Characterization of Microstructure in High-Hardness Surface Layer of Low-Carbon Steel
Abstract
1. Introduction
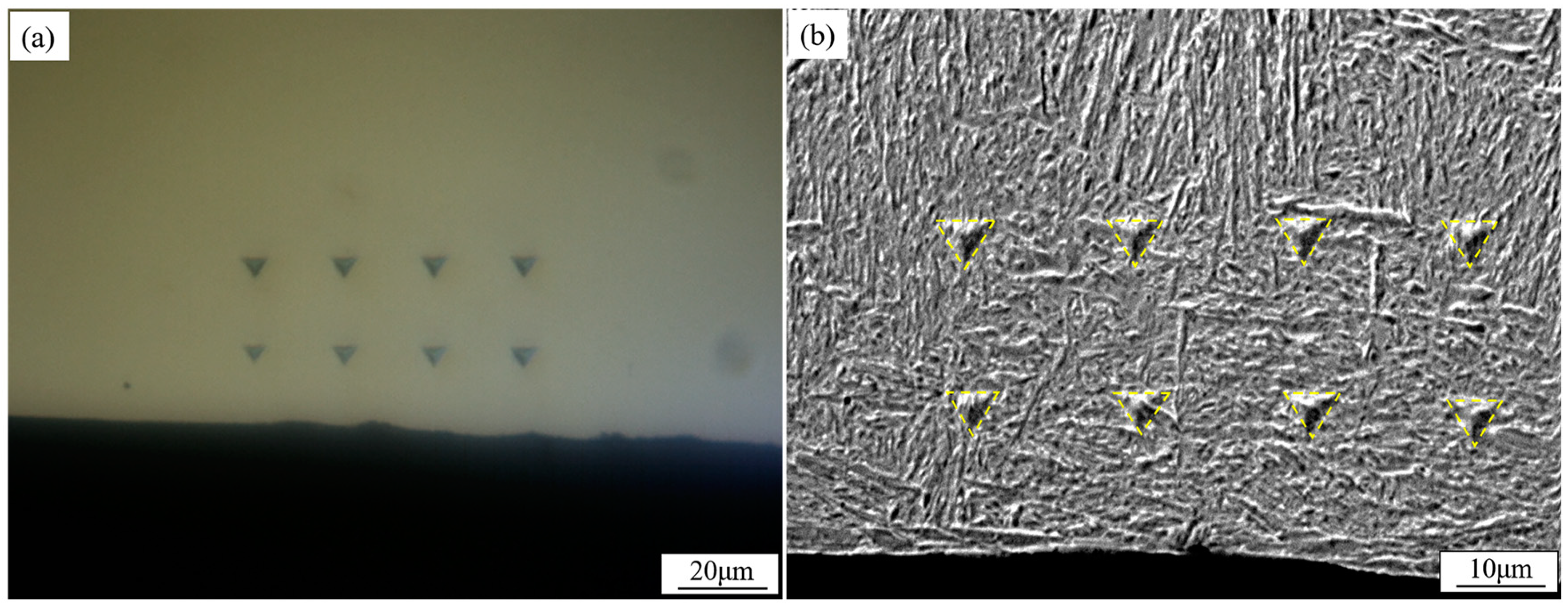
2. Materials and Methods
3. Results
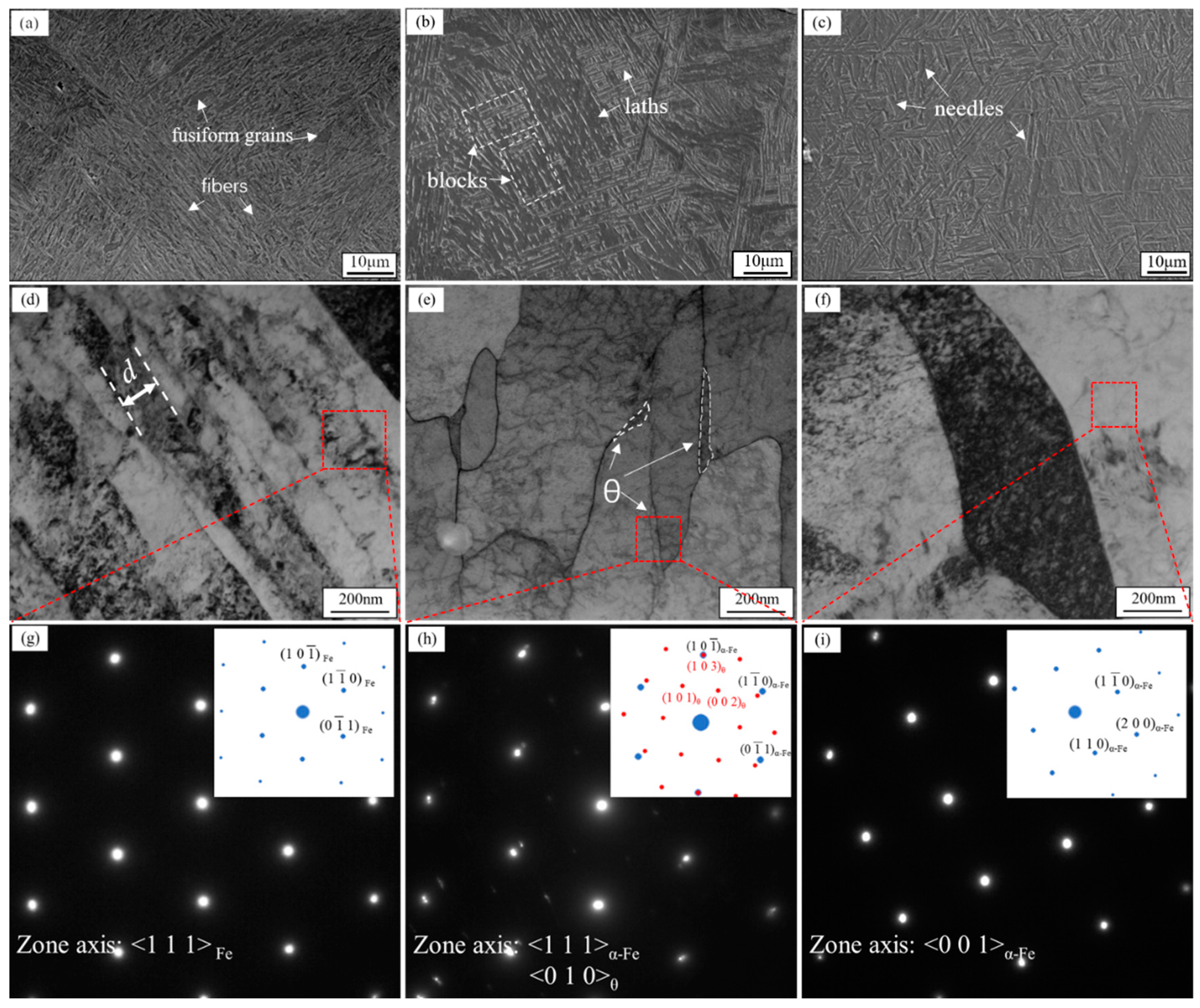
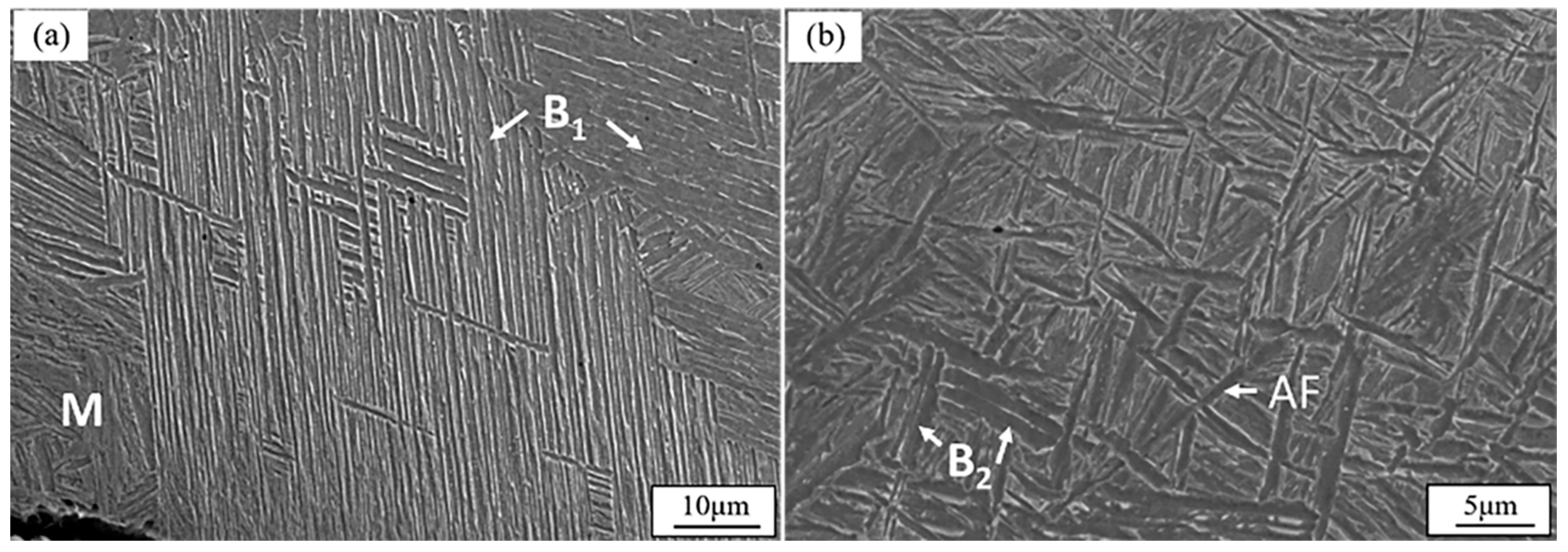
3.1. Microstructure
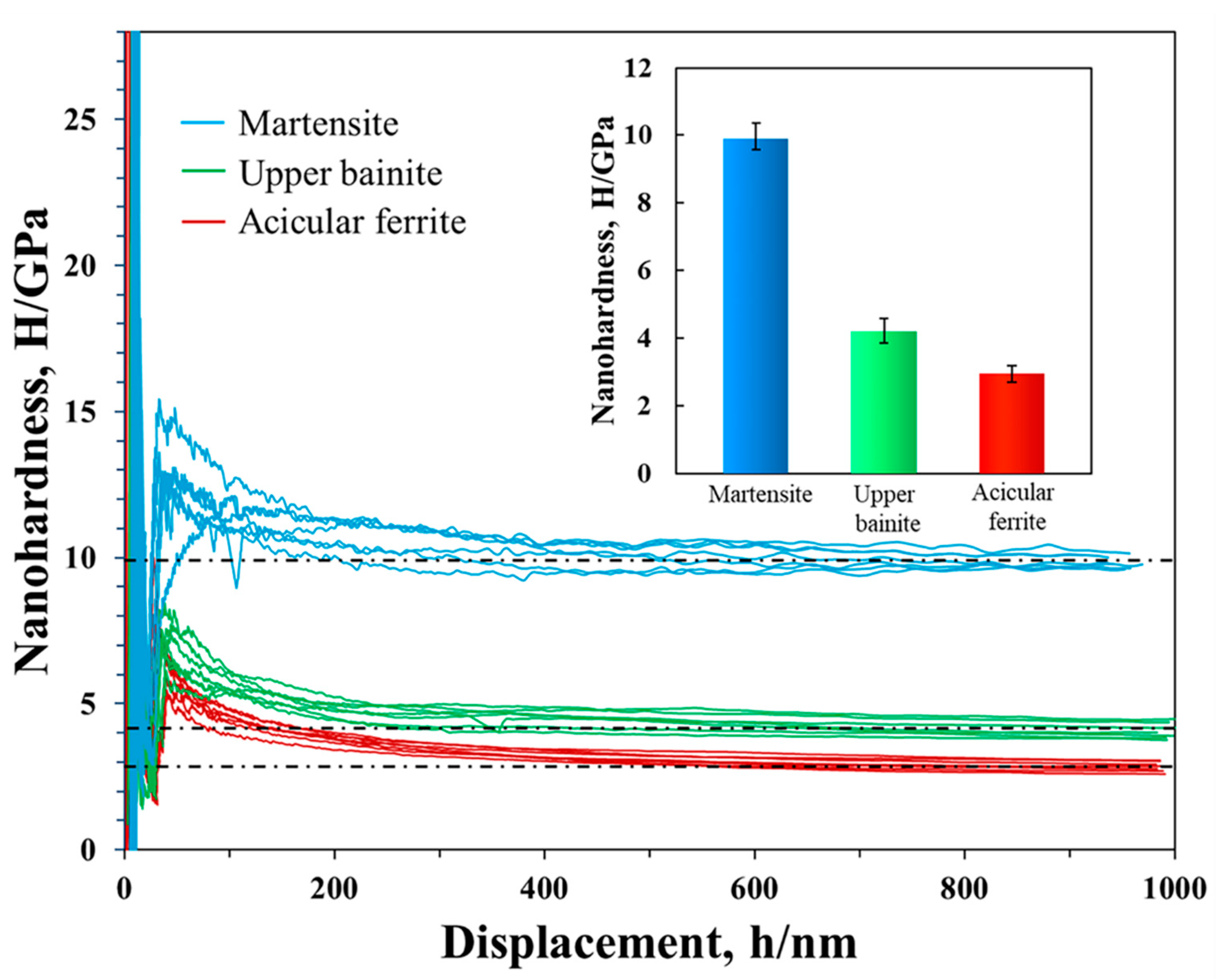
3.2. Nanohardness and Yield Strength
4. Discussion
5. Conclusions
- 1.
- In the thin surface layer of sub-rapid-solidified low-carbon steel (0.023 wt% C), we confirmed the presence of not only upper bainite and acicular ferrite but also lath martensite. B1 upper bainite formed prior to lath martensite, and both of them were in 0–50 μm depth. B2 upper bainite formed after acicular ferrite, and both of them were in 50–200 μm depth. B2 upper bainite was shorter than B1.
- 2.
- The unexpectedly high nanohardness of martensite in this low-carbon steel was 9.87 ± 0.51 GPa, equivalent to the nanohardness level reported for steel with twenty times the carbon content. This could be attributed to the fineness of the martensite laths with width of only 128 nm, which was nearly as low as the minimum width given for martensite. The nanohardness was 4.18 ± 0.39 GPa for upper bainite and 2.93 ± 0.30 GPa for acicular ferrite.
- 3.
