Intergranular to Intragranular Pitting Corrosion Transition Mechanism of Sensitized AA5083 at 150 °C
Abstract
1. Introduction
2. Experimental
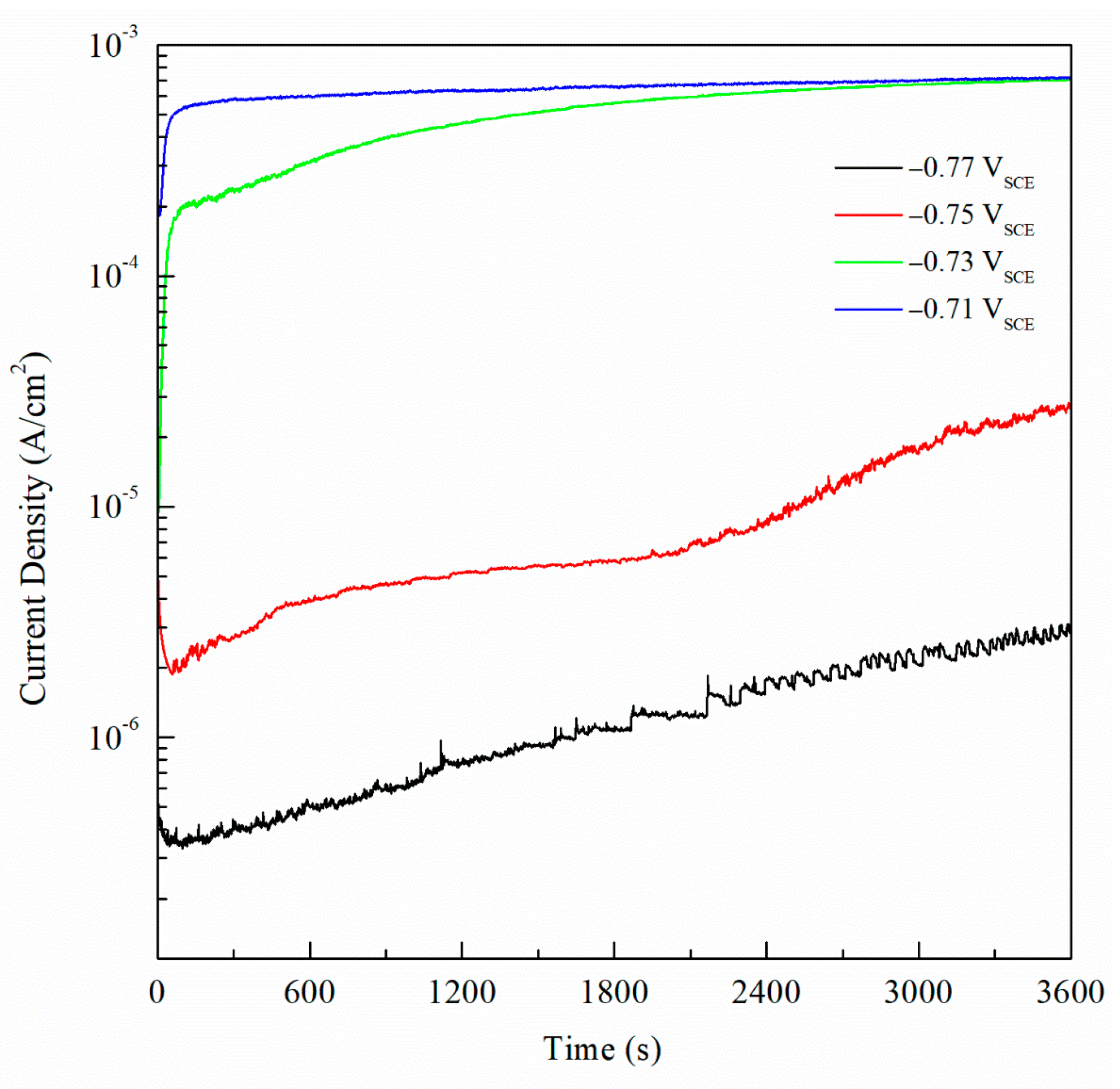
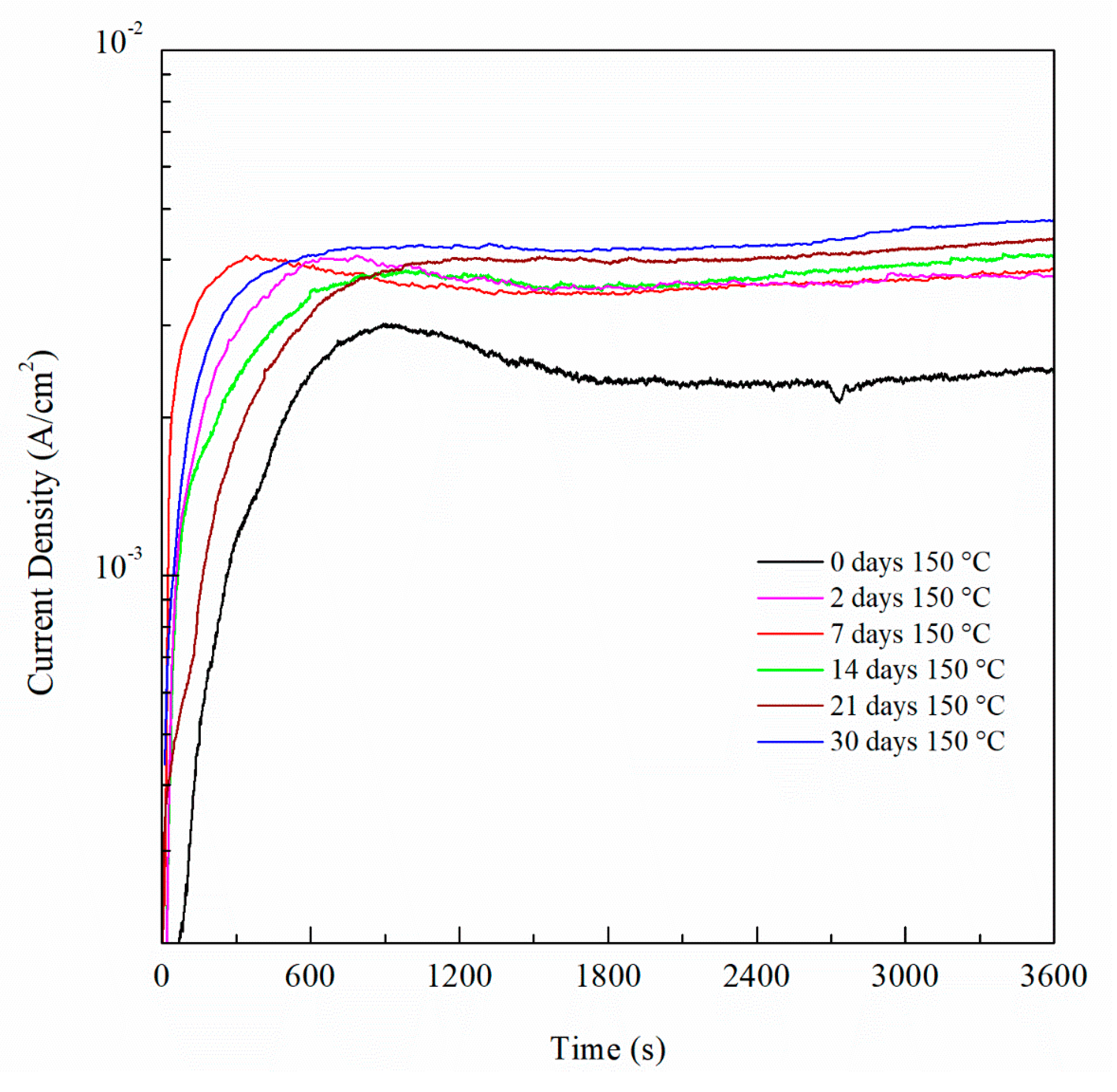
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mondolfo, L.F. Aluminum Alloys; Butterworth-Heinemann: London, UK, 1976. [Google Scholar]
- Polmear, I.J. Light Alloys, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Muller, I.L.; Galvele, J.R. Pitting potential of high purity binary aluminium alloys—II. AlMg and AlZn alloys. Corros. Sci. 1977, 17, 995–1007. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminum alloys: An experimental survey and discussion. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef]
- Buchheit, R.G. A compilation of corrosion potentials reported for intermetallic phases in aluminum alloys. J. Electrochem. Soc. 1995, 142, 3994–3999. [Google Scholar] [CrossRef]
- Grimm, M.; Lohmüller, A.; Singer, R.F.; Virtanen, S. Influence of the microstructure on the corrosion behaviour of cast Mg-Al alloys. Corros. Sci. 2019, 155, 195–208. [Google Scholar] [CrossRef]
- Davenport, A.J.; Yuan, Y.; Ambat, R.; Connolly, B.J.; Strangwood, M.; Afseth, A.; Scamans, G.M. Intergranular corrosion and stress corrosion cracking of sensitised AA5182. Mater. Sci. Forum 2006, 519, 641–646. [Google Scholar] [CrossRef]
- Yi, G.; Zhu, Y.; Cullen, D.A.; Derrick, A.T.; Free, M.L. Light Metals; Springer: San Diego, CA, USA, 2014. [Google Scholar]
- ASTM G67–18 Standard Test Method for Determining the Susceptibility to Intergranular Corrosion of 5XXX Series Aluminum Alloys by Mass Loss after Exposure to Nitric Acid (NAMLT Test); ASTM International: West Conshohocken, PA, USA, 2018.
