Stabilizing Arsenic in Copper Heap Leaching Residues
Abstract
1. Introduction
2. Materials and Methods
3. Results
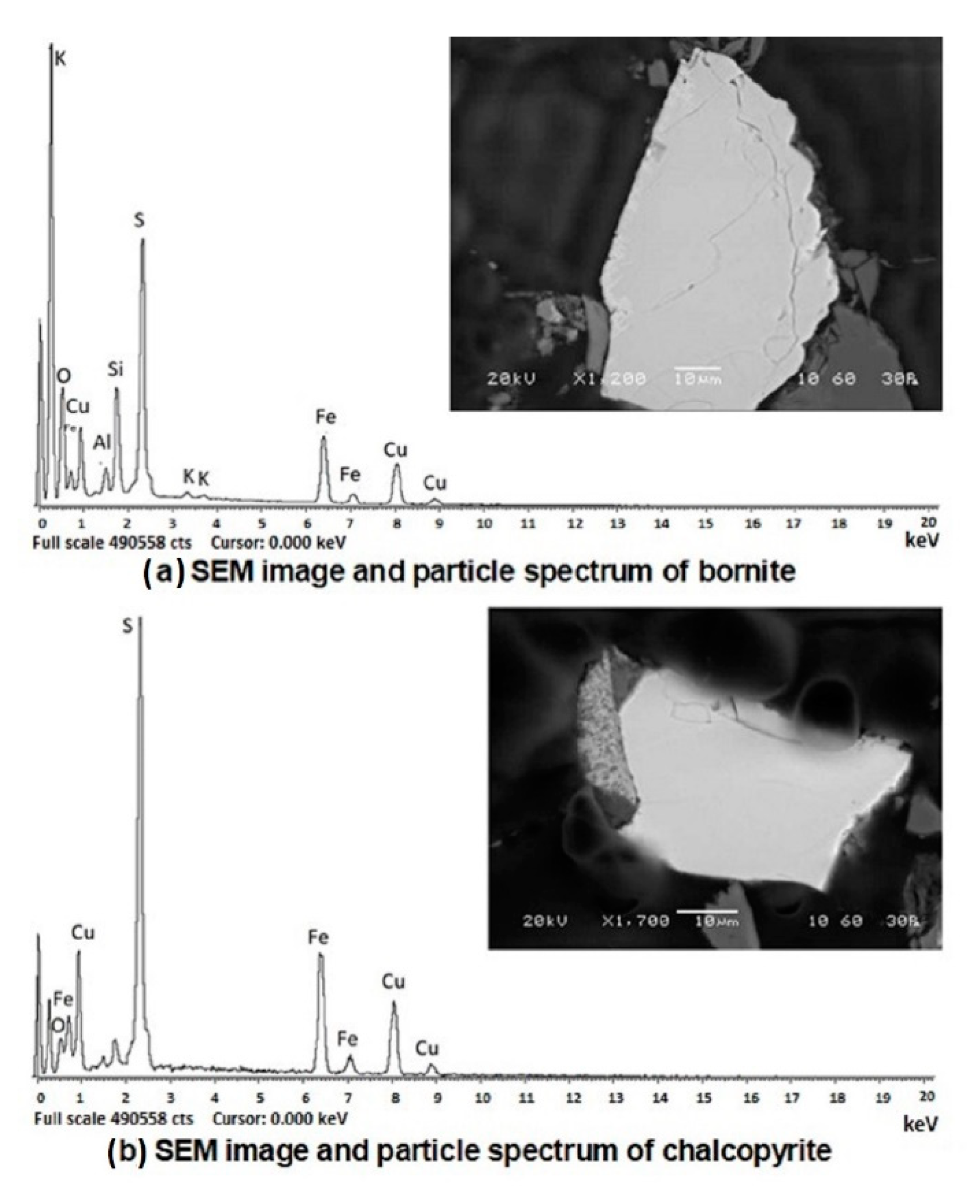
3.1. Sample Characterization
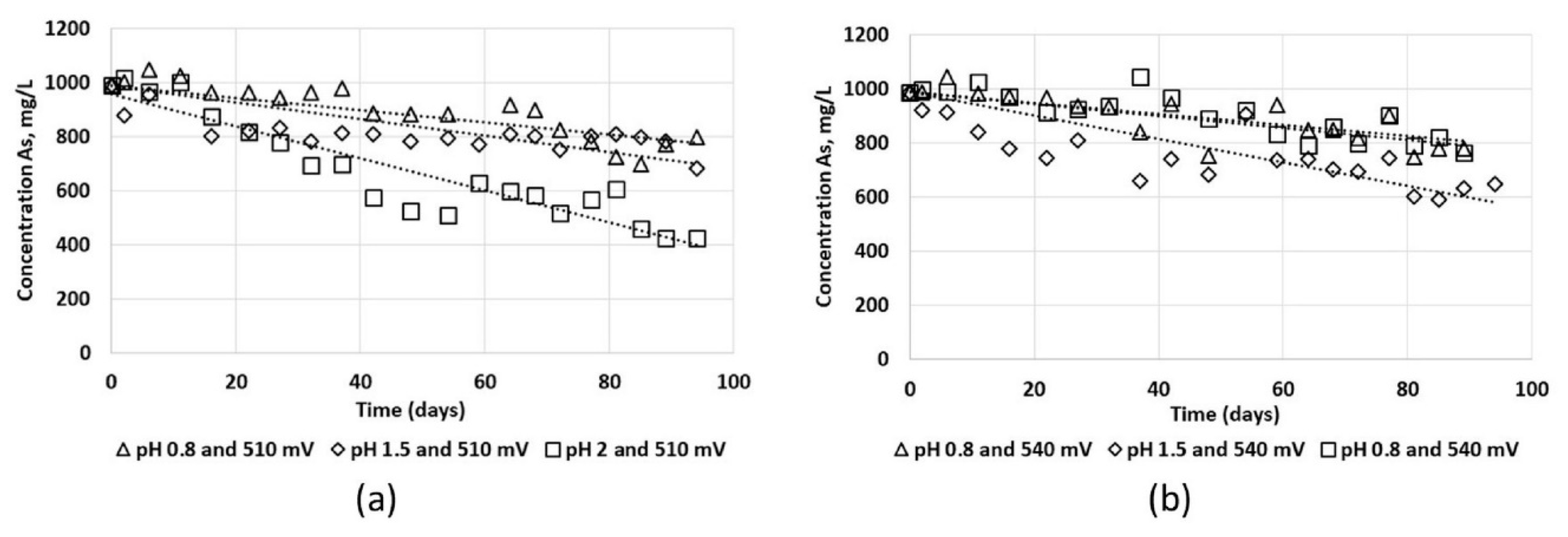
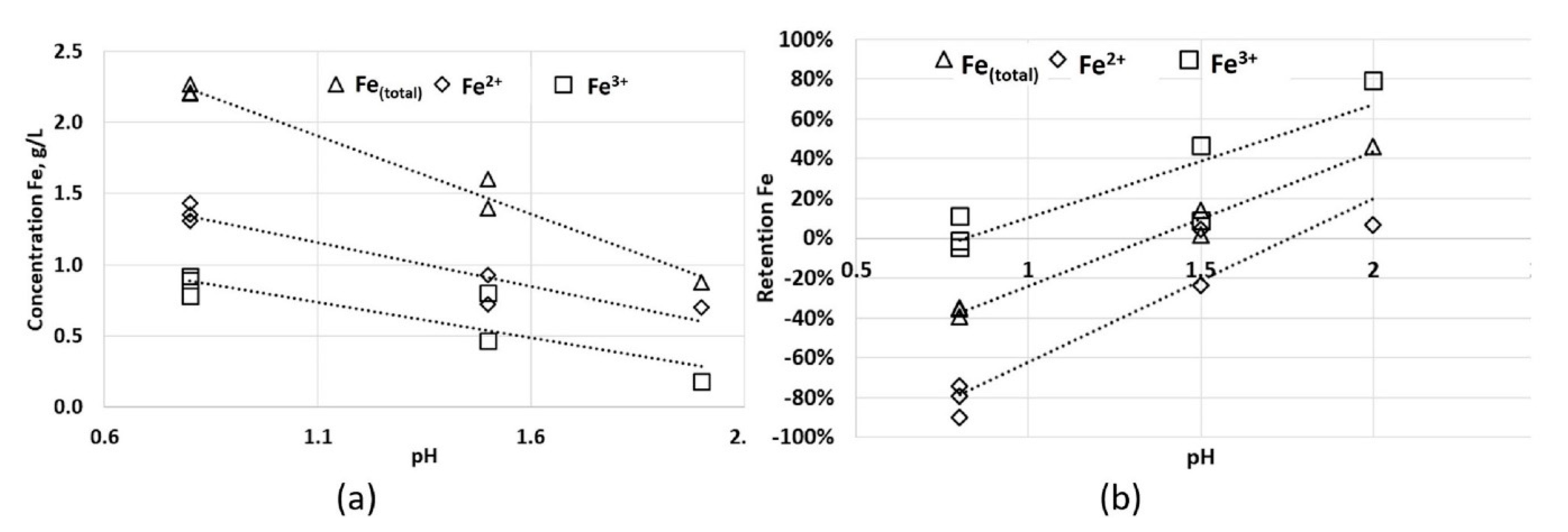
3.2. Column Leaching Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cánovas, M.; Valenzuela, J.; Romero, L.; González, P. Characterization of electroosmotic drainage: Application to mine tailings and solid residues from leaching. J. Mater. Res. Technol. 2020, 9, 2960–2968. [Google Scholar] [CrossRef]
- Valenzuela-Elgueta, J.; Cánovas, M.; García, A.; Zárate, R. Electrocoalescence of emulsions in raffinate from the solvent extraction phase under AC electrical fields. J. Mater. Res. Technol. 2020, 9, 490–497. [Google Scholar] [CrossRef]
- Beiza, L.; Quezada, V.; Melo, E.; Valenzuela, G. Electrochemical Behaviour of Chalcopyrite in Chloride Solutions. Metals 2019, 9, 67. [Google Scholar] [CrossRef]
- Cochilco. Proyección de la Producción de Cobre en Chile 2019–2030. Santiago de Chile. 2019. Available online: https://www.cochilco.cl/Listado%20Temtico/Proyecci%C3%B3n%20de%20la%20producci%C3%B3n%20esperada%20de%20cobre%202019%20-%202030%20Vfinal.pdf (accessed on 24 July 2020).
- Velásquez-Yévenes, L.; Quezada-Reyes, V. Influence of seawater and discard brine on the dissolution of copper ore and copper concentrate. Hydrometallurgy 2018, 180, 88–95. [Google Scholar] [CrossRef]
- Benavente, O.; Hernández, M.; Melo, E.; Núñez, D.; Quezada, V.; Zepeda, Y. Copper Dissolution from Black Copper Ore under Oxidizing and Reducing Conditions. Metals 2019, 9, 799. [Google Scholar] [CrossRef]