- Martensite at the surface had a yield strength of 2378 ± 123 MPa, substantially higher than upper bainite (1007 ± 94 MPa) or acicular ferrite (706 ± 72 MPa). The high yield strength of martensite in the surface layer gave the steel exceptional resistance to abrasion, to a degree that would be unachievable without additional heat treatment in other steels with similar carbon content.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nisbett, E. Factors Affecting the Notch Toughness of Carbon and Low-Alloy Steel Forgings for Pressure Vessel and Piping Applications. J. Eng. Mater. Technol. 1978, 100, 338–347. [Google Scholar] [CrossRef]
- Ponsot, A.; Maynier, P.; Comon, J.; Bastien, P. Application of an Equivalence between Tempering Time and Temperature to the Study of the Hardness of Martensite for C and Low-Alloy Steels. Rev. Met. 1971, 68, 441–448. [Google Scholar] [CrossRef]
- Stokes, J.L. Magnetic Properties of Iron and Low Carbon Steel for Soft Magnet Applications. J. Test. Eval. 1981, 9, 354–358. [Google Scholar]
- Lee, J.H.; Jang, J.H.; Joo, B.D.; Son, Y.M.; Moon, Y.H. Laser Surface Hardening of Aisi H13 Tool Steel. Trans. Nonferrous Met. Soc. China 2009, 19, 137–140. [Google Scholar] [CrossRef]
- Li, H.G.; Wang, L.X.; Xiao, H.T.; Xu, J.L.; Zheng, S.B.; Zhai, Q.J.; Han, K. Hardening Low-Carbon Steels by Engineering the Size and Distribution of Inclusions. Metall. Mater. Trans. A 2019, 50, 336–347. [Google Scholar] [CrossRef]
- Krauss, G.; Thompson, S.W. Ferritic Microstructures in Continuously Cooled Low-and Ultralow-Carbon Steels. ISIJ Int. 1995, 35, 937–945. [Google Scholar] [CrossRef]
- Araki, T.; Enomoto, M.; Shibata, K. Microstructural Aspects of Bainitic and Bainite-Like Ferritic Structures of Continuously Cooled Low Carbon (<0.1%) Hsla Steels. Mater. Trans. JIM 1991, 32, 729–736. [Google Scholar] [CrossRef][Green Version]
- Kitahara, H.; Ueji, R.; Tsuji, N.; Minamino, Y. Crystallographic Features of Lath Martensite in Low-Carbon Steel. Acta Mater. 2006, 54, 1279–1288. [Google Scholar] [CrossRef]
- Morito, S.; Huang, X.; Furuhara, T.; Maki, T.; Hansen, N. The Morphology and Crystallography of Lath Martensite in Alloy Steels. Acta Mater. 2006, 54, 5323–5331. [Google Scholar] [CrossRef]
- Smallman, R.E.; Ngan, A.H.W. Chapter 12—Steel Transformations; Elsevier Ltd.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Miyamoto, G.; Takayama, N.; Furuhara, T. Accurate Measurement of the Orientation Relationship of Lath Martensite and Bainite by Electron Backscatter Diffraction Analysis. Scr. Mater. 2009, 60, 1113–1116. [Google Scholar] [CrossRef]
- Cheng, L.; Bottger, A.; Dekeijser, T.H.; Mittemeijer, E.J. Lattice-Parameters of Iron-Carbon and Iron-Nitrogen Martensites and Austenites. Scr. Metall. Mater. 1990, 24, 509–514. [Google Scholar] [CrossRef]
- Han, K.; Van Genderen, M.; Böttger, A.; Zandbergen, H.; Mittemeijer, E. Initial Stages of Fe-C Martensite Decomposition. Philos. Mag. A 2001, 81, 741–757. [Google Scholar] [CrossRef]
- Jack, K.H. Structural Transformations in the Tempering of High-Carbon Martensitic Steels. J. Iron Steel Inst. 1951, 169, 26. [Google Scholar]
- Kajiwara, S.; Uehara, S.; Nakamura, Y. Structure Images of Unaged Martensite Containing a High-Carbon Content. Philos. Mag. Lett. 1989, 60, 147–153. [Google Scholar] [CrossRef]