- Zhang, R.; Knight, S.P.; Holtz, R.L.; Goswami, R.; Davies, C.H.J.; Birbilis, N. A survey of sensitization in 5xxx series aluminum alloys. Corrosion 2015, 72, 144–159. [Google Scholar] [CrossRef]
- Lyndon, J.A.; Gupta, R.K.; Gibson, M.A.; Birbilis, N. Electrochemical behaviour of the β-phase intermetallic (Mg2Al3) as a function of pH as relevant to corrosion of aluminium–magnesium alloys. Corros. Sci. 2013, 70, 290–293. [Google Scholar] [CrossRef]
- Oguocha, I.N.A.; Adigun, O.J.; Yannacopoulos, S. Effect of sensitization heat treatment on properties of Al–Mg alloy AA5083-H116. J. Mater. Sci. 2008, 43, 4208–4214. [Google Scholar] [CrossRef]
- Yan, J.; Heckman, N.M.; Velasco, L.; Hodge, A.M. Improve sensitization and corrosion resistance of an Al-Mg alloy by optimization of grain boundaries. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Lim, M.L.C.; Matthews, R.; Oja, M.; Tryon, R.; Kelly, R.G.; Scully, J.R. Model to predict intergranular corrosion propagation in three dimensions in AA5083-H131. Mater. Des. 2016, 96, 131–142. [Google Scholar] [CrossRef]
- ASTM standard B928/B928M-15: Standard Specification for High Magnesium Aluminum-Alloy Products for Marine Service and Similar Environments; ASTM International: West Conshohocken, PA, USA, 2018.
- Goswami, R.; Spanos, G.; Pao, P.S.; Holtz, R.L. Precipitation behavior of the ß phase in Al-5083. Mater. Sci. Eng. 2010, 527, 1089–1095. [Google Scholar] [CrossRef]
- Massalski, T.B.; Murray, J.L.; Bennett, L.H.; Baker, H. Binary Alloy Phase Diagrams; American Society for Metals: Metals Park, OH, USA, 1986. [Google Scholar]
- Ding, Y.; Gao, K.; Huang, H.; Wen, S.; Wu, X.; Nie, Z.; Guo, S.; Shao, R.; Huang, C.; Zhou, D. Nucleation and evolution of β phase and corresponding intergranular corrosion transition at 100–230 °C in 5083 alloy containing Er and Zr. Mater. Des. 2019, 174, 107778. [Google Scholar] [CrossRef]
- Steiner, M.A.; Agnew, S.R. Modeling sensitization of Al–Mg alloys via β-phase precipitation kinetics. Scri. Mater. 2015, 102, 55–58. [Google Scholar] [CrossRef]