- Quezada, V.; Roca, A.; Benavente, O.; Cruells, M.; Keith, B.; Melo, E. Effect of pretreatment prior to leaching on a chalcopyrite mineral in acid media using NaCl and KNO3. J. Mater. Res. Technol. 2020, 9, 10316–10324. [Google Scholar] [CrossRef]
- Pantuzzo, F.L.; Ciminelli, V.S.T. Arsenic association and stability in long-term disposed arsenic residues. Water Res. 2010, 44, 5631–5640. [Google Scholar] [CrossRef]
- Raghav, M.; Shan, J.; Sáez, A.E.; Ela, W.P. Scoping candidate minerals for stabilization of arsenic-bearing solid residuals. J. Hazard. Mater. 2013, 263, 525–532. [Google Scholar] [CrossRef]
- Xiu, W.; Guo, H.; Shen, J.; Liu, S.; Ding, S.; Hou, W.; Ma, J.; Dong, H. Stimulation of Fe(II) Oxidation, Biogenic Lepidocrocite Formation, and Arsenic Immobilization by Pseudogulbenkiania Sp. Strain 2002. Environ. Sci. Technol. 2016, 50, 6449–6458. [Google Scholar] [CrossRef]
- Lihareva, N. Arsenic solubility, mobility and speciation in the deposits from a copper production waste storage. Microchem. J. 2005, 81, 177–183. [Google Scholar] [CrossRef]
- Phenrat, T.; Marhaba, T.F.; Rachakornkij, M. Leaching behaviors of arsenic from arsenic-iron hydroxide sludge during TCLP. J. Environ. Eng. 2008, 134, 671–682. [Google Scholar] [CrossRef]
- Otgon, N.; Zhang, G.; Zhang, K.; Yang, C. Removal and fixation of arsenic by forming a complex precipitate containing scorodite and ferrihydrite. Hydrometallurgy 2019, 186, 58–65. [Google Scholar] [CrossRef]
