xu, R.D.; He, S.W.; Chen, H.S.; Li, K.; Zhu, Y.; Shen, Q.F. φ–pH diagram of As–N–Na–H2O system for arsenic removal during alkaline pressure oxidation leaching of lead anode slime. Trans. Nonferrous Met. Soc. China 2017, 27, 676–685. [Google Scholar] [CrossRef]
- Jia, G.B.; Yang, B.; Liu, D.C. Deeply removing lead from Pb-Sn alloy with vacuum distillation. Trans. Nonferrous Met. Soc. China 2013, 23, 1822–1831. [Google Scholar] [CrossRef]
- Dai, Y.N.; Yang, B. Non-Ferrous Metal Vacuum Metallurg; Metallurgical Industry Press: Beijing, China, 2009. (In Chinese) [Google Scholar]
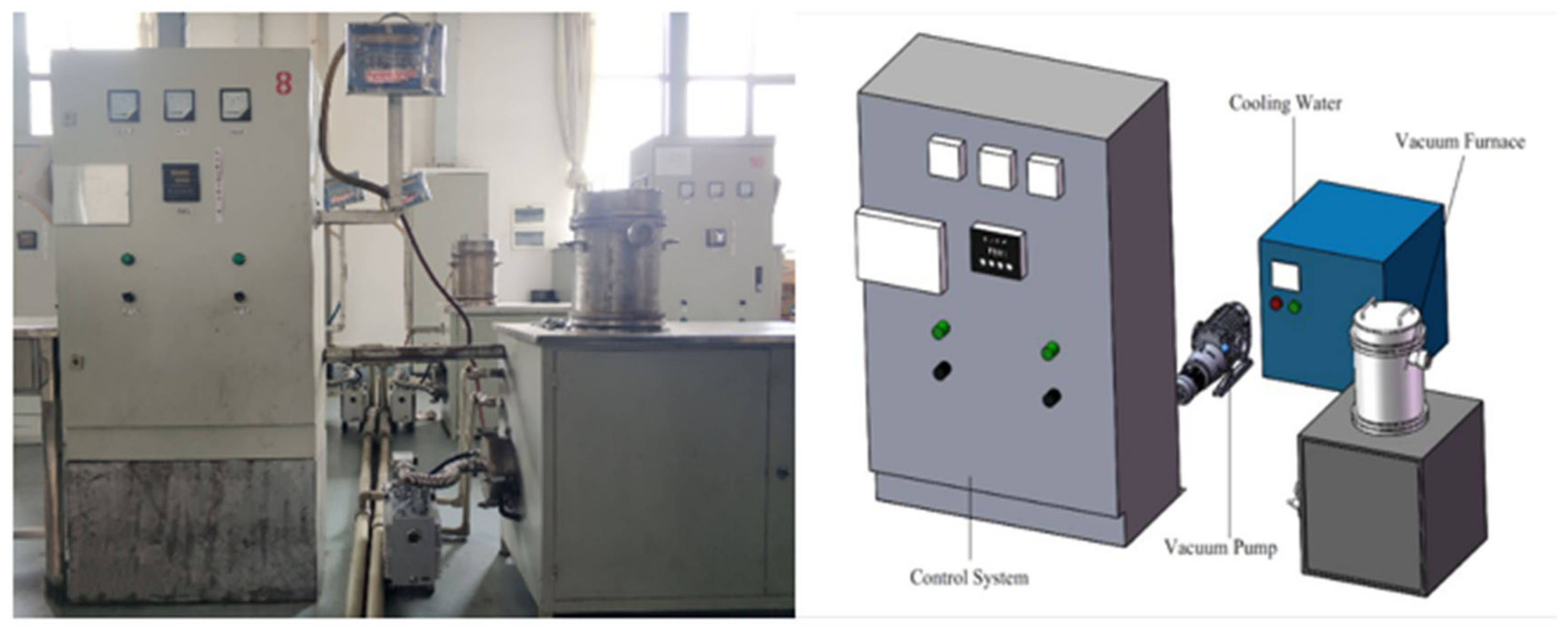

| Component | As | Ag | Te | Bi | Pb | Sb | Cu | Au |
|---|---|---|---|---|---|---|---|---|
| wt/% | 2.4 | 9.2 | 0.21 | 16 | 15 | 38 | 2.2 | 670 (g/t) |
| Element | Pb | Bi | As | Sb | Te | Cu | Ag | Au |
|---|---|---|---|---|---|---|---|---|
| A | −10,130 | −10,400 | −6160 | −6500 | −7830 | −17,520 | −14,400 | −19,280 |
| B | −0.985 | −1.26 | 0 | 0 | −4.27 | −1.21 | −0.85 | −1.01 |
| C × 103 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D | 13.28 | 14.47 | 11.94 | 8.49 | 24.41 | 15.33 | 13.82 | 14.50 |
| Component | As | Ag | Te | Bi | Sb | Pb |
|---|---|---|---|---|---|---|
| wt/% | 6.9 | 2.8 | 3.0 | 43 | 35 | 9.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Kong, X.; Yi, J.; Yang, B.; Xu, B.; Liu, D.; Wu, J.; Xiong, H. Vacuum Gasification-Directional Condensation for Separation of Tellurium from Lead Anode Slime. Metals 2021, 11, 1535. https://doi.org/10.3390/met11101535
Gao Z, Kong X, Yi J, Yang B, Xu B, Liu D, Wu J, Xiong H. Vacuum Gasification-Directional Condensation for Separation of Tellurium from Lead Anode Slime. Metals. 2021; 11(10):1535. https://doi.org/10.3390/met11101535
Chicago/Turabian StyleGao, Zhe, Xiangfeng Kong, Jiafei Yi, Bin Yang, Baoqiang Xu, Dachun Liu, Jian Wu, and Heng Xiong. 2021. "Vacuum Gasification-Directional Condensation for Separation of Tellurium from Lead Anode Slime" Metals 11, no. 10: 1535. https://doi.org/10.3390/met11101535
APA StyleGao, Z., Kong, X., Yi, J., Yang, B., Xu, B., Liu, D., Wu, J., & Xiong, H. (2021). Vacuum Gasification-Directional Condensation for Separation of Tellurium from Lead Anode Slime. Metals, 11(10), 1535. https://doi.org/10.3390/met11101535