- Holroyd, N.J.H.; Burnett, T.L.; Seifi, M.; Lewandowski, J.J. Improved understanding of environment-induced cracking (EIC) of sensitized 5XXX series aluminium alloys. Mater. Sci. Eng. 2017, 682, 613–621. [Google Scholar] [CrossRef]
- Goswami, R.; Holtz, R.L. Transmission electron microscopic investigations of grain boundary beta phase precipitation in Al 5083 aged at 373 K (100 °C). Metall. Mater. Trans. A 2013, 44, 1279–1289. [Google Scholar] [CrossRef]
- Dix, E.H., Jr.; Anderson, W.A.; Shumaker, M.B. Influence of service temperature on the resistance of wrought aluminum-magnesium alloys to corrosion. Corrosion 1959, 15, 19–26. [Google Scholar] [CrossRef]
- Searles, J.L.; Gouma, P.I.; Buchheit, R.G. Stress corrosion cracking of sensitized AA5083 (Al-4.5Mg-1.0Mn). Metall. Mater. Trans. A 2001, 32, 2859–2867. [Google Scholar] [CrossRef]
- Scotto D’Antuono, D.; Gaies, J.; Golumbfskie, W.; Taheri, M.L. Direct measurement of the effect of cold rolling on β phase precipitation kinetics in 5xxx series aluminum alloys. Acta Mater. 2017, 123, 264–271. [Google Scholar] [CrossRef]
- Zhang, R.; Steiner, M.A.; Agnew, S.R.; Kairy, S.K.; Davies, C.H.J.; Birbilis, N. Experiment-based modelling of grain boundary β-phase (Mg2Al3) evolution during sensitisation of aluminum alloy AA5083. Sci. Rep. 2017, 7, 2961. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Hashimoto, T.; Liu, B. Localized corrosion in AA2024-T351 aluminium alloy: Transition from intergranular corrosion to crystallographic pitting. Mater. Charact. 2017, 130, 230–236. [Google Scholar] [CrossRef]
- Zhang, W.; Frankel, G.S. Transitions between pitting and intergranular corrosion in AA2024. Electrochim. Acta 2003, 48, 1193–1210. [Google Scholar] [CrossRef]
- Jain, S.; Hudson, J.L.; Scully, J.R. Effects of constituent particles and sensitization on surface spreading of intergranular corrosion on a sensitized AA5083 alloy. Electrochim. Acta 2013, 108, 253–264. [Google Scholar] [CrossRef]