- Quansah, R.; Armah, F.A.; Essumang, D.K.; Luginaah, I.; Clarke, E.; Marfoh, K.; Cobbina, S.J.; Edward, N.-A.; Namujju, P.B.; Obiri, S.; et al. Association of Arsenic with Adverse Pregnancy Outcomes/Infant Mortality: A Systematic Review and Meta-Analysis. Enviromental Heal. Perspect. 2015, 123, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Twidwell, L. Arsenic Hydrometallurgy; Fundamentals, Technology And Applications. In Arsenic Hydrometallurgy; Fundamentals, Technology and Applications: Vancouver, BC, Canada, 2014; p. 17. [Google Scholar]
- Viñals, J.; Roca, A.; Hernández, M.C.; Benavente, O. Topochemical transformation of enargite into copper oxide by hypochlorite leaching. Hydrometallurgy 2003, 68, 183–193. [Google Scholar] [CrossRef]
- Long, G.; Peng, Y.; Bradshaw, D. A review of copper-arsenic mineral removal from copper concentrates. Miner. Eng. 2012, 179–186. [Google Scholar] [CrossRef]
- Riveros, P.A.; Dutrizac, J.E.; Spencer, P. Arsenic disposal practices in the metallurgical industry. Can. Met. Q. 2001, 40, 395–420. [Google Scholar] [CrossRef]
- Nishimura, T.; Umetsu, Y. Oxidative precipitation of arsenic(III) with manganese(II) and iron(II) in dilute acidic solution by ozone. Hydrometallurgy 2001, 62, 83–92. [Google Scholar] [CrossRef]
- Filippou, D.; Demopoulos, G.P. Arsenic immobilization by controlled scorodite precipitation. JOM 1997, 49, 52–55. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Ito, M.; Hiroyoshi, N. Hematite-catalysed scorodite formation as a novel arsenic immobilisation strategy under ambient conditions. Chemosphere 2019, 233, 946–953. [Google Scholar] [CrossRef]
- Rong, Z.; Tang, X.; Wu, L.; Chen, X.; Dang, W.; Li, X.; Huang, L.; Wang, Y. The effect of precursor speciation on the growth of scorodite in an atmospheric scorodite synthesis. R. Soc. Open Sci. 2020, 7. [Google Scholar] [CrossRef]