- Naraghi, R.; Selleby, M.; Agren, J. Thermodynamics of Stable and Metastable Structures in Fe-C System. Calphad 2014, 46, 148–158. [Google Scholar] [CrossRef]
- Preciado, M.; Pellizzari, M. Influence of Deep Cryogenic Treatment on the Thermal Decomposition of Fe-C Martensite. J. Mater. Sci. 2014, 49, 8183–8191. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Christian, J. Bainite in Steels. Metall. Trans. A 1990, 21, 767–797. [Google Scholar] [CrossRef]
- Ricks, R.; Howell, P.; Barritte, G. The Nature of Acicular Ferrite in Hsla Steel Weld Metals. J. Mater. Sci. 1982, 17, 732–740. [Google Scholar] [CrossRef]
- Bahl, S.; Suwas, S.; Ungàr, T.; Chatterjee, K. Elucidating Microstructural Evolution and Strengthening Mechanisms in Nanocrystalline Surface Induced by Surface Mechanical Attrition Treatment of Stainless Steel. Acta Mater. 2017, 122, 138–151. [Google Scholar] [CrossRef]
- Xin, Y.; Kynoch, J.; Han, K.; Liang, Z.; Lee, P.J.; Larbalestier, D.C.; Su, Y.-F.; Nagahata, K.; Aoki, T.; Longo, P. Facility Implementation and Comparative Performance Evaluation of Probe-Corrected Tem/Stem with Schottky and Cold Field Emission Illumination. Microsc. Microanal. 2013, 19, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Kurdjumov, G.; Kaminsky, E. X-ray Studies of the Structure of Quenched Carbon Steel. Nature 1928, 122, 475–477. [Google Scholar]
- Clarke, A.J.; Speer, J.G.; Miller, M.K.; Hackenberg, R.E.; Edmonds, D.V.; Matlock, D.K.; Rizzo, F.C.; Clarke, K.D.; Moor, E.D. Carbon Partitioning to Austenite from Martensite or Bainite During the Quench and Partition (Q&P) Process: A Critical Assessment. Acta Mater. 2008, 56, 16–22. [Google Scholar]
- Zhang, P.; Chen, Y.; Xiao, W.; Ping, D.; Zhao, X. Twin Structure of the Lath Martensite in Low Carbon Steel. Prog. Nat. Sci. Mater. Int. 2016, 26, 169–172. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, D.; Liu, D.; Kang, J.; Yuan, G.; Mao, Q.J.; Misra, R.D.K.; Wang, G.D. Combined Thermo-Mechanical Controlled Processing and Dynamic Carbon Partitioning of Low Carbon Si/Al-Mn Steels. Mater. Sci. Eng. A 2018, 732, 298–310. [Google Scholar] [CrossRef]
- Morito, S.; Ohba, T.; Maki, T. Comparison of Deformation Structure of Lath Martensite in Low Carbon and Ultra-Low Carbon Steels. Mater. Sci. Forum 2007, 558–559, 933–938. [Google Scholar] [CrossRef]
- Maki, T. Morphology and Substructure of Martensite in Steels. Gangtie 2012, 2, 34–58. [Google Scholar]
- He, B.B.; Huang, M.X. Revealing the Intrinsic Nanohardness of Lath Martensite in Low Carbon Steel. Metall. Mater. Trans. A 2014, 46, 688–694. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Steels: Microstructure and Properties; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Ohmura, T.; Hara, T.; Tsuzaki, K. Evaluation of Temper Softening Behavior of Fe–C Binary Martensitic Steels by Nanoindentation. Scr. Mater. 2003, 49, 1157–1162. [Google Scholar] [CrossRef]
- Ohmura, T.; Tsuzaki, K.; Matsuoka, S. Nanohardness Measurement of High-Purity Fe–C Martensite. Scr. Mater. 2001, 45, 889–894. [Google Scholar] [CrossRef]
- He, B.B.; Zhu, K.; Huang, M.X. On the Nanoindentation Behaviour of Complex Ferritic Phases. Philos. Mag. Lett. 2014, 94, 439–446. [Google Scholar] [CrossRef]