- Seong, J.; Yang, F.; Scheltens, F.; Frankel, G.S.; Sridhar, N. Influence of the altered surface layer on the corrosion of AA5083. J. Electrochem. Soc. 2015, 162, C209–C218. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Marcos, M. CeCl3 and LaCl3 binary solutions as environment-friendly corrosion inhibitors of AA5083 Al–Mg alloy in NaCl solutions. J. Alloys Compd. 2001, 323, 855–858. [Google Scholar] [CrossRef]
- Li, Y.; Cai, J.M.; Guan, L.; Wang, G. pH-dependent electrochemical behaviour of Al3Mg2 in NaCl solution. Appl. Surf. Sci. 2019, 467, 619–633. [Google Scholar] [CrossRef]
- Südholz, A.D.; Kirkland, N.T.; Buchheit, R.G.; Birbilis, N. Electrochemical properties of intermetallic phases and common impurity elements in magnesium alloys. Electrochem. Solid-State Lett. 2011, 14, C5–C7. [Google Scholar] [CrossRef]
- Jain, S.; Lim, M.L.C.; Hudson, J.L.; Scully, J.R. Spreading of intergranular corrosion on the surface of sensitized Al-4.4Mg alloys: A general finding. Corros. Sci. 2012, 59, 136–147. [Google Scholar] [CrossRef]
- Gupta, R.K.; Sukiman, N.L.; Cavanaugh, M.K.; Hinton, B.R.W.; Hutchinson, C.R.; Birbilis, N. Metastable pitting characteristics of aluminium alloys measured using current transients during potentiostatic polarisation. Electrochim. Acta 2012, 66, 245–254. [Google Scholar] [CrossRef]
- Foley, D.L.; Leff, A.; Lang, A.C.; Taheri, M.L. Evolution of β-phase precipitates in an aluminum-magnesium alloy at the nanoscale. Acta Mater. 2019, 185, 279–286. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. Localized alkaline corrosion of alloy AA5083 in neutral 3.5% NaCl solution. Corros. Sci. 2001, 43, 1657–1674. [Google Scholar] [CrossRef]
- Zhang, W.; Frankel, G.S. Localized corrosion growth kinetics in AA2024 alloys. J. Electrochem. Soc. 2002, 149, B510–B519. [Google Scholar] [CrossRef]
- Comotti, I.M.; Trueba, M.; Trasatti, S.P. The pit transition potential in the repassivation of aluminium alloys. Surf. Interface Anal. 2013, 45, 1575–1584. [Google Scholar] [CrossRef]
- Pride, S.T.; Scully, J.R.; Hudson, J.L. Metastable pitting of aluminum and criteria for the transition to stable pit growth. J. Electrochem. Soc. 1994, 141, 3028–3039. [Google Scholar] [CrossRef]