- Paktunc, D.; Bruggeman, K. Solubility of nanocrystalline scorodite and amorphous ferric arsenate: Implications for stabilization of arsenic in mine wastes. Appl. Geochem. 2010, 25, 674–683. [Google Scholar] [CrossRef]
- Ke, P.-C.; Liu, Z. Synthesis, in-situ coating and characterization of scorodite with high leaching stability. Trans. Nonferrous Met. Soc. China 2019, 29, 876–892. [Google Scholar] [CrossRef]
- Taboada, M.E.; Hernández, P.; Flores, E.; Graber, T. Crystallization of Arsenic Salts. Soc. Química Del Perú 2008, 74, 343–349. [Google Scholar]
- Paktunc, D.; Dutrizac, J.; Gertsman, V. Synthesis and phase transformations involving scorodite, ferric arsenate and arsenical ferrihydrite: Implications for arsenic mobility. Geochim. Cosmochim. Acta 2008, 72, 2649–2672. [Google Scholar] [CrossRef]
- Twidwell, L.G. Treatment of Arsenic-Bearing Minerals and Fixation of Recovered Arsenic Products: A Review. Sme Miner. Process. Extr. Met. Handb. 2018. [Google Scholar] [CrossRef]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Gomez, M.A.; Becze, L.; Cutler, J.N.; Demopoulos, G.P. Hydrothermal reaction chemistry and characterization of ferric arsenate phases precipitated from Fe2(SO4)3-As 2O5-H2SO4 solutions. Hydrometallurgy 2011, 107, 74–90. [Google Scholar] [CrossRef]
- Monhemius, J. The Scorodite Process: A Technology for the disposal of arsenic in the 21st century. In Effluent Treatment in the Minning Industry; Castro, S., Vergara, F., Sánchez, M., Eds.; Universidad de Concepción: Concepción, Chile, 1998; pp. 119–161. [Google Scholar]
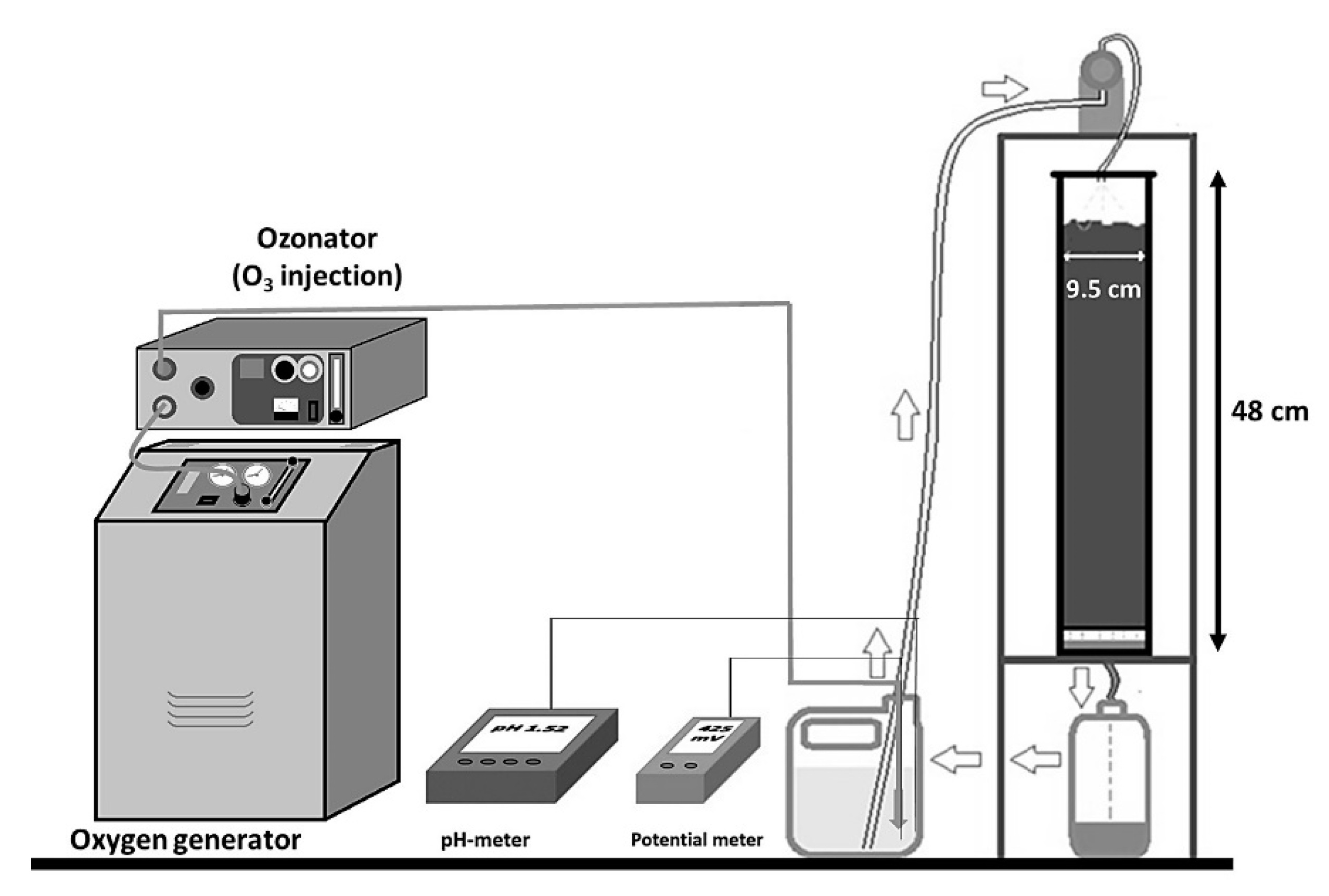




| Variable | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 |
|---|---|---|---|---|---|---|
