Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges
Abstract
1. Introduction
2. Powder Metallurgical Processing of γ-TiAl Alloys
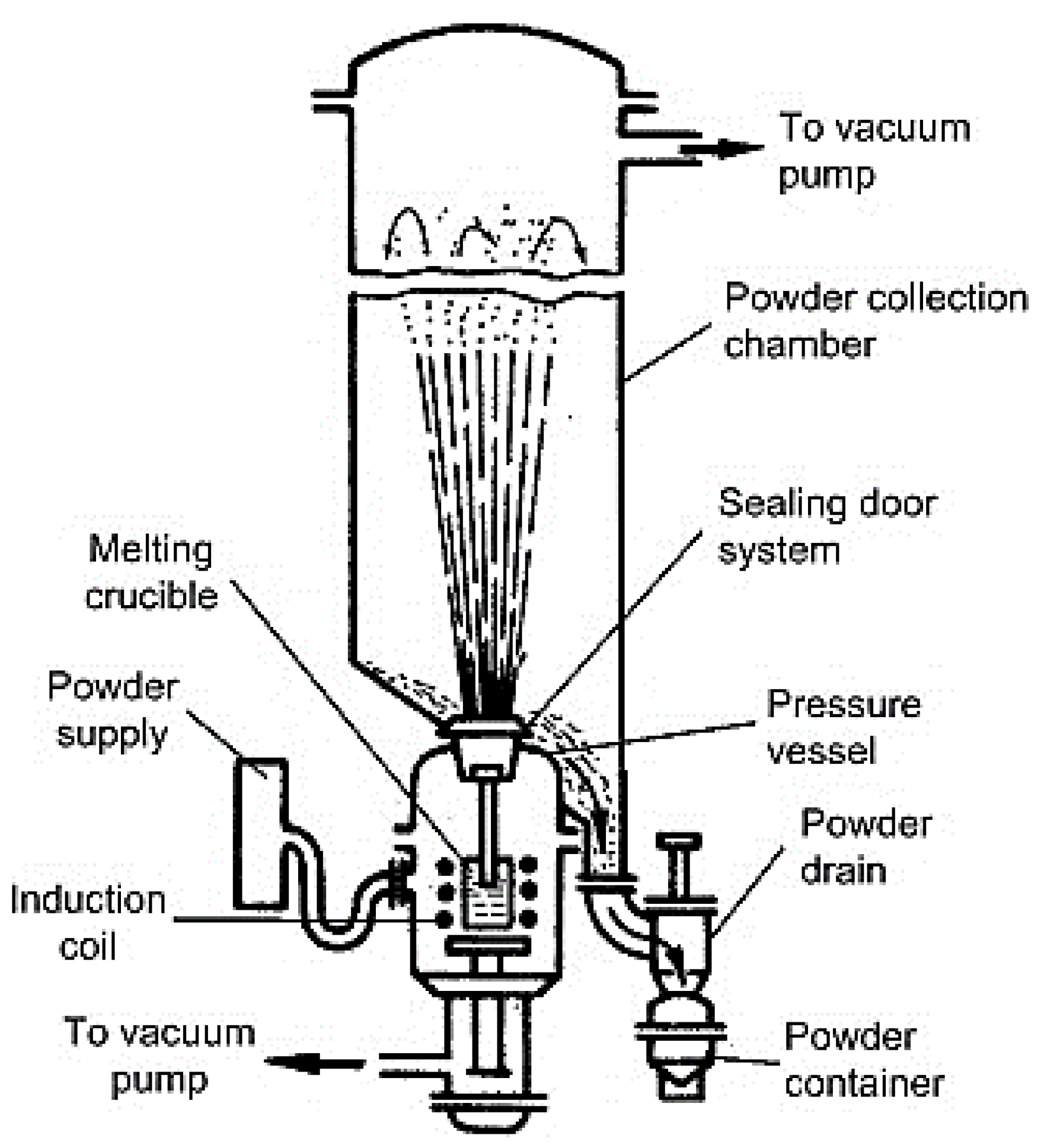
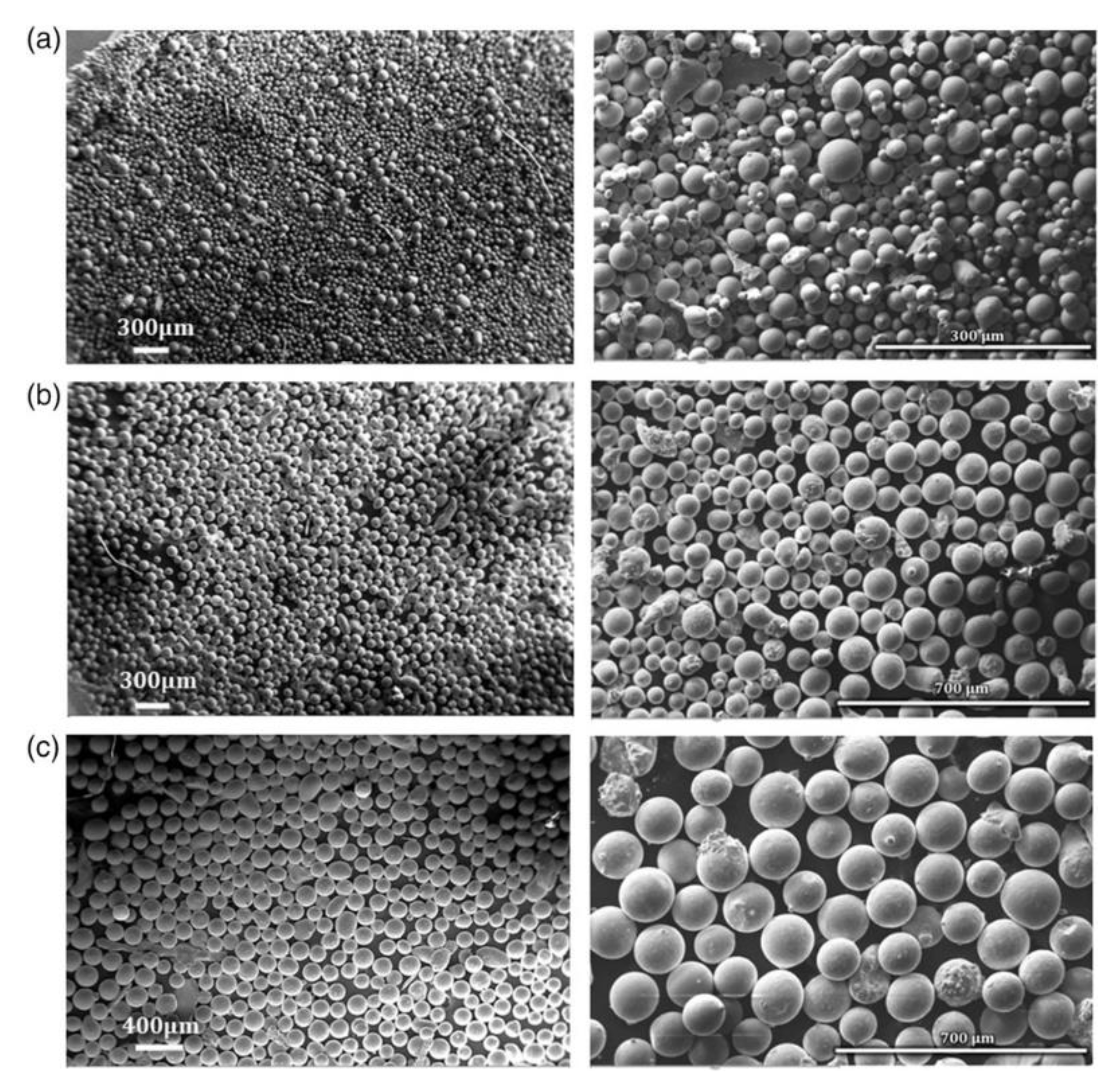
2.1. Gas Atomization (GA)
2.2. Mechanical Milling (MM)
2.3. Mechanical Alloying (MA)
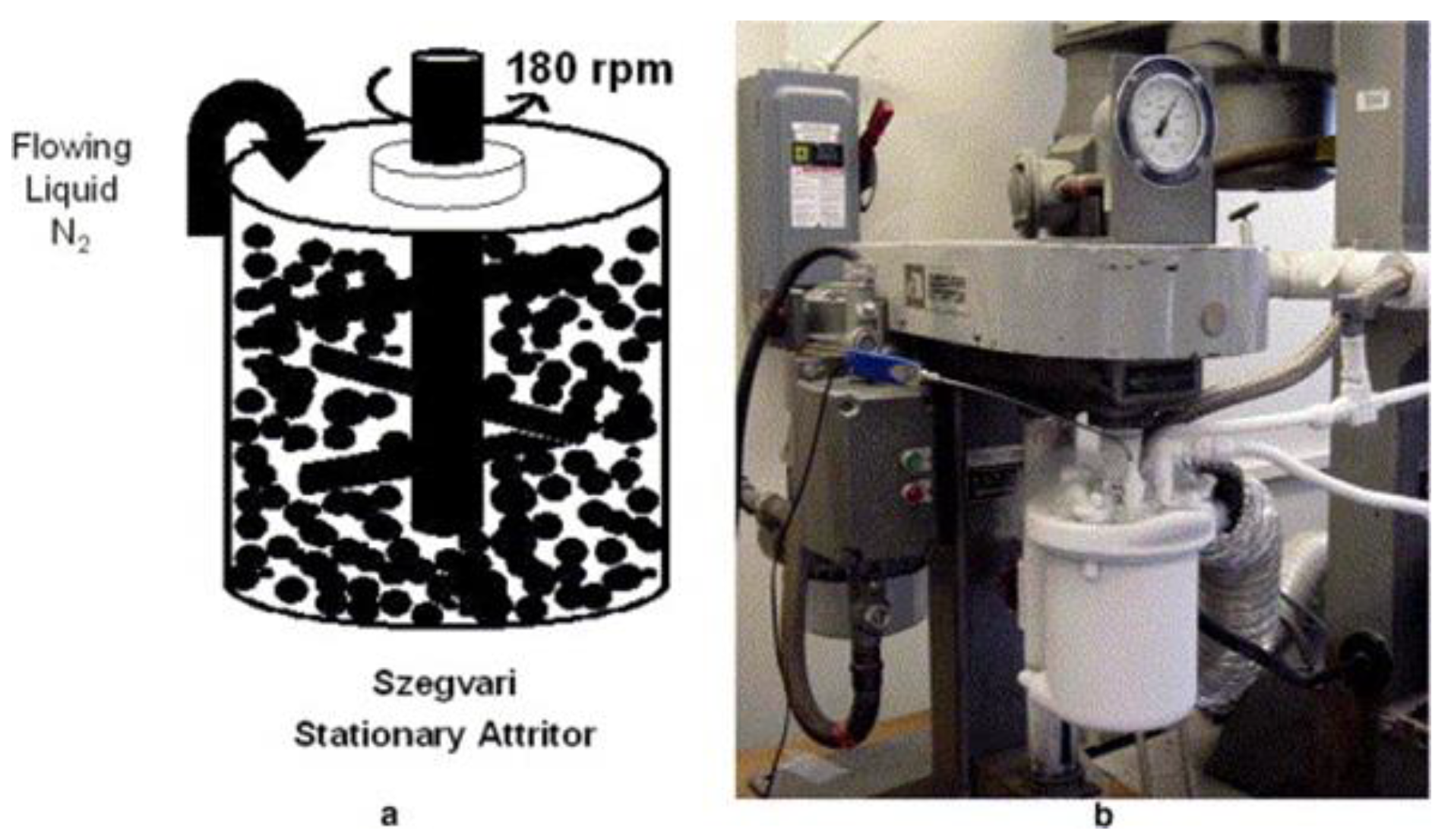
2.4. Cryomilling (CM)
3. Powder Consolidation by SPS
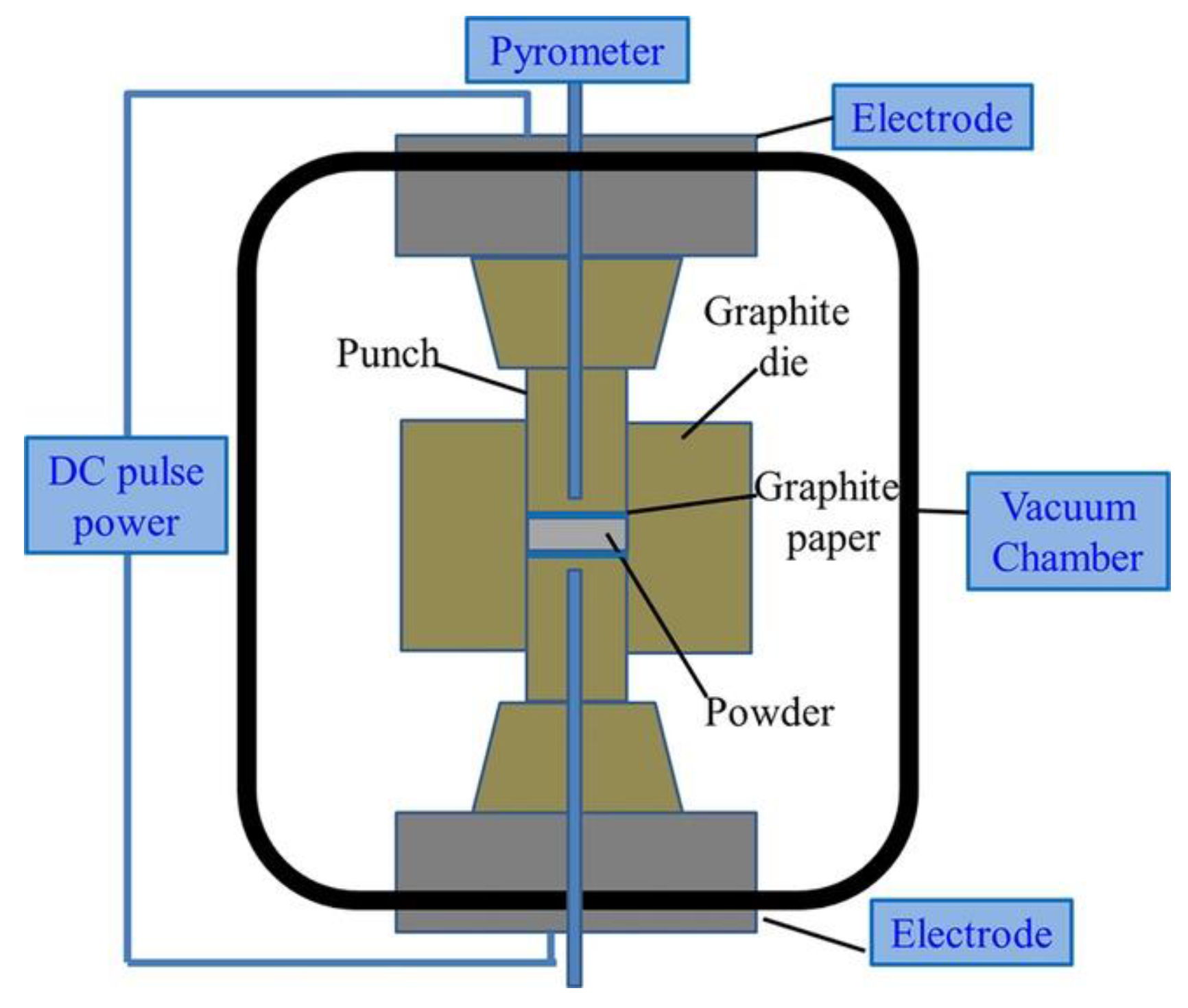
3.1. Basic Operating Principles
3.2. Mechanisms of Sintering in SPS
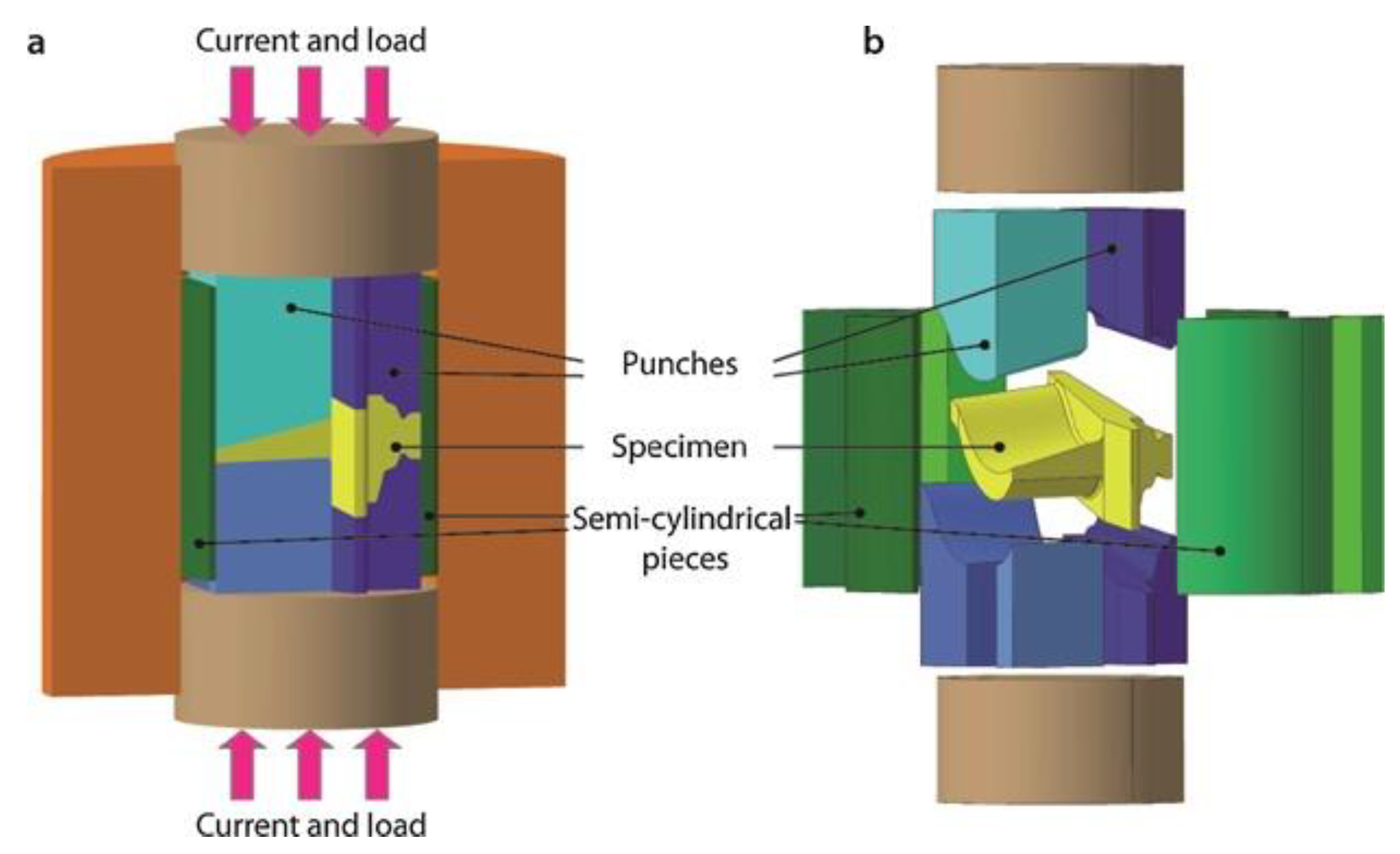
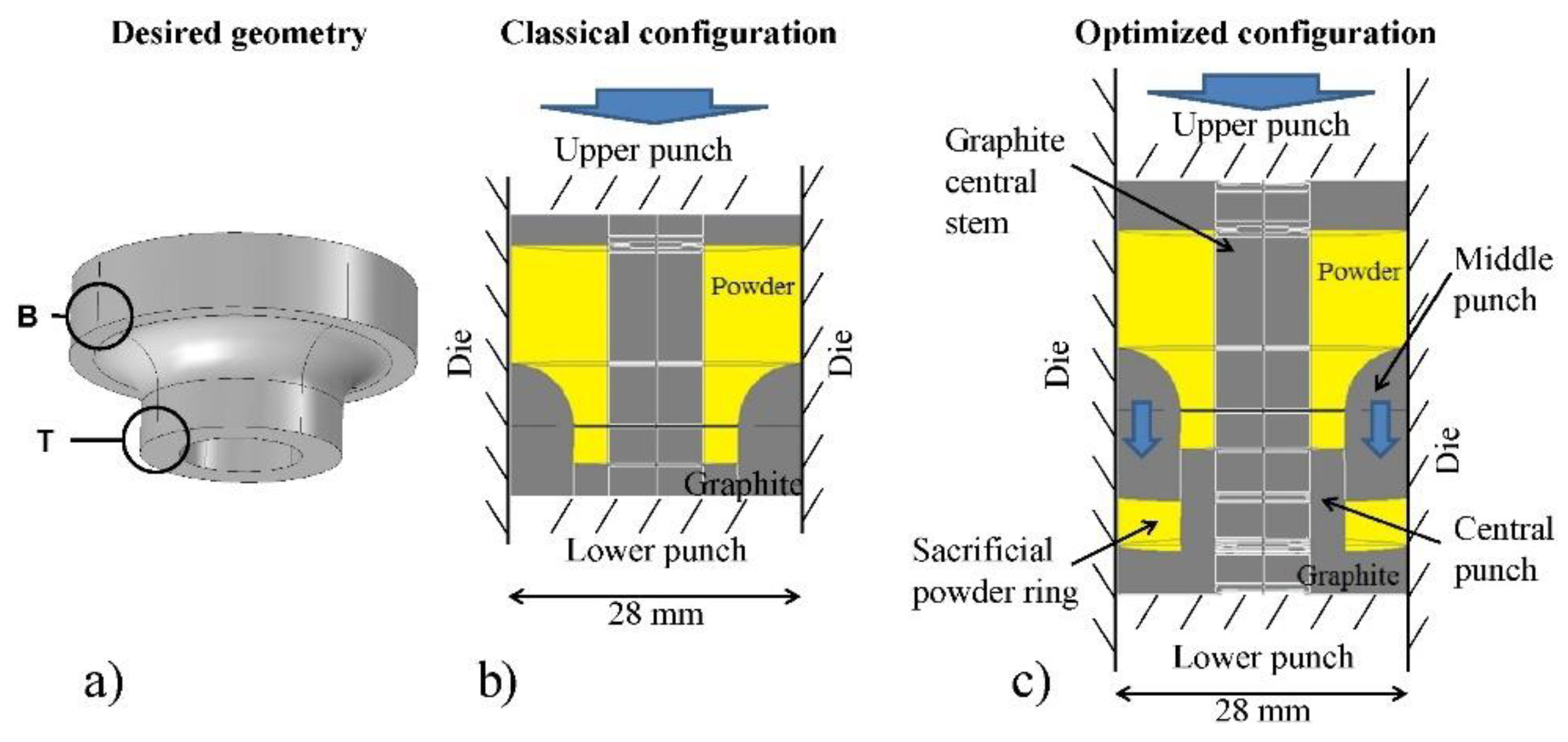
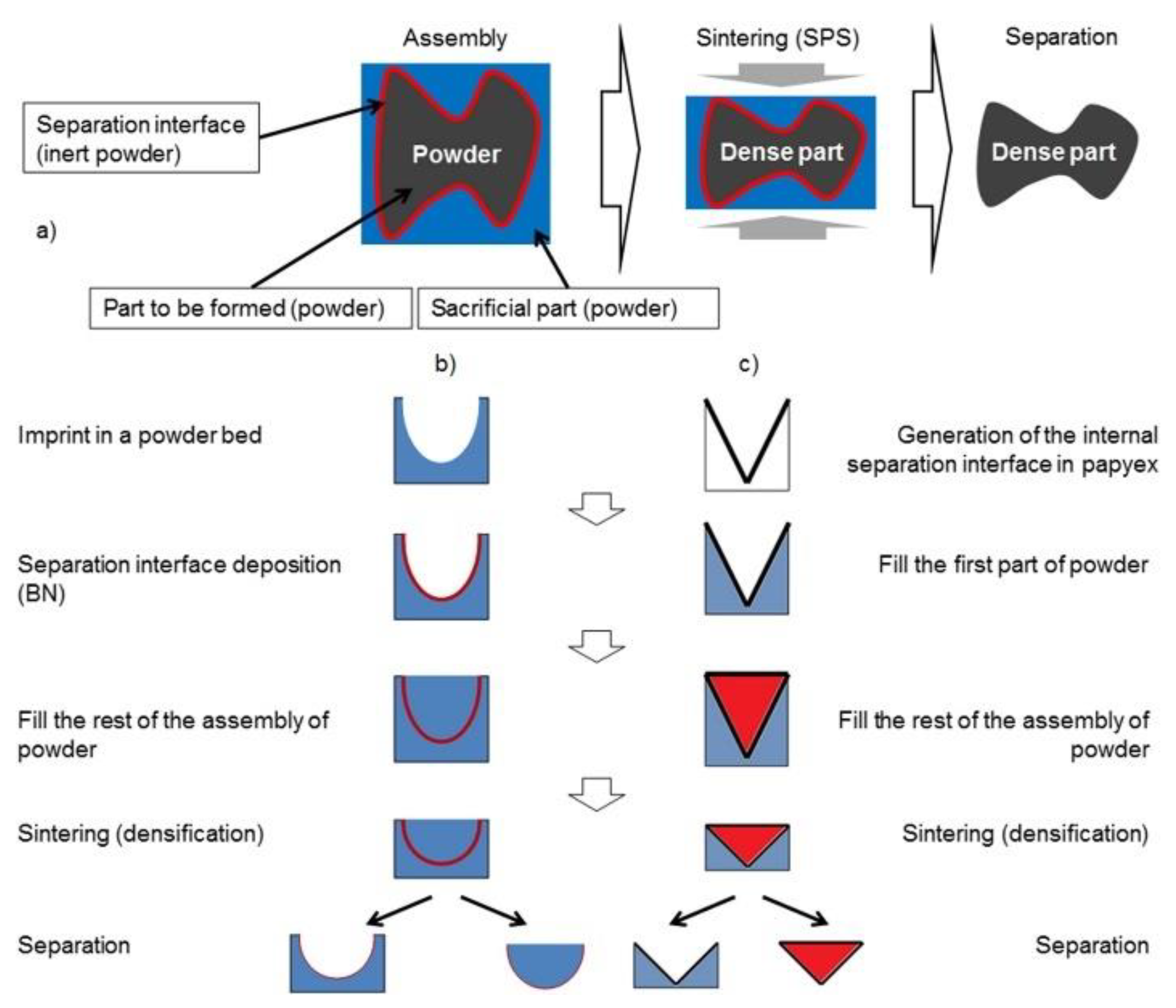