- Yasuda, M.; Weinberg, F.; Tromans, D. Pitting corrosion of Al and Al-Cu single crystals. J. Electrochem. Soc. 1990, 137, 3708. [Google Scholar] [CrossRef]
- Moore, K.L.; Sykes, J.M.; Grant, P.S. An electrochemical study of repassivation of aluminium alloys with SEM examination of the pit interiors using resin replicas. Corros. Sci. 2008, 50, 3233–3240. [Google Scholar] [CrossRef]
- Kramer, L.; Phillippi, M.; Tack, W.; Wong, C. Locally reversing sensitization in 5xxx aluminum plate. J. Mater. Eng. 2012, 21, 1025–1029. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhang, C.; Zhang, T.; Yu, B.; Meng, G.; Shao, Y.; Wang, F.; Liu, L. Protection of AA5083 by a zirconium-based conversion coating. J. Electrochem. Soc. 2016, 163, C576–C586. [Google Scholar] [CrossRef]
- Seong, J.; Frankel, G.S.; Sridhar, N. Corrosion inhibition of sensitized and solutionized AA5083. J. Electrochem. Soc. 2015, 162, C449–C456. [Google Scholar] [CrossRef]
- Xia, D.-H.; Pan, C.; Qin, Z.; Fan, B.; Song, S.; Jin, W. Covalent surface modification of LY12 aluminum alloy surface by self-assembly dodecyl phosphate film towards corrosion proteciton. Prog. Org. Coat. 2020, 143, 105638. [Google Scholar] [CrossRef]
- Zhang, R.; Li, J.; Li, Q.; Qi, Y.; Zeng, Z.; Qiu, Y.; Chen, X.; Kairy, S.K.; Thomas, S.; Birbilis, N. Analysing the degree of sensitisation in 5xxx series aluminium alloys using artificial neural networks: A tool for alloy design. Corros. Sci. 2019, 150, 268–278. [Google Scholar] [CrossRef]
- Li, F.; Xiang, D.; Qin, Y.; Pond, R.B.; Slusarski, K. Measurements of degree of sensitization (DoS) in aluminum alloys using EMAT ultrasound. Ultrasonics 2011, 51, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Buczynski, J. Electrochemical Analyses of Etchants Used to Detect Sensitization in Marine-Grade 5XXX Aluminum-Magnesium Alloys. Master’s Thesis, University of Virginia, Charlottesville, VA, USA, 2012. [Google Scholar]