| pH | 0.8 | 1.5 | 2.0 | 0.8 | 1.5 | 0.8 |
| Eh (mV) | 510 | 510 | 510 | 540 | 540 | 540 |
| Element | As | Fe(total) | Fe2+ | Fe3+ | Cu(total) | Cu(Soluble) |
|---|---|---|---|---|---|---|
| Mass (%) | 0.003 | 2.96 | 0.56 | 2.40 | 0.45 | 0.31 |
| Mineral | Volume (%) |
|---|---|
| Chalcocite (Cu2S) | 50 |
| Chalcopyrite (CuFeS2) | 20 |
| Covellite (CuS) | 15 |
| Bornite (Cu5FeS4) | 10 |
| Pyrite (FeS2) | 3 |
| Hematite (Fe2O3) | 2 |
| Element | Particle (a) % Atomic | Particle (b) % Atomic |
|---|---|---|
| S | 50.76 | 50.52 |
| Fe | 14.86 | 24.72 |
| Cu | 34.38 | 24.76 |
| Element | Unit | Leach Sol. | PLS-P | Mix Sol. |
|---|---|---|---|---|
| H2SO4 | g/L | 5.86 | 109 | 11.8 |
| As(total) | mg/L | 29.0 | 12,040 | 988 |
| As3+ | mg/L | 29.0 | 12,020 | 973 |
| As5+ | mg/L | <0.001 | 20.0 | 15.0 |
| Fe(total) | mg/L | 1383 | 2206 | 1630 |
| Fe2+ | mg/L | 642 | 2192 | 752 |
| Fe3+ | mg/L | 741 | 14.0 | 880 |
| Cu2+ | mg/L | 140 | 9477 | 910 |
| Column | As(total) g/kg | Fe(total) g/kg |
|---|---|---|
| C-1 | 1.52 | 28.76 |
| C-2 | 1.56 | 35.61 |
| C-3 | 6.16 | 45.84 |
| C-4 | 1.52 | 28.96 |
| C-5 | 1.19 | 34.20 |
| C-6 | 1.43 | 30.00 |
| O | Fe | S | Ca | Si | Cu | As | Al | K | Na | P | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 55.13 | 9.96 | 9.34 | 1.97 | 4.63 | 0.40 | 5.31 | 3.47 | 1.10 | 2.95 | 0.45 | 1.24 |
| Name | Formula | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 |
|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | ||
| Quartz | SiO2 | 24.9 | 31.5 | 31.7 | 26.7 | 23.6 | 32.4 |
| Orthoclase | KAlSi3O8 | 17.3 | 19.8 | 21.7 | 18.5 | 15.39 | 18.2 |
| Pyrite | FeS2 | 0.00 | 0.28 | 1.11 | 0.61 | 0.25 | 0.46 |