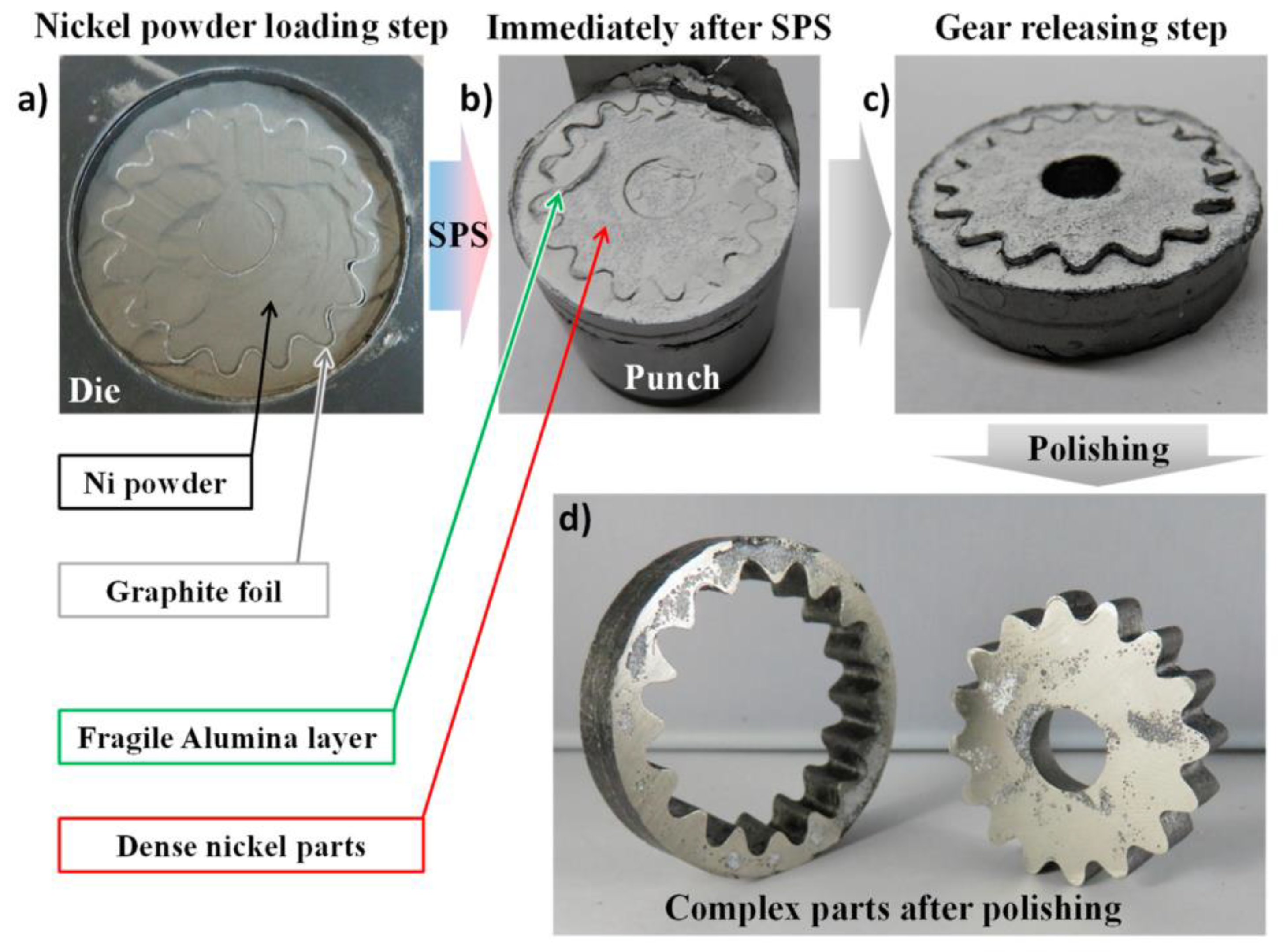
3.3. SPS Modeling for Complex-Net Shaping
3.4. Carburation at High Temperature during SPS
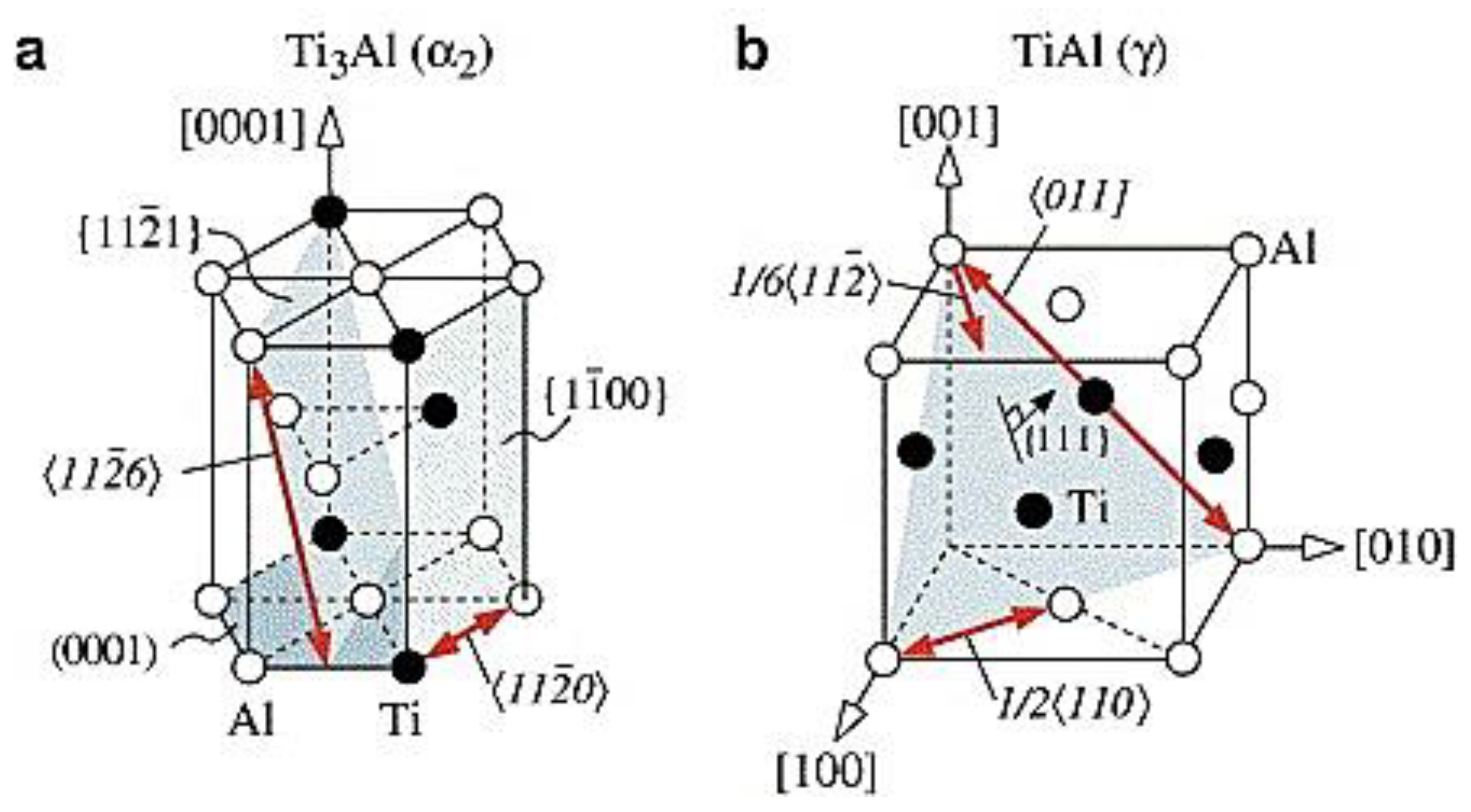
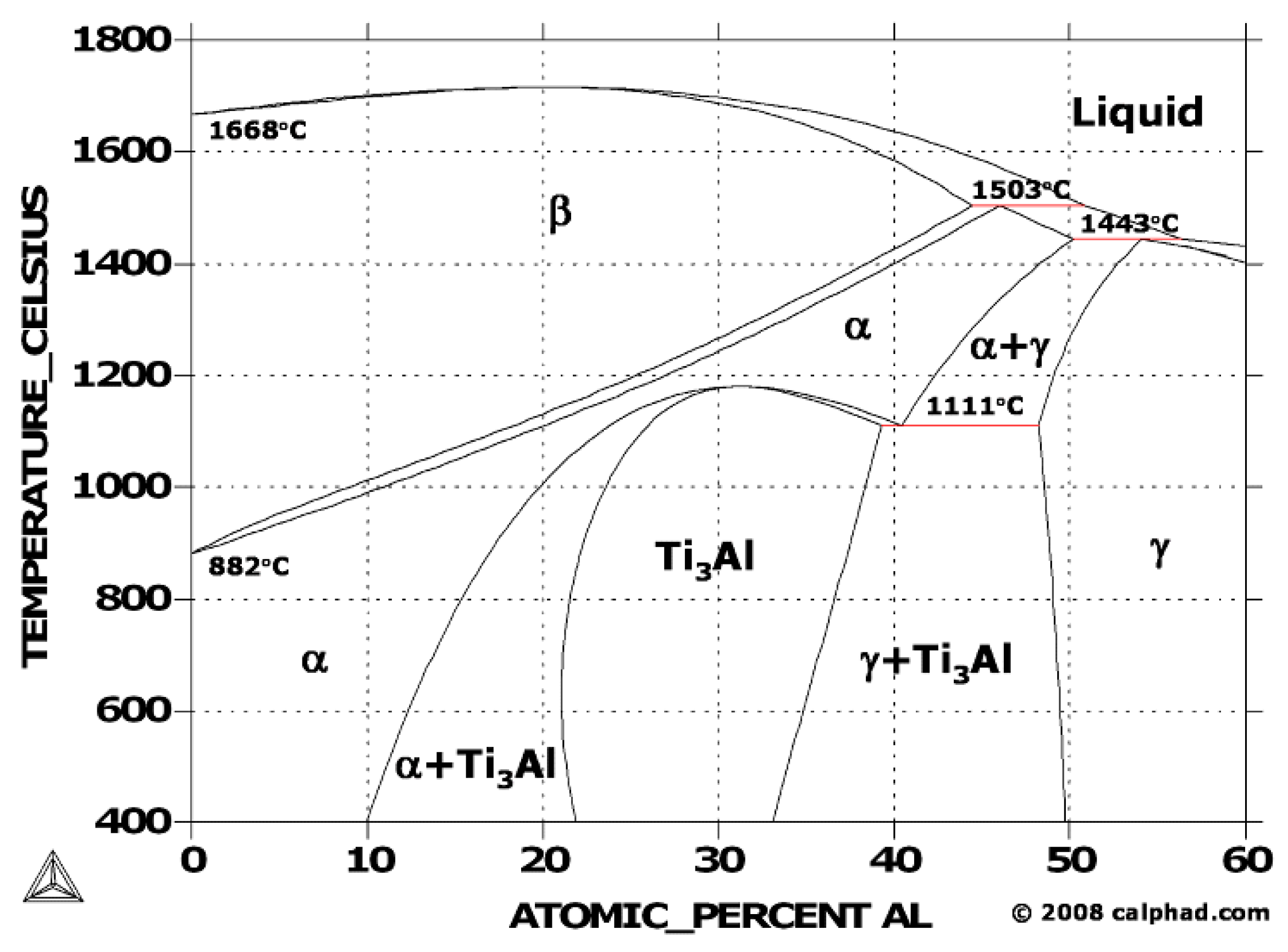
4. Crystallographic Phases and Microstructures
4.1. Crystallographic Phases
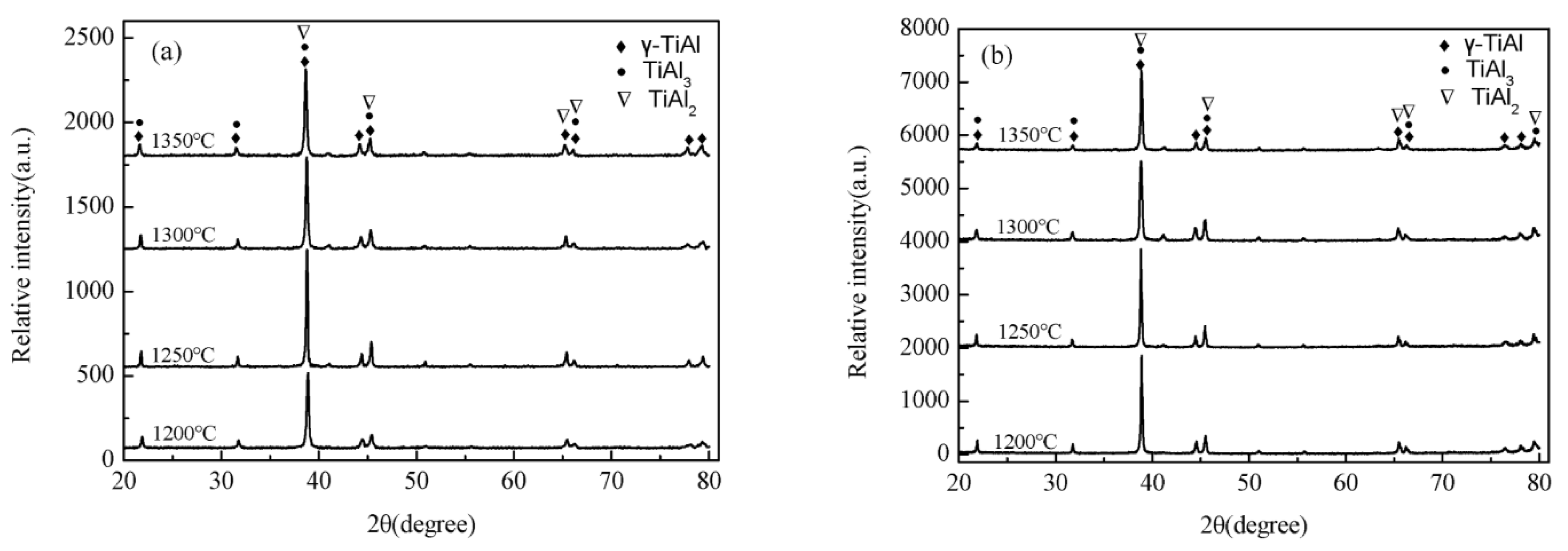
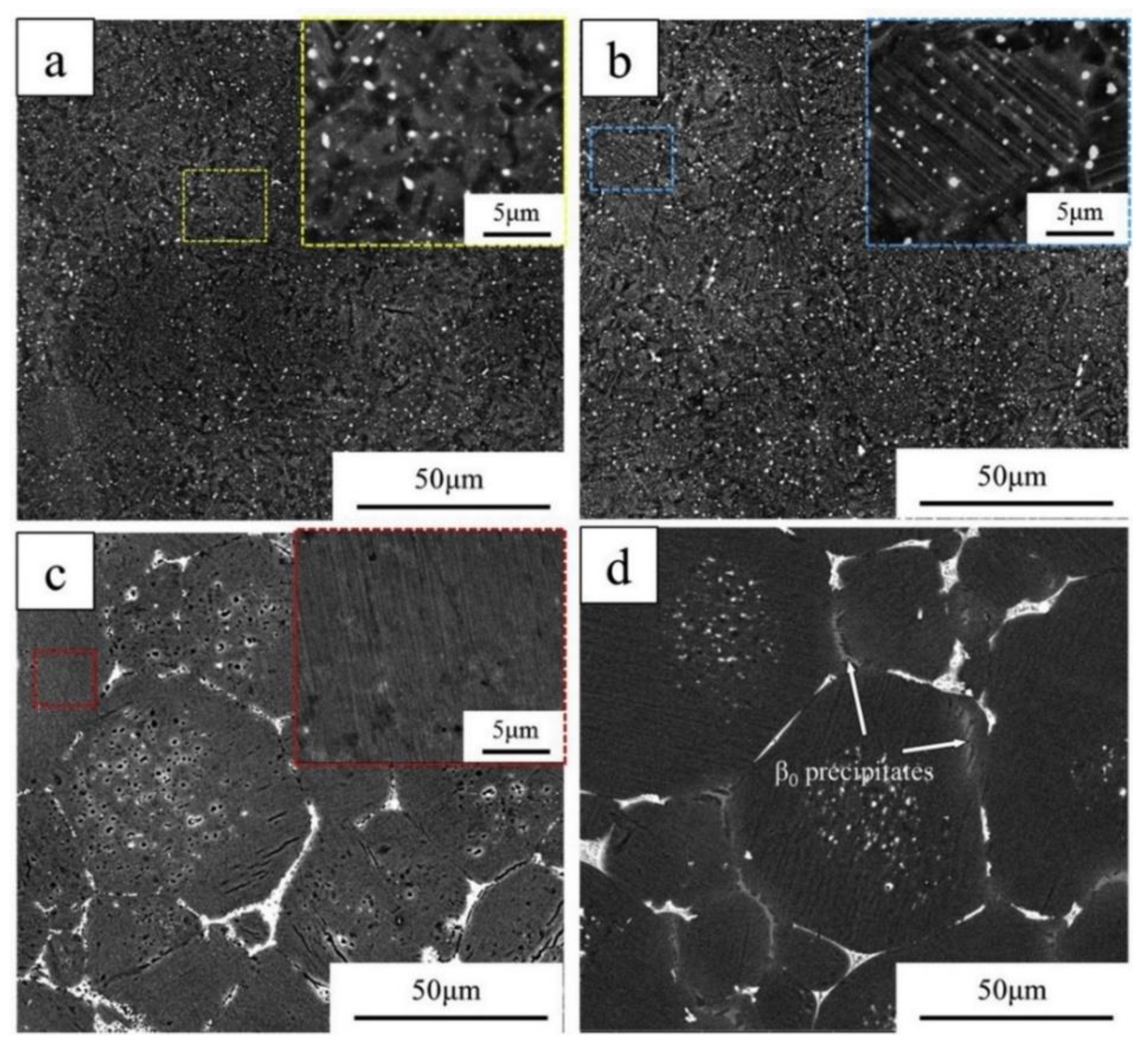
4.2. Microstructures of TiAl
5. Microstructure-Property Relations
6. Alloy Development
6.1. 1st and 2nd Generation of TiAl alloys
6.2. 3rd and 4th Generation of TiAl Alloys
7. Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taub, A.I.; Fleischer, R.L. Intermetallic compounds for high-temperature structural use. Science 1989, 243, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Wolff, I.M.; Hill, P.J. Platinum metals-based intermetallics for high-temperature service. Platin. Met. Rev. 2000, 44, 158–166. [Google Scholar]
- Muktinutalapati, N.R. Materials for gas turbines–an overview. In Advances in Gas Turbine Technology; IntechOpen: London, UK, 2011; Available online: https://www.intechopen.com/books/advances-in-gas-turbine-technology/materials-for-gas-turbines-an-overview (accessed on 1 May 2020).
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Clemens, H.; Smarsly, W. Light-weight intermetallic titanium aluminides–status of research and development. Adv. Mater. Res. 2011, 278, 551–556. [Google Scholar] [CrossRef]
- Voice, W. The future use of gamma titanium aluminides by Rolls-Royce. Aircr. Eng. Aerosp. Technol. 1999, 71, 337–340. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Weimer, M.; Kelly, T.; Suzuki, A.; Subramanian, P.R. The science, technology, and implementation of TiAl alloys in commercial aircraft engines. MRS Online Proc. Libr. Arch. 2013, 1516, 49–58. [Google Scholar] [CrossRef]
- Loria, E.A. Quo vadis gamma titanium aluminide. Intermetallics 2001, 9, 997–1001. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y. 27-Powder metallurgy titanium aluminide alloys. In Titanium Powder Metallurgy; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 515–531. [Google Scholar]
- Lamirand, M.; Bonnentien, J.L.; Ferrière, G.; Guérin, S.; Chevalier, J.P. Relative effects of chromium and niobium on microstructure and mechanical properties as a function of oxygen content in TiAl alloys. Scr. Mater. 2007, 56, 325–328. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, H.; Zhang, H.; Chen, R.; Guo, J.; Fu, H. Influence of Mn addition on the microstructure and mechanical properties of a directionally solidified γ-TiAl alloy. Mater. Charact. 2018, 137, 133–141. [Google Scholar] [CrossRef]
- Imayev, V.; Oleneva, T.; Imayev, R.; Christ, H.J.; Fecht, H.J. Microstructure and mechanical properties of low and heavy alloyed γ-TiAl + α2-Ti3Al based alloys subjected to different treatments. Intermetallics 2012, 26, 91–97. [Google Scholar] [CrossRef]
- Larsen, J.M.; Worth, B.D.; Balsone, S.J.; Jones, J.W. An Overview of the Structural Capability of Available Gamma Titanium Aluminide Alloys; U.S. Department of EnergyOffice of Scientific and Technical Informatio: Oak Ridge, TN, USA, 1995.