- Yasuda, M. Pitting Corrosion and Intergranular Corrosion of Al and Al-Cu Alloy Single Crystals and Bicrystals. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1988. [Google Scholar]
- Craig, H.L., Jr. Nitric Acid Weight Loss Test for the H116 and H117 Tempers of 5086 and 5456 Aluminum Alloys. In Localized Corrosion—Cause of Metal Failure; ASTM International: West Conshohocken, PA, USA, 1972; pp. 17–37. [Google Scholar]
- Shaw, B.A.; McCosby, M.M.; Abdullah, A.M.; Pickering, H.W. The localized corrosion of Al 6XXX alloys. JOM 2001, 53, 42–46. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. Study of Al alloy corrosion in neutral NaCl by the pitting scan technique. Mater. Chem. Phys. 2010, 121, 523–533. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M.; Sánchez-Amaya, J.M.; González-Rovira, L. Using EIS to analyse specimens of Al–Mg alloy AA5083 treated by thermal activation in cerium salt baths. Corros. Sci. 2008, 50, 1376–1384. [Google Scholar] [CrossRef]
- Ohta, K.; Ishida, H. Comparison among several numerical integration methods for Kramers-Kronig transformation. Appl. Spectrosc. 1988, 42, 952–957. [Google Scholar] [CrossRef]
- Bohren, C.F. What did Kramers and Kronig do and how did they do it? Eur. J. Phys. 2010, 31, 573–578. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. On the mixed nature of cerium conversion coatings. Mater. Corros. 2002, 53, 176–184. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. Inhibition of the corrosion process of alloy AA5083 (Al-Mg) in seawater by cerium cations. An EIS study. Mater. Corros. 2001, 52, 344–350. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interf. Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Hsu, C.H.; Mansfeld, F. Technical note: Concerning the conversion of the constant phase element parameter Y0 into a capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Yao, M.; Shan, W. Dielectric behavior of alumina thin film under high DC electric field prepared by sol–gel method. J. Adv. Dielectr. 2013, 3, 1350017. [Google Scholar] [CrossRef]
