Fabrication of Two-Layered Aluminum Foam with Closed-Cell and Open-Cell Structures and Shaping of Closed-Cell Layer by Press Forming Immediately after Foaming
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication Procedure for Sintered Sample
2.2. Temperature Measurement Procedure during Foaming
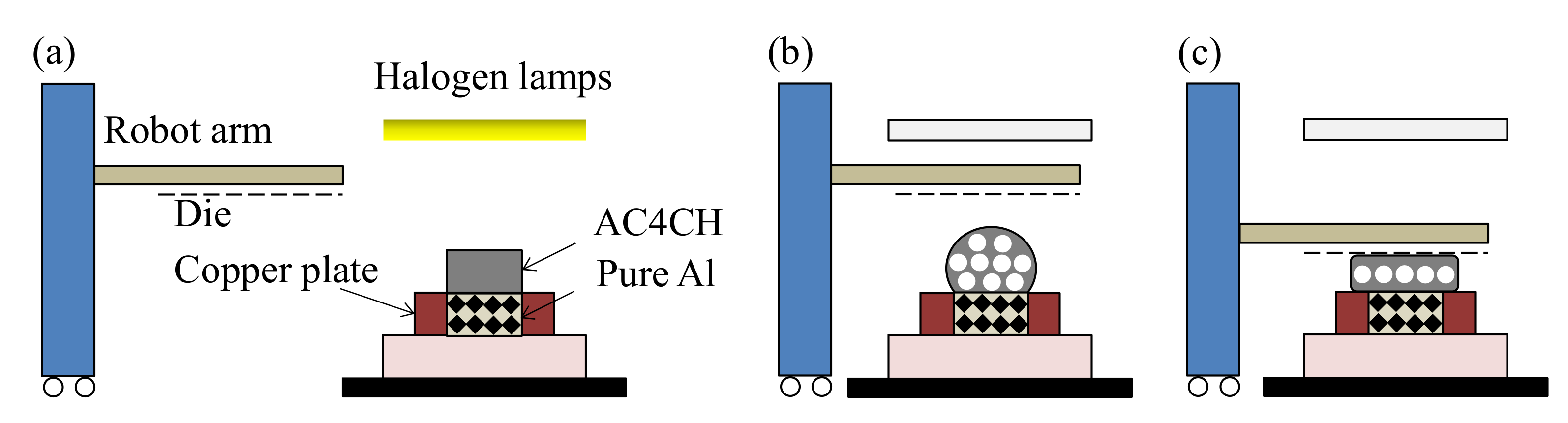
2.3. Press Forming Procedure
3. Results
3.1. Temperature Measurement during Foaming
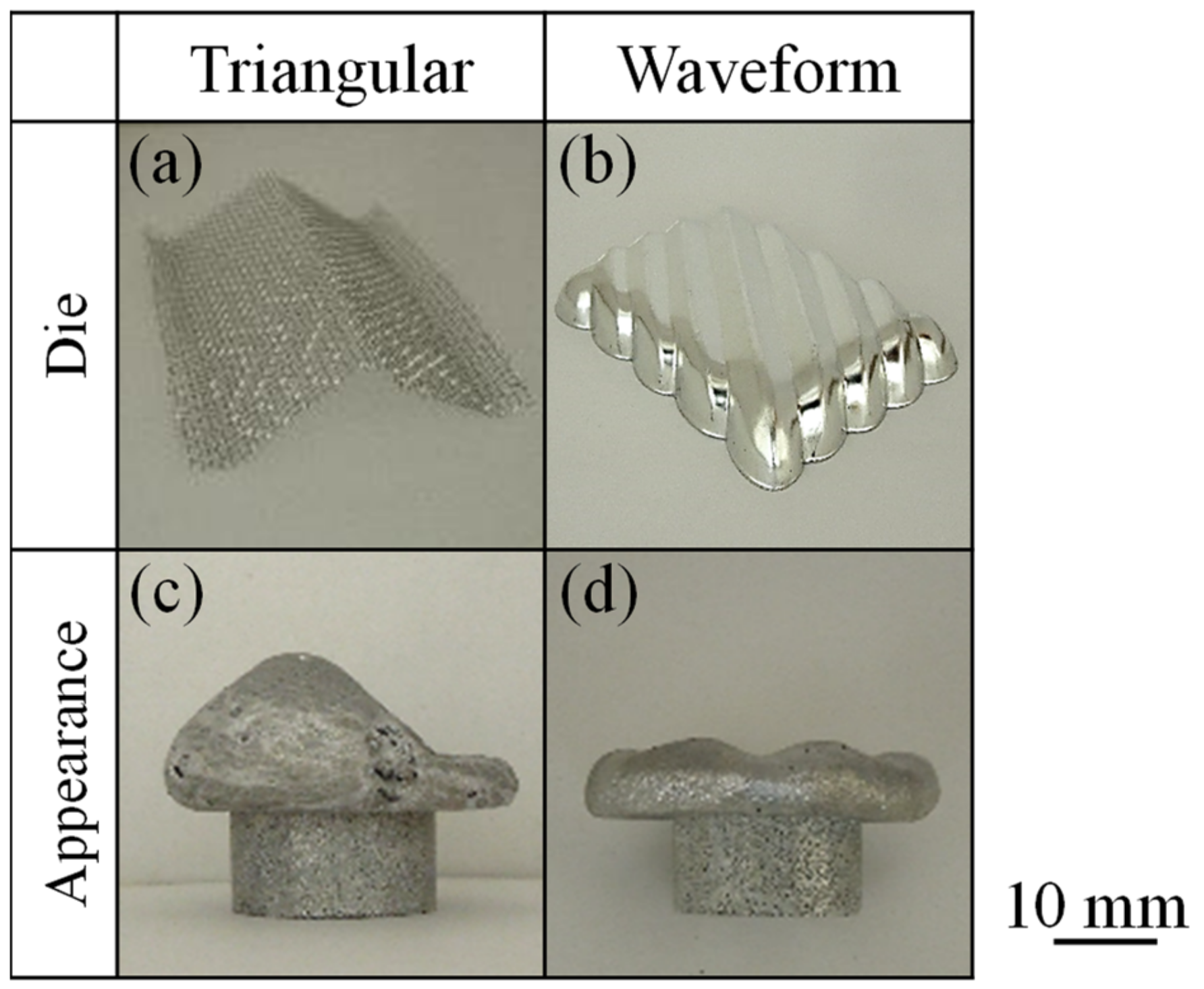
3.2. Press Forming of Closed-Cell Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- García-Moreno, F. Commercial Applications of Metal Foams: Their Properties and Production. Materials 2016, 9, 85. [Google Scholar] [CrossRef]
- Gibson, L.J. Mechanical behavior of metallic foams. Annu. Rev. Mater. Sci. 2000, 30, 191–227. [Google Scholar] [CrossRef]
- Baumgartner, F.; Duarte, I.; Banhart, J. Industrialization of powder compact foaming process. Adv. Eng. Mater. 2000, 2, 168–174. [Google Scholar] [CrossRef]
- Duarte, I.; Banhart, J. A study of aluminium foam formation—Kinetics and microstructure. Acta Mater. 2000, 48, 2349–2362. [Google Scholar] [CrossRef]
- Duarte, I.; Oliveira, M.; Garcia-Moreno, F.; Mukherjee, M.; Banhart, J. Foaming of AA 6061 using multiple pieces of foamable precursor. Colloids Surf. A Physicochem. Eng. Asp. 2013, 438, 47–55. [Google Scholar] [CrossRef]
- Duarte, I.; Vesenjak, M.; Vide, M.J. Automated Continuous Production Line of Parts Made of Metallic Foams. Metals 2019, 9, 531. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Sun, D.X. A novel sintering-dissolution process for manufacturing Al foams. Scr. Mater. 2001, 44, 105–110. [Google Scholar] [CrossRef]
- Stanev, L.; Kolev, M.; Drenchev, B.; Drenchev, L. Open-Cell Metallic Porous Materials Obtained through Space Holders-Part I: Production Methods. A Review. J. Manuf. Sci. Eng. Trans. ASME 2017, 139. [Google Scholar] [CrossRef]