- Choi, B.W.; Seo, D.H.; Jang, J.I. A Nanoindentation Study on the Micromechanical Characteristics of Api X100 Pipeline Steel. Met. Mater. Int. 2009, 15, 373–378. [Google Scholar] [CrossRef]
- Choi, B.W.; Seo, D.H.; Yoo, J.Y.; Jang, J.I. Predicting Macroscopic Plastic Flow of High-Performance, Dual-Phase Steel through Spherical Nanoindentation on Each Microphase. J. Mater. Res. 2011, 24, 816–822. [Google Scholar] [CrossRef]
- Shang, C.J.; Liang, X.; Wang, X.M.; He, X.L.; Liu, H. The Nanohardness of Acicular Ferrite and Bainitic Ferrite in Low Carbon Microalloying Steel. In Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2007. [Google Scholar]
- McFarlan, W.; Taylor, H.L. Properties and Applications of Low Carbon Martensitic Steel Sheets. SAE Trans. 1969, 78, 104. [Google Scholar]
- McNitt, L.F. Application of Low-Carbon, High-Strength Steels. Des. News 1970, 25, 150. [Google Scholar]
- Shen, Z.Y.; Wang, B.L.; Liang, G.F.; Zhang, Y.H.; Han, K.; Song, C.J. Grain Boundary Pop-in, Yield Point Phenomenon and Carbon Segregation in Aged Low Carbon Steel. ISIJ Int. 2018, 58, 373–375. [Google Scholar] [CrossRef]
- Pohl, F. Pop-in Behavior and Elastic-to-Plastic Transition of Polycrystalline Pure Iron during Sharp Nanoindentation. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Li, Z.M.; Li, X.; Yang, L.; Shen, Z.Y.; Wang, B.L.; Zhao, S.L.; Liang, G.F.; Song, C.J. Effect of Coiling and Annealing Temperatures on Yield Point Behavior of Low-Carbon Steel. J. Iron Steel Res. Int. 2020, 27, 325–333. [Google Scholar] [CrossRef]
- Rodríguez, R.; Gutierrez, I. Correlation between Nanoindentation and Tensile Properties: Influence of the Indentation Size Effect. Mater. Sci. Eng. A 2003, 361, 377–384. [Google Scholar] [CrossRef]
- Jun, H.J.; Kang, J.S.; Seo, D.H.; Kang, K.B.; Park, C.G. Effects of Deformation and Boron on Microstructure and Continuous Cooling Transformation in Low Carbon Hsla Steels. Mater. Sci. Eng. A 2006, 422, 157–162. [Google Scholar] [CrossRef]
- Yang, J.; Huang, C.; Huang, C.; Aoh, J. Influence of Acicular Ferrite and Bainite Microstructures on Toughness for an Ultra-Low-Carbon Alloy Steel Weld Metal. J. Mater. Sci. Lett. 1993, 12, 1290–1293. [Google Scholar] [CrossRef]

| Element | C | Mn | Si | Ti | Fe |
|---|---|---|---|---|---|
| Mass Fraction/% | 0.023 | 0.64 | 0.21 | 0.016 | Balance |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Zheng, S.; Xin, Y.; Xu, J.; Han, K.; Li, H.; Zhai, Q. Characterization of Microstructure in High-Hardness Surface Layer of Low-Carbon Steel. Metals 2020, 10, 995. https://doi.org/10.3390/met10080995
Xiao H, Zheng S, Xin Y, Xu J, Han K, Li H, Zhai Q. Characterization of Microstructure in High-Hardness Surface Layer of Low-Carbon Steel. Metals. 2020; 10(8):995. https://doi.org/10.3390/met10080995
Chicago/Turabian StyleXiao, Haitao, Shaobo Zheng, Yan Xin, Jiali Xu, Ke Han, Huigai Li, and Qijie Zhai. 2020. "Characterization of Microstructure in High-Hardness Surface Layer of Low-Carbon Steel" Metals 10, no. 8: 995. https://doi.org/10.3390/met10080995
APA StyleXiao, H., Zheng, S., Xin, Y., Xu, J., Han, K., Li, H., & Zhai, Q. (2020). Characterization of Microstructure in High-Hardness Surface Layer of Low-Carbon Steel. Metals, 10(8), 995. https://doi.org/10.3390/met10080995