- Hirschorna, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musianid, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Benoit, M.; Bataillon, C.; Gwinner, B.; Miserque, F.; Orazem, M.E.; Sanchez-Sanchez, C.M.; Tribollet, B.; Vivier, V. Comparison of different methods for measuring passive film thickness on metals. Electrochim. Acta 2016, 201, 340–347. [Google Scholar] [CrossRef]
- Gao, W.; Wang, D.; Seifi, M.; Lewandowski, J.J. Anisotropy of corrosion and environmental cracking in AA5083-H128 Al-Mg alloy. Mater. Sci. Eng. A 2018, 730, 367–379. [Google Scholar] [CrossRef]
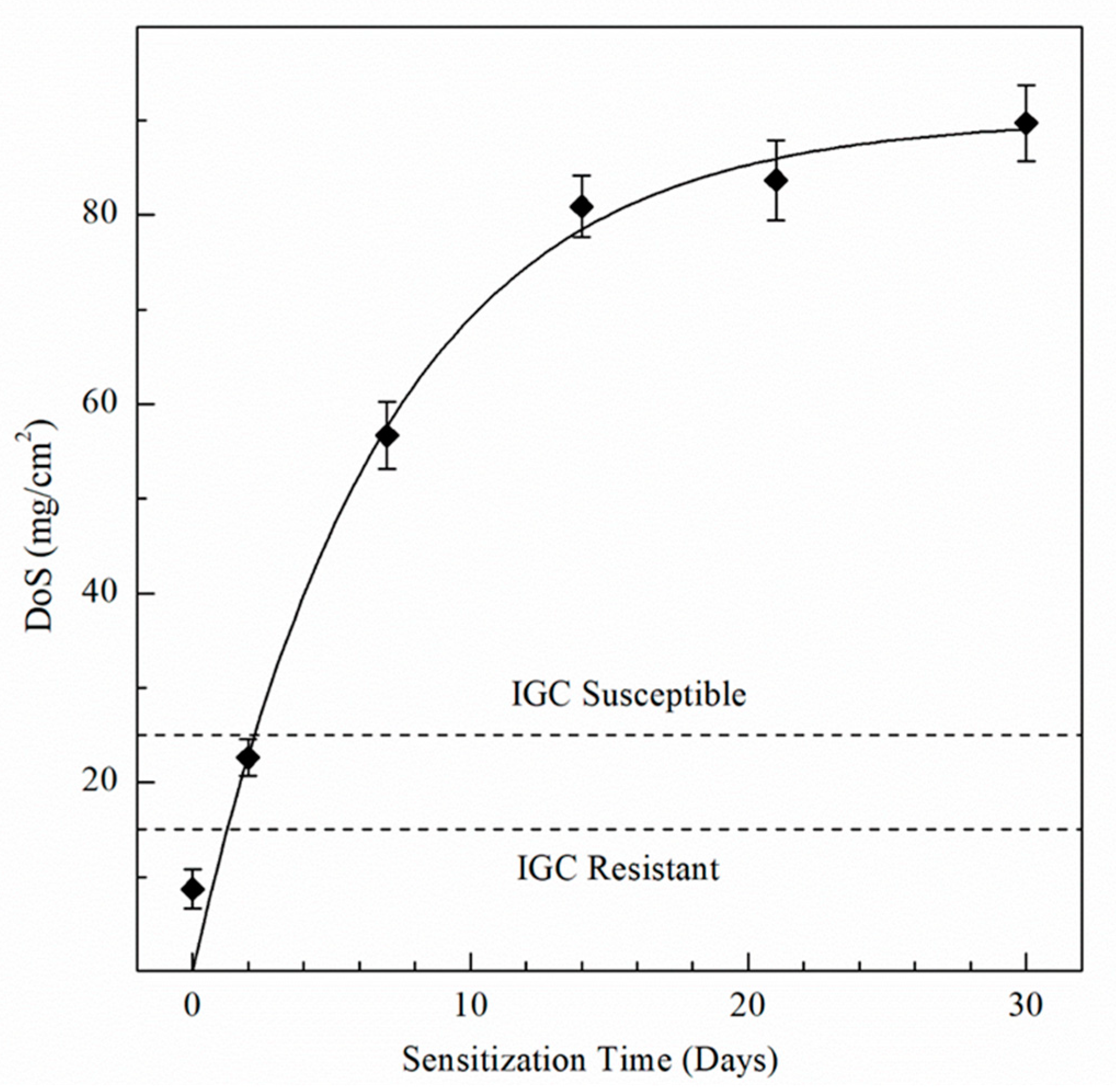

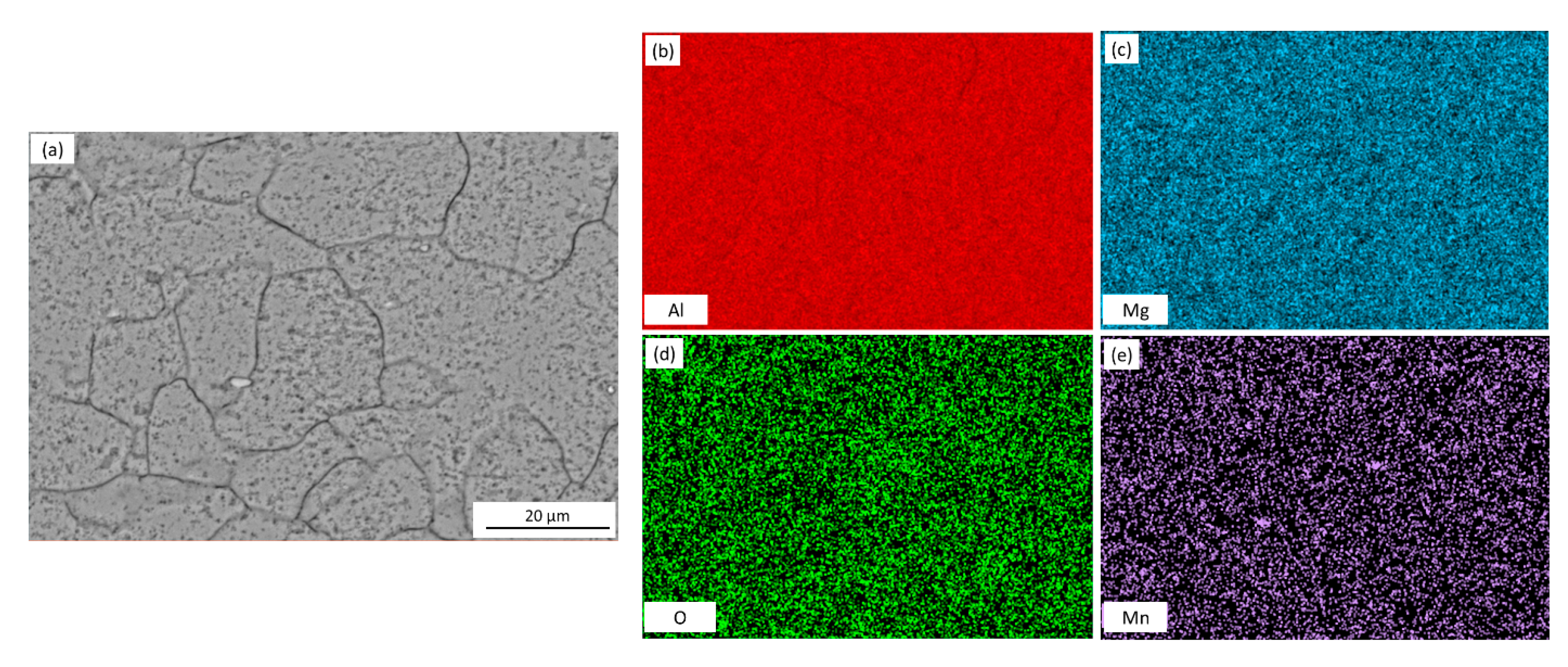


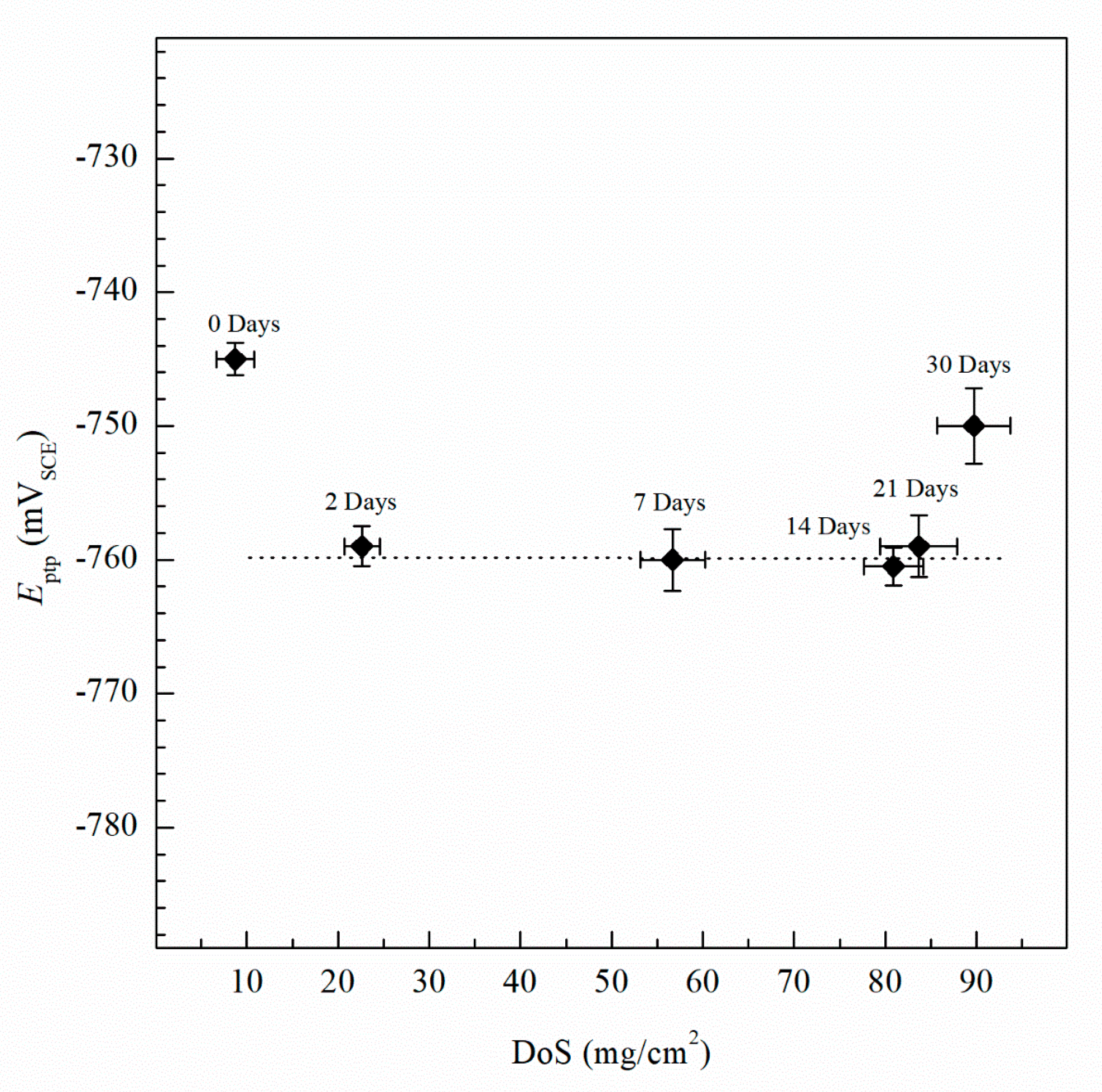

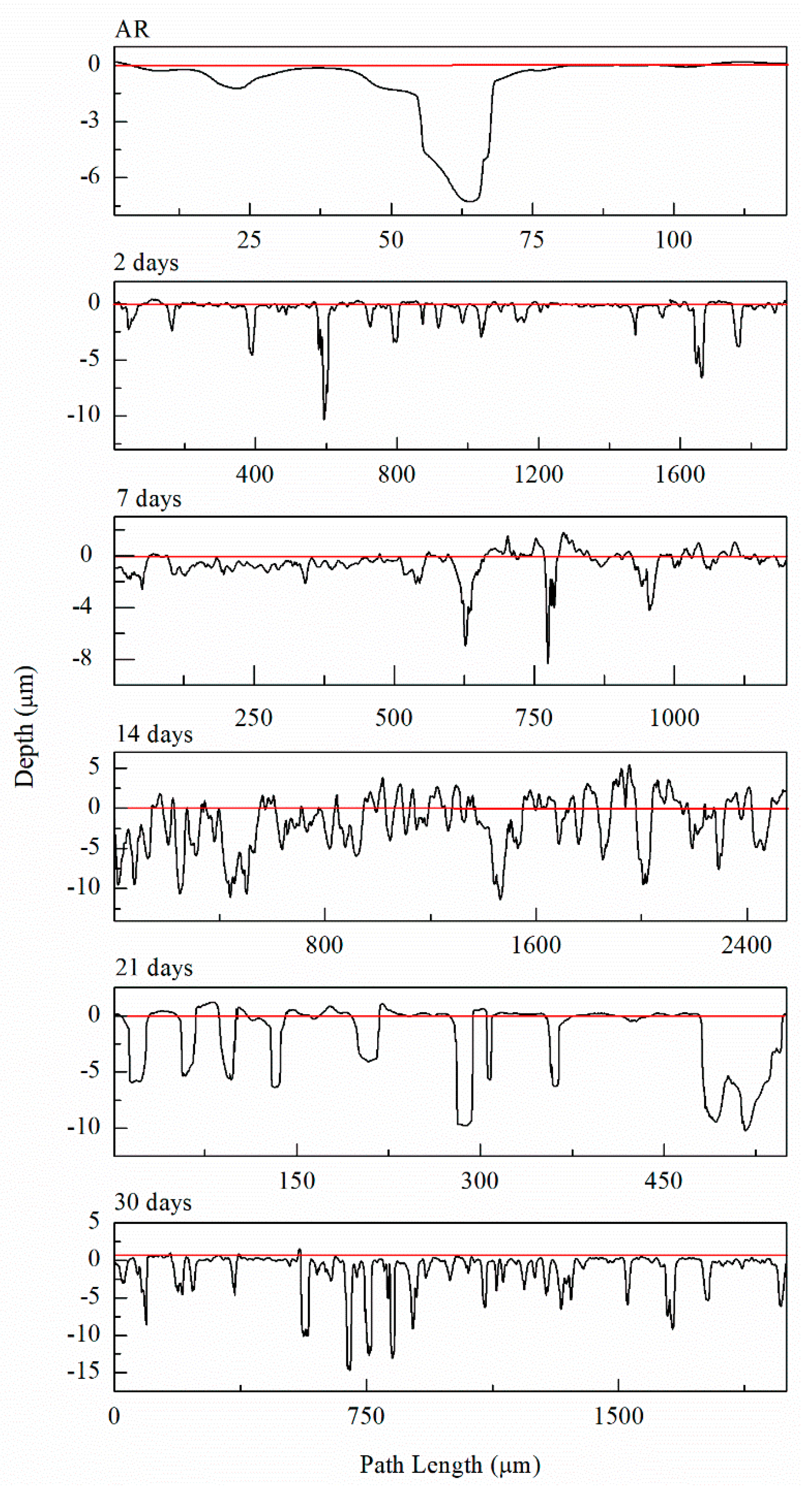

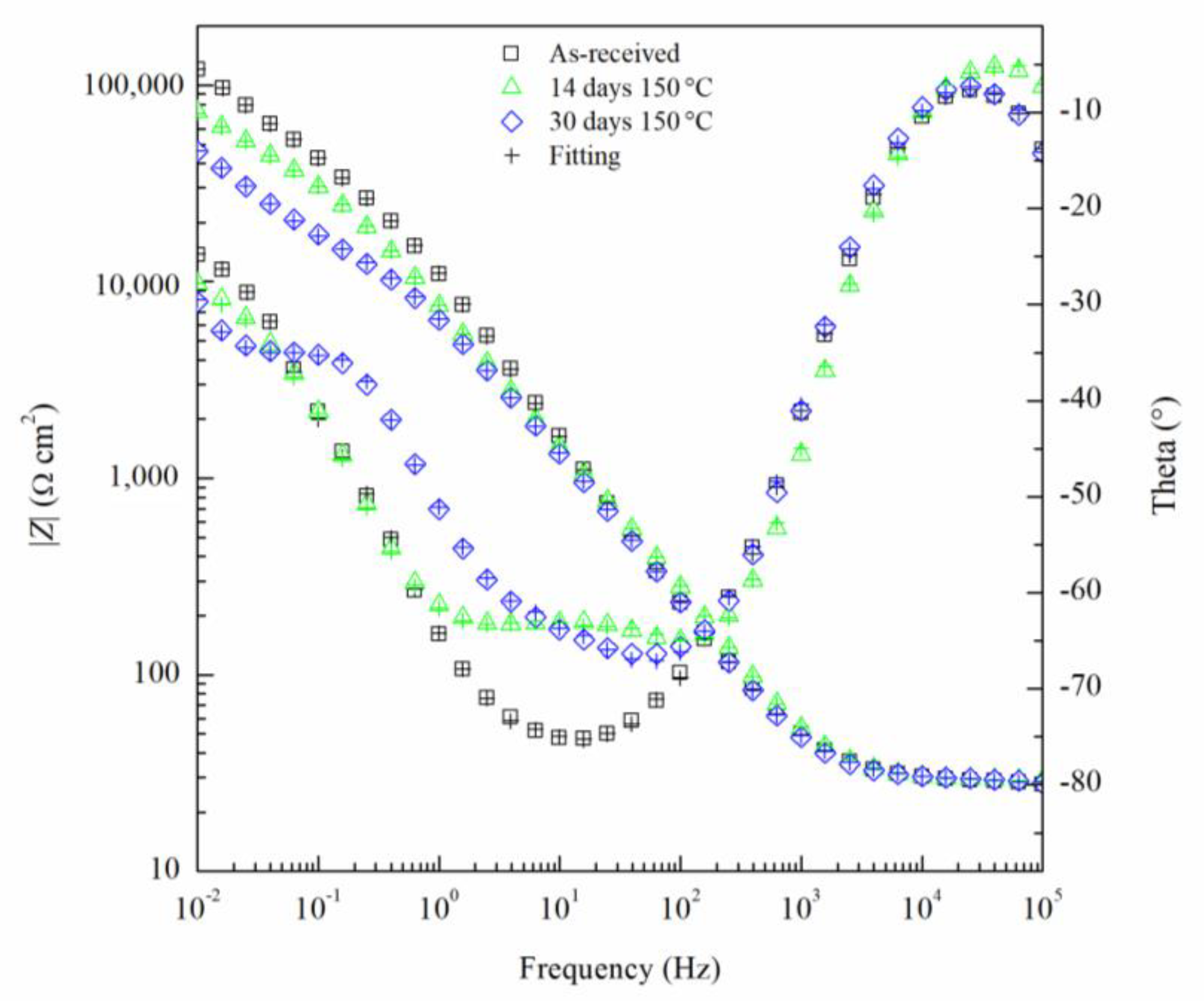
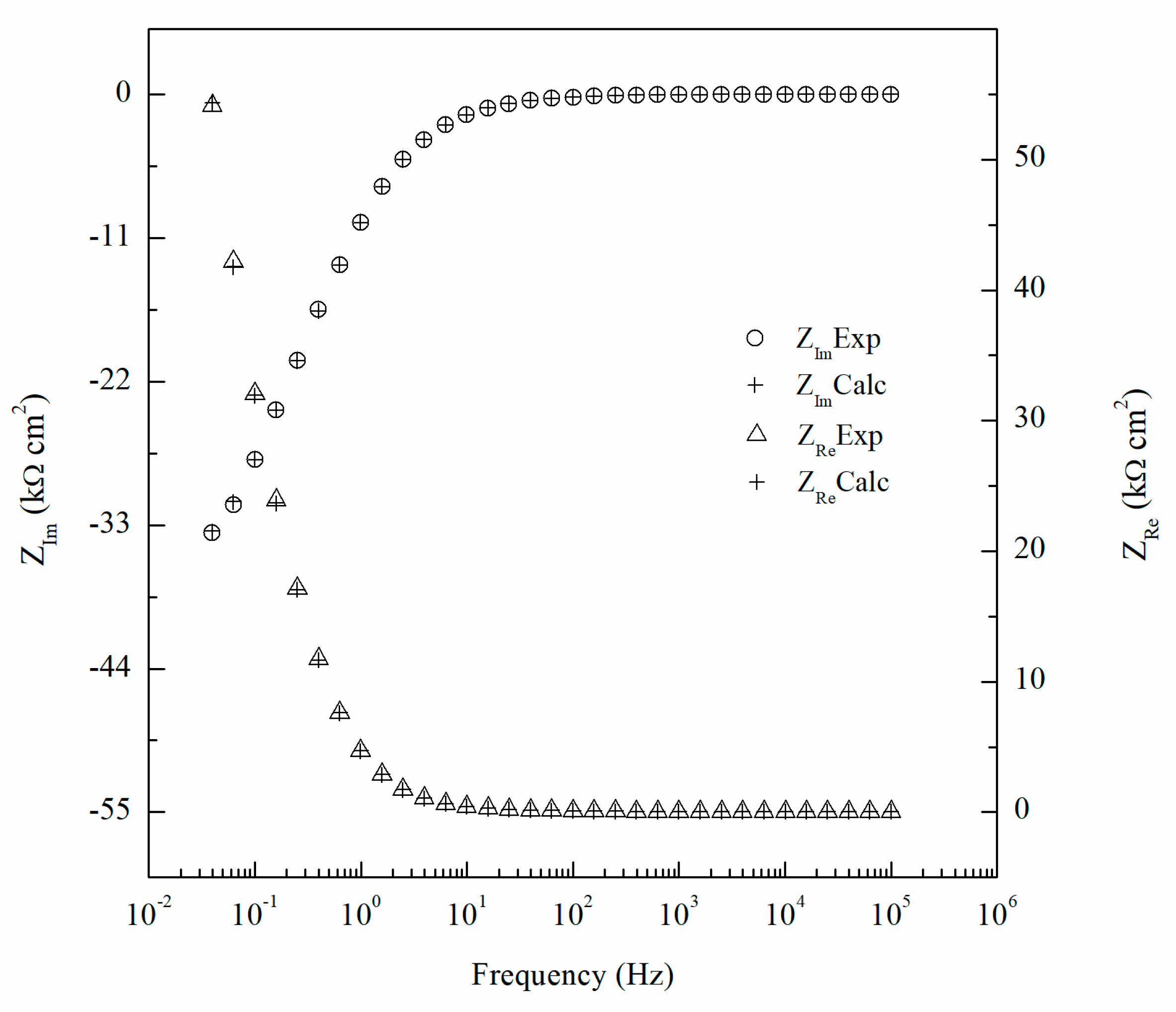
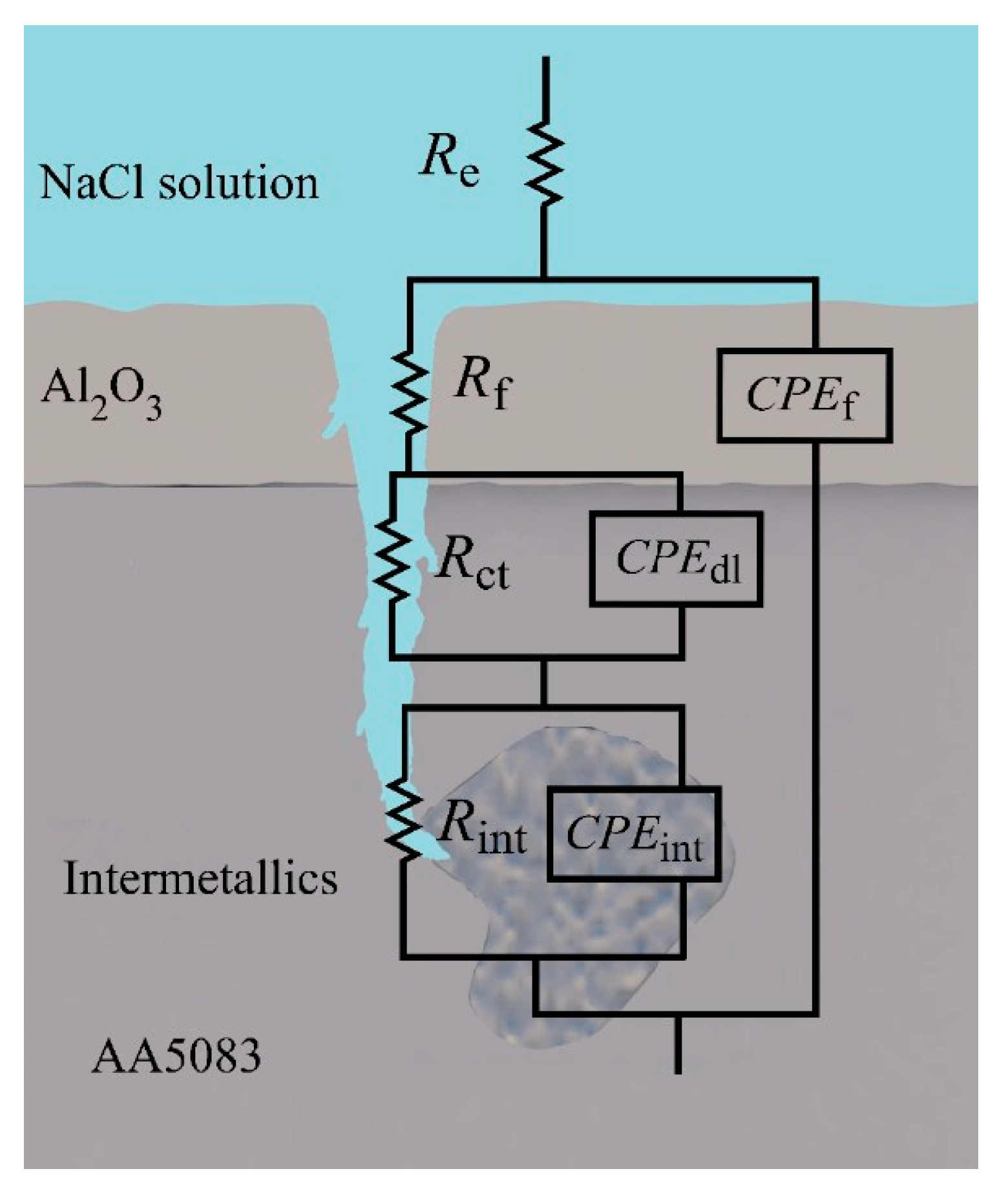
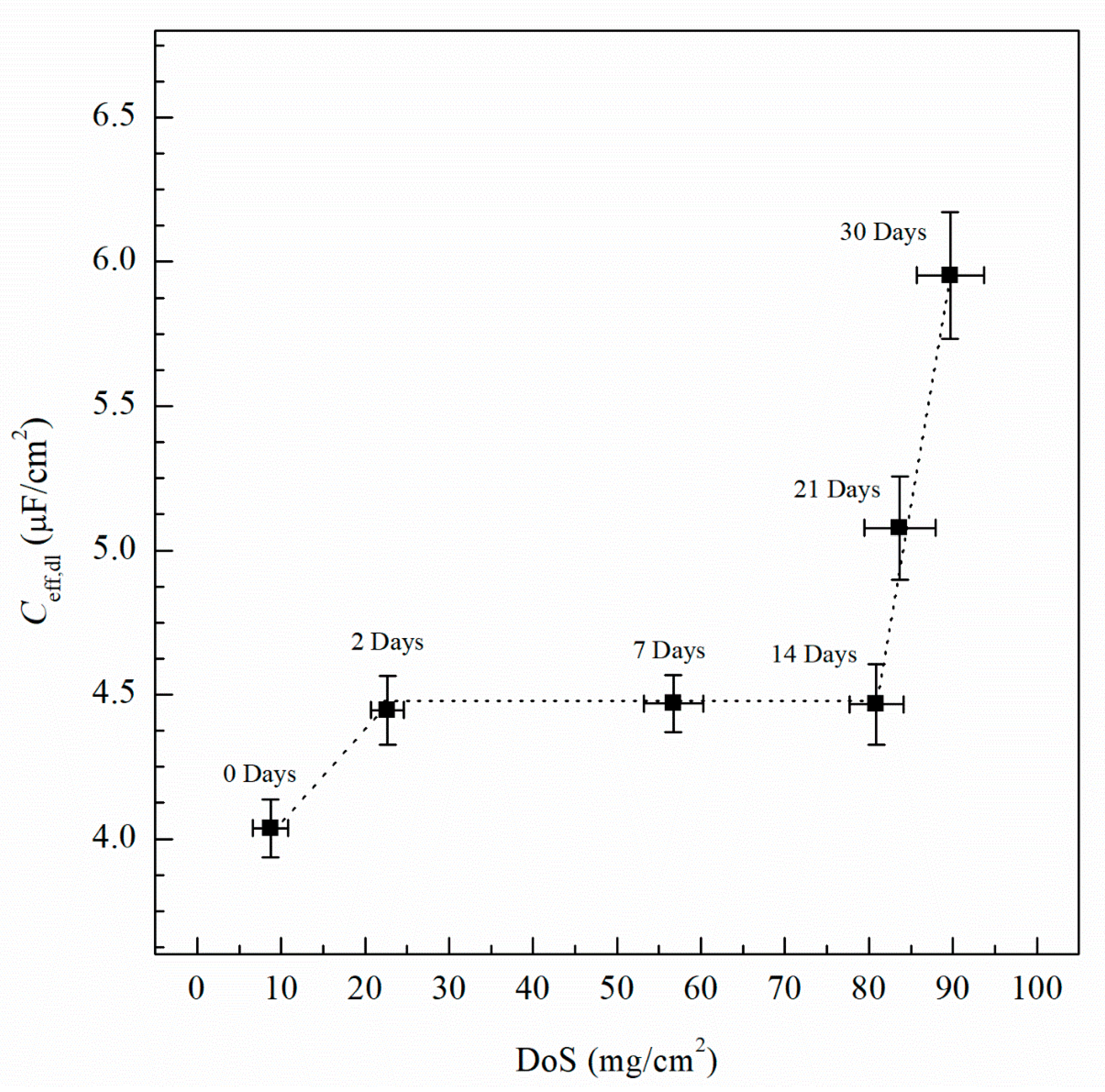
| Element | Mg | Si | Fe | Cu | Mn | Zn | Cr | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 4.4 | 0.08 | 0.19 | 0.026 | 0.56 | 0.004 | 0.077 | 0.015 | Bal. |
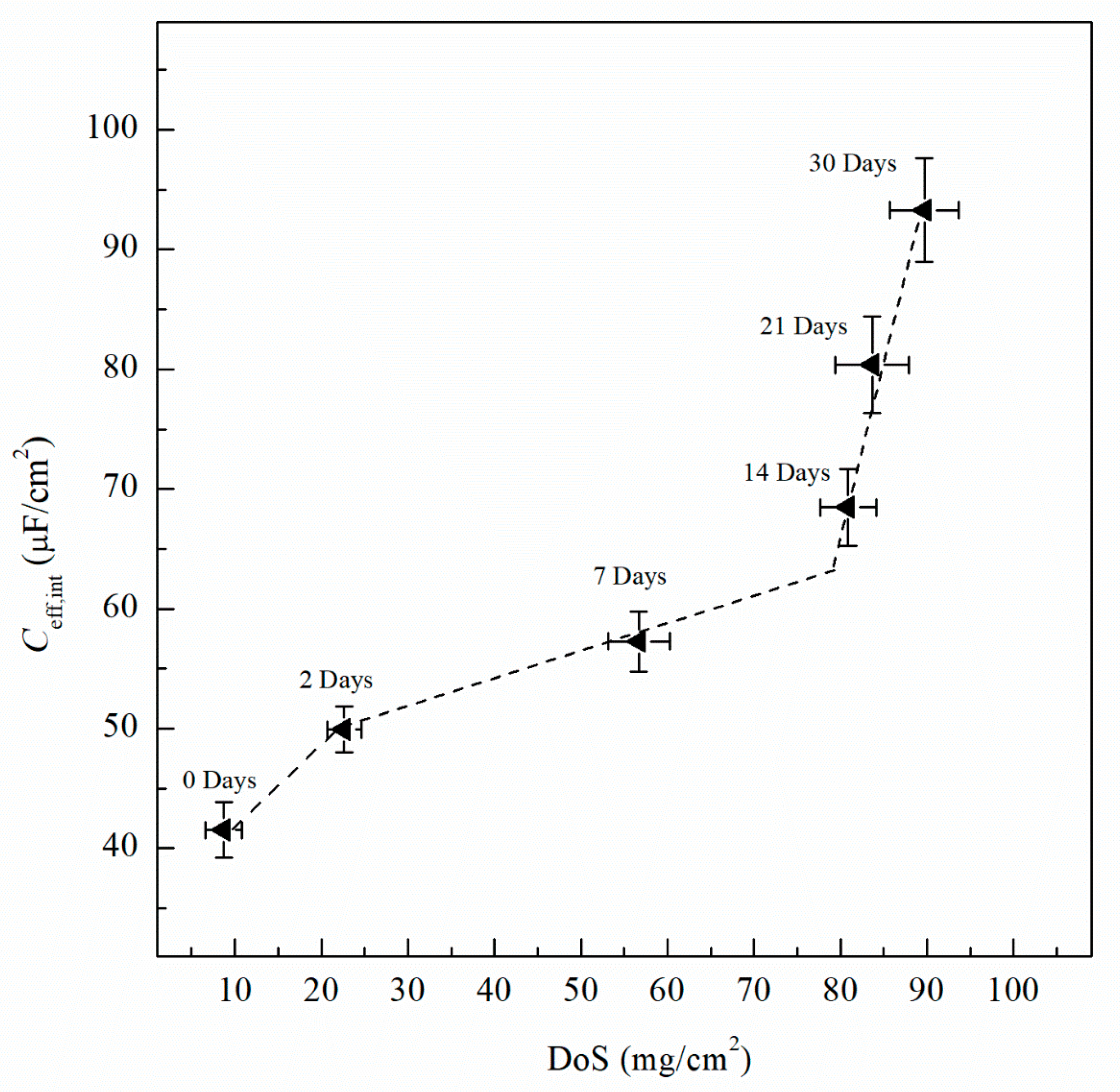
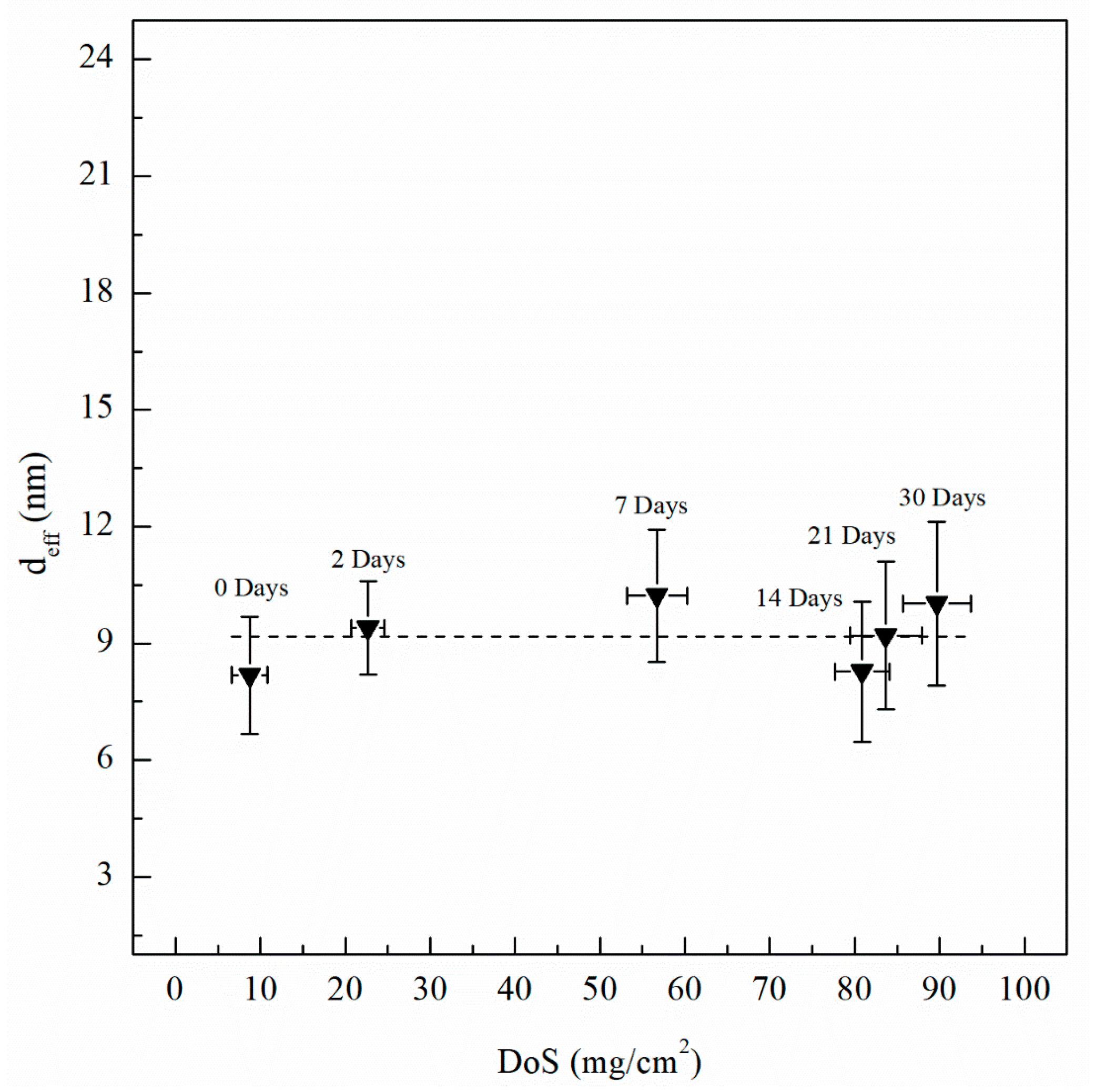
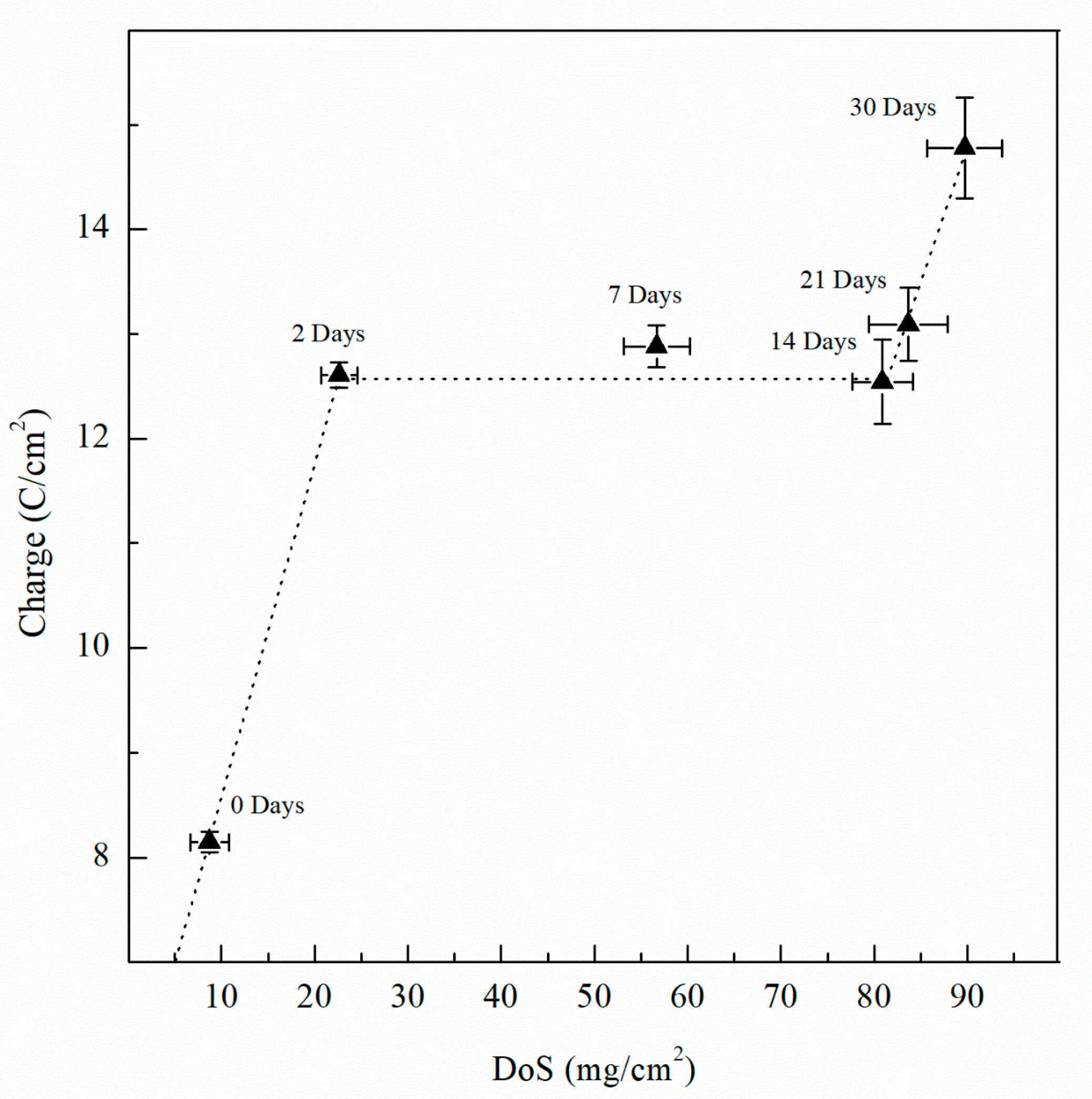
| Sensitization Time, Days | DoS mg/cm2 | icorr A/cm2 | Ecorr mVSCE | Epit mVSCE | Eptp mVSCE | Ceff,f µF/cm2 | deff nm | Ceff,int µF/cm2 | Ceff,dl µF/cm2 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 8 (±2.1) | 1.12 × 10−7 (±3.2 × 10−8) | −737 (±8) | −728 (±3) | −750 (±1) | 33.8 (±3.4) | 8 (±1.5) | 41.9 (±2.3) | 4.01 (±0.10) |
| 2 | 23 (±1.9) | 5.08 × 10−7 (±2.4 × 10−8) | −851 (±12) | −730 (±3) | −759 (±2) | 38.9 (±3.1) | 9 (±1.2) | 50.0 (±1.9) | 4.46 (±0.15) |
| 7 | 57 (±3.5) | 5.71 × 10−7 (±2.6 × 10−8) | −874 (±12) | −734 (±6) | −759 (±2) | 44.5 (±3.5) | 11 (±1.7) | 57.3 (±2.5) | 4.47 (±0.11) |
| 14 | 81 (±3.2) | 6.16 × 10−7 (±3.5 × 10−8) | −884 (±18) | −745 (±8) | −760 (±3) | 34.2 (±3.4) | 8 (±1.7) | 68.5 (±3.2) | 4.49 (±0.15) |
| 21 | 83 (±4.3) | 9.79 × 10−7 (±5.2 × 10−8) | −852 (±16) | −750 (±5) | −758 (±2) | 38.0 (±3.8) | 9 (±1.9) | 80.4 (±4.0) | 5.08 (±0.18) |