- Stanev, L.; Kolev, M.; Drenchev, B.; Drenchev, L. Open-Cell Metallic Porous Materials Obtained Through Space Holders-Part II: Structure and Properties. A Review. J. Manuf. Sci. Eng. Trans. ASME 2017, 139. [Google Scholar] [CrossRef]
- Hangai, Y.; Ikeda, H.; Amagai, K.; Suzuki, R.; Matsubara, M.; Yoshikawa, N. Fabrication of two-layered aluminum foam having layers with closed-cell and open-cell pores. Metall. Mater. Trans. A 2018, 49, 4452–4455. [Google Scholar] [CrossRef]
- Hangai, Y.; Ando, M.; Ohashi, M.; Amagai, K.; Suzuki, R.; Matsubara, M.; Yoshikawa, N. Compressive properties of two-layered aluminum foams with closed-cell and open-cell structures. Mater. Today Commun. 2020, 24. [Google Scholar] [CrossRef]
- Matsumoto, R.; Tsuruoka, H.; Otsu, M.; Utsunomiya, H. Fabrication of skin layer on aluminum foam surface by friction stir incremental forming and its mechanical properties. J. Mater. Process. Technol. 2015, 218, 23–31. [Google Scholar] [CrossRef]
- Matsumoto, R.; Mori, S.; Otsu, M.; Utsunomiya, H. Formation of skin surface layer on aluminum foam by friction stir powder incremental forming. Int. J. Adv. Manuf. Technol. 2018, 99, 1853–1861. [Google Scholar] [CrossRef]
- Banhart, J.; Seeliger, H.W. Aluminium Foam Sandwich Panels: Manufacture, Metallurgy and Applications. Adv. Eng. Mater. 2008, 10, 793–802. [Google Scholar] [CrossRef]
- Banhart, J.; Seeliger, H.W. Recent Trends in Aluminum Foam Sandwich Technology. Adv. Eng. Mater. 2012, 14, 1082–1087. [Google Scholar] [CrossRef]
- Hangai, Y.; Ohashi, M.; Nagahiro, R.; Amagai, K.; Utsunomiya, T.; Yoshikawa, N. Press forming of aluminum foam during foaming of precursor. Mater. Trans. 2019, 60, 2464–2469. [Google Scholar] [CrossRef]
- Hangai, Y.; Kawato, D.; Ando, M.; Ohashi, M.; Morisada, Y.; Ogura, T.; Fujii, H.; Nagahiro, R.; Amagai, K.; Utsunomiya, T.; et al. Nondestructive observation of pores during press forming of aluminum foam by X-ray radiography. Mater. Charact. 2020, 170, 110631. [Google Scholar] [CrossRef]