- Tang, J.; Huang, B.; Liu, W.; He, Y.; Zhou, K.; Wu, A.; Peng, K.; Qin, W.; Du, Y. A high ductility TiAl alloy made by two-step heat treatment. Mater. Res. Bull. 2003, 38, 2019–2024. [Google Scholar] [CrossRef]
- Lasalmonie, A. Intermetallics: Why is it so difficult to introduce them in gas turbine engines? Intermetallics 2006, 14, 1123–1129. [Google Scholar] [CrossRef]
- Lagos, M.A.; Agote, I. SPS synthesis and consolidation of TiAl alloys from elemental powders: Microstructure evolution. Intermetallics 2013, 36, 51–56. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Couret, A.; Molénat, G.; Galy, J.; Thomas, M. Microstructures and mechanical properties of TiAl alloys consolidated by spark plasma sintering. Intermetallics 2008, 16, 1134–1141. [Google Scholar] [CrossRef]
- Jaramillo, D.; Cuenca, R.; Juárez, F. Sintering comparison of NiCoCrAl-Ta powder processed by hot pressing and spark plasma. Powder Technol. 2012, 221, 264–270. [Google Scholar]
- Chang, I.; Zhao, Y. Advances in Powder Metallurgy: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Martín, A.; Cepeda-Jiménez, C.M.; Pérez-Prado, M.T. Gas atomization of γ-TiAl Alloy Powder for Additive Manufacturing. Adv. Eng. Mater. 2020, 22, 1900594. [Google Scholar] [CrossRef]
- Neikov, O.D. Chapter 4-Atomization and Granulation. In Handbook of Non-Ferrous Metal Powders: Technologies and Applications; Neikov, O.D., Naboychenko, S.S., Yefimov, N.A., Second, E., Eds.; Elsevier: Oxford, UK, 2019; pp. 125–185. [Google Scholar]
- Gerling, R.; Clemens, H.; Schimansky, F.P. Powder Metallurgical Processing of Intermetallic Gamma Titanium Aluminides. Adv. Eng. Mater. 2004, 6, 23–38. [Google Scholar] [CrossRef]
- Sun, P.; Fang, Z.Z.; Zhang, Y.; Xia, Y. Review of the methods for production of spherical Ti and Ti alloy powder. JOM 2017, 69, 1853–1860. [Google Scholar] [CrossRef]
- Antony, L.V.M.; Reddy, R.G. Processes for production of high-purity metal powders. JOM 2003, 55, 14–18. [Google Scholar] [CrossRef]
- Franz, H.; Plochl, L.; Schimansky, F.P. Recent advances of titanium alloy powder production by ceramic-free inert gas atomization. In Proceedings of the Titanium 2008, Las Vegas, NV, USA, 21–24 September 2008. [Google Scholar]
- Gu, X.; Cao, F.; Liu, N.; Zhang, G.; Yang, D.; Shen, H.; Zhang, D.; Song, H.; Sun, J. Microstructural evolution and mechanical properties of a high yttrium containing TiAl based alloy densified by spark plasma sintering. J. Alloy. Compd. 2020, 819, 153264. [Google Scholar] [CrossRef]
- Liu, B.; Wang, M.; Du, Y.; Li, J. Size-Dependent Structural Properties of a High-Nb TiAl Alloy Powder. Materials 2020, 13, 161. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhao, J.L.; Qian, M. Spark Plasma Sintering of Ti-48Al-2Cr-2Nb Alloy Powder and Characterization of an Unexpected Phase. JOM 2019, 71, 2556–2563. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liu, Y.; Li, J.; He, L.; Zhang, C. Densification and microstructural evolution of a high niobium containing TiAl alloy consolidated by spark plasma sintering. Intermetallics 2015, 64, 70–77. [Google Scholar] [CrossRef]
- Xiao, S.L.; Jing, T.; Xu, L.J.; Chen, Y.Y.; Yu, H.B.; Han, J.C. Microstructures and mechanical properties of TiAl alloy prepared by spark plasma sintering. Trans. Nonferrous Met. Soc. China 2009, 19, 1423–1427. [Google Scholar] [CrossRef]
- Hadef, F. Synthesis and disordering of B2 TM-Al (TM=Fe, Ni, Co) intermetallic alloys by high energy ball milling: A review. Powder Technol. 2017, 311, 556–578. [Google Scholar] [CrossRef]
- Aliofkhazraei, M. Handbook of Mechanical Nanostructuring; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V.V. The science and technology of mechanical alloying. Mater. Sci. Eng. A 2001, 304, 151–158. [Google Scholar] [CrossRef]
- Yadav, T.P.; Yadav, R.M.; Singh, D.P. Mechanical milling: A top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Oehring, M.; Klassen, T.; Bormann, R. The formation of metastable Ti–Al solid solutions by mechanical alloying and ball milling. J. Mater. Res. 1993, 8, 2819–2829. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, L.; Lai, M.O. Study of thermal stability of mechanically alloyed Ti–75% Al powders. J. Alloy. Compd. 2000, 297, 211–218. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liu, Y.; Zhao, S.; Li, J. Microstructure characterization and mechanical properties of TiAl-based alloys prepared by mechanical milling and spark plasma sintering. Mater. Charact. 2017, 128, 75–84. [Google Scholar] [CrossRef]
- Shulong, X.; Lijuan, X.; Hongbao, Y.; Yuyong, C. Effect of Y Addition on Microstructure and Mechanical Properties of TiAl-based Alloys Prepared by SPS. Rare Met. Mater. Eng. 2013, 42, 23–27. [Google Scholar] [CrossRef]
- Xiao, S.; Xu, L.; Chen, Y.; Yu, H. Microstructure and mechanical properties of TiAl-based alloy prepared by double mechanical milling and spark plasma sintering. Trans. Nonferrous Met. Soc. China 2012, 22, 1086–1091. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying: A Novel Technique to Synthesize Advanced Materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Clemens, H.; Mayer, S. Design, Processing, Microstructure, Properties, and Applications of Advanced Intermetallic TiAl Alloys. Adv. Eng. Mater. 2013, 15, 191–215. [Google Scholar] [CrossRef]
- Lu, L.; Lai, M.O.; Froes, F.H. The mechanical alloying of titanium aluminides. JOM 2002, 54, 62–64. [Google Scholar] [CrossRef]
- Forouzanmehr, N.; Karimzadeh, F.; Enayati, M.H. Study on solid-state reactions of nanocrystalline TiAl synthesized by mechanical alloying. J. Alloy. Compd. 2009, 471, 93–97. [Google Scholar] [CrossRef]
- Sim, K.H.; Wang, G.; Son, R.C.; Choe, S.L. Influence of mechanical alloying on the microstructure and mechanical properties of powder metallurgy Ti2AlNb-based alloy. Powder Technol. 2017, 317, 133–141. [Google Scholar] [CrossRef]
- Bohn, R.; Klassen, T.; Bormann, R. Mechanical behavior of submicron-grained γ-TiAl-based alloys at elevated temperatures. Intermetallics 2001, 9, 559–569. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Bellon, P.; Averback, R.S.; Hales, S.J. Nanocrystalline TiAl powders synthesized by high-energy ball milling: Effects of milling parameters on yield and contamination. J. Alloy. Compd. 2004, 368, 187–196. [Google Scholar] [CrossRef]
- Lavernia, E.J.; Han, B.Q.; Schoenung, J.M. Cryomilled nanostructured materials: Processing and properties. Mater. Sci. Eng. 2008, 493, 207–214. [Google Scholar] [CrossRef]
- Witkin, D.B.; Lavernia, E.J. Synthesis and mechanical behavior of nanostructured materials via cryomilling. Prog. Mater. Sci. 2006, 51, 1–60. [Google Scholar] [CrossRef]