| Muscovite | KAl2(Si3Al)O10(OH,F)2 | 10.2 | 5.93 | 17.3 | 14.0 | 16.6 | 11.3 |
| Kaolinite | Al2Si2O5(OH)4 | 5.51 | 3.60 | 0.00 | 0.00 | 0.00 | 2.32 |
| Albite | NaCaAlSi3O8 | 11.1 | 10.2 | 6.52 | 9.86 | 13.2 | 9.18 |
| Chalcocite | Cu2S | 0.95 | 1.43 | 1.08 | 1.18 | 1.12 | 0.00 |
| Chlorite | (Mg,Fe)3(Si,Al)4O10(OH)2 (Mg,Fe)3(OH)6 | 3.95 | 6.74 | 8.20 | 7.50 | 3.87 | 10.5 |
| Jarosite | KFe+33(SO4)2(OH)6 | 3.22 | 1.39 | 0.20 | 1.26 | 0.94 | 1.22 |
| Alunite | KAl3(SO4)2(OH)6 | 0.17 | 0.76 | 1.58 | 1.89 | 2.94 | 0.81 |
| Gypsum | CaSO4·2H2O | 13.9 | 8.07 | 7.19 | 8.17 | 8.92 | 9.46 |
| Scorodite | Fe3+AsO4·2H2O | 0.26 | 0.62 | 0.92 | 1.53 | 2.40 | 0.78 |
| Nontronite | Na0.3Fe2Si4O10(OH)2·4H2O | 8.57 | 9.69 | 2.50 | 5.76 | 9.10 | 1.72 |
| Bassanite | CaSO4·0.5H2O | 0.00 | 0.00 | 0.00 | 3.12 | 1.74 | 1.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavente, O.; Hernández, M.C.; Melo, E.; Quezada, V.; Sepúlveda, Y.; Zepeda, Y. Stabilizing Arsenic in Copper Heap Leaching Residues. Metals 2020, 10, 1242. https://doi.org/10.3390/met10091242
Benavente O, Hernández MC, Melo E, Quezada V, Sepúlveda Y, Zepeda Y. Stabilizing Arsenic in Copper Heap Leaching Residues. Metals. 2020; 10(9):1242. https://doi.org/10.3390/met10091242
Chicago/Turabian StyleBenavente, Oscar, María Cecilia Hernández, Evelyn Melo, Víctor Quezada, Yan Sepúlveda, and Yuri Zepeda. 2020. "Stabilizing Arsenic in Copper Heap Leaching Residues" Metals 10, no. 9: 1242. https://doi.org/10.3390/met10091242
APA StyleBenavente, O., Hernández, M. C., Melo, E., Quezada, V., Sepúlveda, Y., & Zepeda, Y. (2020). Stabilizing Arsenic in Copper Heap Leaching Residues. Metals, 10(9), 1242. https://doi.org/10.3390/met10091242