- Hangai, Y.; Morita, T.; Utsunomiya, T. Functionally graded aluminum foam consisting of dissimilar aluminum alloys fabricated by sintering and dissolution process. Mater. Sci. Eng. A 2017, 696, 544–551. [Google Scholar] [CrossRef]
- Hangai, Y.; Amagai, K.; Omachi, K.; Tsurumi, N.; Utsunomiya, T.; Yoshikawa, N. Forming of aluminum foam using steel mesh as die during foaming of precursor by optical heating. Opt. Laser Technol. 2018, 108, 496–501. [Google Scholar] [CrossRef]
- The Japan Institute of Light Metals. Structures and Properties of Aluminum; The Japan Institute of Light Metals: Tokyo, Japan, 1991. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hangai, Y.; Ando, M.; Ohashi, M.; Amagai, K. Fabrication of Two-Layered Aluminum Foam with Closed-Cell and Open-Cell Structures and Shaping of Closed-Cell Layer by Press Forming Immediately after Foaming. Metals 2021, 11, 140. https://doi.org/10.3390/met11010140
Hangai Y, Ando M, Ohashi M, Amagai K. Fabrication of Two-Layered Aluminum Foam with Closed-Cell and Open-Cell Structures and Shaping of Closed-Cell Layer by Press Forming Immediately after Foaming. Metals. 2021; 11(1):140. https://doi.org/10.3390/met11010140
Chicago/Turabian StyleHangai, Yoshihiko, Mizuki Ando, Masataka Ohashi, and Kenji Amagai. 2021. "Fabrication of Two-Layered Aluminum Foam with Closed-Cell and Open-Cell Structures and Shaping of Closed-Cell Layer by Press Forming Immediately after Foaming" Metals 11, no. 1: 140. https://doi.org/10.3390/met11010140
APA StyleHangai, Y., Ando, M., Ohashi, M., & Amagai, K. (2021). Fabrication of Two-Layered Aluminum Foam with Closed-Cell and Open-Cell Structures and Shaping of Closed-Cell Layer by Press Forming Immediately after Foaming. Metals, 11(1), 140. https://doi.org/10.3390/met11010140