| 30 | 89 (±4.0) | 2.33 × 10−6 (±1.8 × 10−7) | −891 (±15) | −756 (±4) | −757 (±4) | 41.4 (±4.2) | 10 (±2.1) | 92.7 (±4.3) | 5.95 (±0.22) |
| Sensitization Time, Days | Re Ω·cm2 | Rf Ω·cm2 | Rct Ω·cm2 | Rint Ω·cm2 | Yf S·cm−2·snint | nint | Ydl S·cm−2·snf | nf | Yint S·cm−2·sndl | ndl | χ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 28.3 | 1589 | 4.80 × 104 | 4.13 × 104 | 1.70 × 10−5 | 0.85 | 1.33 × 10−5 | 0.87 | 1.14 × 10−4 | 0.85 | 2.73 × 10−4 |
| 2 | 27.2 | 1605 | 3.08 × 104 | 4.19 × 104 | 1.83 × 10−5 | 0.84 | 1.34 × 10−5 | 0.88 | 1.87 × 10−4 | 0.80 | 1.22 × 10−4 |
| 7 | 28.6 | 1750 | 2.89 × 104 | 4.24 × 104 | 2.03 × 10−5 | 0.83 | 1.37 × 10−5 | 0.88 | 1.72 × 10−4 | 0.83 | 2.08 × 10−4 |
| 14 | 28.7 | 1859 | 2.09 × 104 | 5.01 × 104 | 1.57 × 10−5 | 0.83 | 1.77 × 10−5 | 0.85 | 1.75 × 10−4 | 0.85 | 5.55 × 10−4 |
| 21 | 27.3 | 1407 | 1.74 × 104 | 5.15 × 104 | 1.92 × 10−5 | 0.85 | 2.87 × 10−5 | 0.81 | 2.33 × 10−4 | 0.83 | 1.51 × 10−4 |
| 30 | 28.3 | 1528 | 1.53 × 104 | 5.43 × 104 | 1.97 × 10−5 | 0.84 | 2.93 × 10−5 | 0.82 | 2.52 × 10−4 | 0.83 | 2.45 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ress, J.; Martin, U.; Bosch, J.; Gupta, R.K.; Bastidas, D.M. Intergranular to Intragranular Pitting Corrosion Transition Mechanism of Sensitized AA5083 at 150 °C. Metals 2020, 10, 1082. https://doi.org/10.3390/met10081082
Ress J, Martin U, Bosch J, Gupta RK, Bastidas DM. Intergranular to Intragranular Pitting Corrosion Transition Mechanism of Sensitized AA5083 at 150 °C. Metals. 2020; 10(8):1082. https://doi.org/10.3390/met10081082
Chicago/Turabian StyleRess, Jacob, Ulises Martin, Juan Bosch, Rajeev K. Gupta, and David M. Bastidas. 2020. "Intergranular to Intragranular Pitting Corrosion Transition Mechanism of Sensitized AA5083 at 150 °C" Metals 10, no. 8: 1082. https://doi.org/10.3390/met10081082
APA StyleRess, J., Martin, U., Bosch, J., Gupta, R. K., & Bastidas, D. M. (2020). Intergranular to Intragranular Pitting Corrosion Transition Mechanism of Sensitized AA5083 at 150 °C. Metals, 10(8), 1082. https://doi.org/10.3390/met10081082