- Deng, H.; Chen, A.; Chen, L.; Wei, Y.; Xia, Z.; Tang, J. Bulk nanostructured Ti-45Al-8Nb alloy fabricated by cryomilling and spark plasma sintering. J. Alloy. Compd. 2019, 772, 140–149. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Guyon, J.; Monchoux, J.P.; Hazotte, A.; Bouzy, E. On grain refinement of a γ-TiAl alloy using cryo-milling followed by spark plasma sintering. Intermetallics 2015, 66, 141–148. [Google Scholar] [CrossRef]
- Sun, Y.; Kulkarni, K.; Sachdev, A.K.; Lavernia, E.J. Synthesis of γ-TiAl by reactive spark plasma sintering of cryomilled Ti and Al powder blend, part I: Influence of processing and microstructural evolution. Met. Mater. Trans. A 2014, 45, 2750–2758. [Google Scholar] [CrossRef]
- Sun, Y.; Kulkarni, K.; Sachdev, A.K.; Lavernia, E.J. Synthesis of γ-TiAl by reactive spark plasma sintering of cryomilled Ti and Al powder blend: Part II: Effects of electric field and microstructure on sintering kinetics. Met. Mater. Trans. A 2014, 45, 2759–2767. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Guyon, J.; Hazotte, A.; Monchoux, J.P.; Bouzy, E. Effect of powder state on spark plasma sintering of TiAl alloys. Intermetallics 2013, 34, 94–100. [Google Scholar] [CrossRef]
- Chen, F.; Yang, S.; Wu, J.; Galaviz Perez, J.A.; Shen, Q.; Schoenung, J.M.; Lavernia, E.J.; Zhang, L. Spark plasma sintering and densification mechanisms of conductive ceramics under coupled thermal/electric fields. J. Am. Ceram. Soc. 2015, 98, 732–740. [Google Scholar] [CrossRef]
- Matizamhuka, W.R. Fabrication of Fine-Grained Functional Ceramics by Two-Step Sintering or Spark Plasma Sintering (SPS). In Sintering [Working Title]; IntechOpen: London, UK, 2019. [Google Scholar]
- Matizamhuka, W.R. Spark plasma sintering (SPS)-an advanced sintering technique for structural nanocomposite materials. J. South. African Inst. Min. Met. 2016, 116, 1171–1180. [Google Scholar] [CrossRef]
- Suárez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R.; Kessel, H.U.; Hennicke, J.; Kirchner, R.; Kessel, T. Challenges and opportunities for spark plasma sintering: A key technology for a new generation of materials. Sinter. Appl. 2013, 13, 319–342. [Google Scholar]
- Marder, R.; Estournès, C.; Chevallier, G.; Chaim, R. Spark and plasma in spark plasma sintering of rigid ceramic nanoparticles: A model system of YAG. J. Eur. Ceram. Soc. 2015, 35, 211–218. [Google Scholar] [CrossRef]
- Marder, R.; Estournès, C.; Chevallier, G.; Chaim, R. Plasma in spark plasma sintering of ceramic particle compacts. Scr. Mater. 2014, 82, 57–60. [Google Scholar] [CrossRef]
- Saunders, T.; Grasso, S.; Reece, M.J. Plasma formation during electric discharge (50V) through conductive powder compacts. J. Eur. Ceram. Soc. 2015, 35, 871–877. [Google Scholar] [CrossRef]
- Rahaman, M.N. Ceramic Processing and Sintering; CRC press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Olevsky, E.A.; Kandukuri, S.; Froyen, L. Consolidation enhancement in spark-plasma sintering: Impact of high heating rates. J. Appl. Phys. 2007, 102, 114913. [Google Scholar] [CrossRef]
- Manière, C.; Torresani, E.; Olevsky, E.A. Simultaneous spark plasma sintering of multiple complex shapes. Materials 2019, 12, 557. [Google Scholar] [CrossRef]
- Manière, C.; Durand, L.; Weibel, A.; Estournès, C. Spark-plasma-sintering and finite element method: From the identification of the sintering parameters of a submicronic α-alumina powder to the development of complex shapes. Acta Mater. 2016, 102, 169–175. [Google Scholar] [CrossRef]
- Voisin, T.; Monchoux, J.P.; Durand, L.; Karnatak, N.; Thomas, M.; Couret, A. An Innovative Way to Produce γ-TiAl Blades: Spark Plasma Sintering. Adv. Eng. Mater. 2015, 17, 1408–1413. [Google Scholar] [CrossRef]
- Manière, C.; Durand, L.; Weibel, A.; Chevallier, G.; Estournès, C. A sacrificial material approach for spark plasma sintering of complex shapes. Scr. Mater. 2016, 124, 126–128. [Google Scholar] [CrossRef]
- Manière, C.; Nigito, E.; Durand, L.; Weibel, A.; Beynet, Y.; Estournès, C. Spark plasma sintering and complex shapes: The deformed interfaces approach. Powder Technol. 2017, 320, 340–345. [Google Scholar] [CrossRef]
- Olevsky, E.A.; Garcia-Cardona, C.; Bradbury, W.L.; Haines, C.D.; Martin, D.G.; Kapoor, D. Fundamental Aspects of Spark Plasma Sintering: II. Finite Element Analysis of Scalability. J. Am. Ceram. Soc. 2012, 95, 2414–2422. [Google Scholar] [CrossRef]
- Voisin, T.; Durand, L.; Karnatak, N.; Le Gallet, S.; Thomas, M.; Le Berre, Y.; Castagné, J.F.; Couret, A. Temperature control during Spark Plasma Sintering and application to up-scaling and complex shaping. J. Mater. Process. Technol. 2013, 213, 269–278. [Google Scholar] [CrossRef]
- Franceschin, G.; Flores-Martínez, N.; Vázquez-Victorio, G.; Ammar, S.; Valenzuela, R. Sintering and reactive sintering by spark plasma sintering (SPS). In Sintering of Functional Materials; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/books/sintering-of-functional-materials/sintering-and-reactive-sintering-by-spark-plasma-sintering-sps- (accessed on 1 May 2020).
- Isobe, T.; Daimon, K.; Sato, T.; Matsubara, T.; Hikichi, Y.; Ota, T. Spark plasma sintering technique for reaction sintering of Al2O3/Ni nanocomposite and its mechanical properties. Ceram. Int. 2008, 34, 213–217. [Google Scholar] [CrossRef]
- Waseem, O.A.; Lee, J.; Lee, H.M.; Ryu, H.J. The effect of Ti on the sintering and mechanical properties of refractory high-entropy alloy TixWTaVCr fabricated via spark plasma sintering for fusion plasma-facing materials. Mater. Chem. Phys. 2018, 210, 87–94. [Google Scholar] [CrossRef]
- Meir, S.; Kalabukhov, S.; Froumin, N.; Dariel, M.P.; Frage, N. Synthesis and densification of transparent magnesium aluminate spinel by SPS processing. J. Am. Ceram. Soc. 2009, 92, 358–364. [Google Scholar] [CrossRef]
- Martins, D.; Grumbach, F.; Simoulin, A.; Sallot, P.; Mocellin, K.; Bellet, M.; Estournès, C. Spark plasma sintering of a commercial TiAl 48-2-2 powder: Densification and creep analysis. Mater. Sci. Eng. A 2018, 711, 313–316. [Google Scholar] [CrossRef]
- Anselmi-Tamburini, U.; Garay, J.E.; Munir, Z.A. Fast low-temperature consolidation of bulk nanometric ceramic materials. Scr. Mater. 2006, 54, 823–828. [Google Scholar] [CrossRef]
- Lenel, F. Resistance sintering under pressure. JOM 1955, 7, 158–167. [Google Scholar] [CrossRef]
- Weissler, G.A. Resistance sintering with alumina dies. Int. J. Powder Met. Powder Technol. 1981, 17, 107–118. [Google Scholar]
- Herrmann, M.; Schulz, I.; Bales, A.; Sempf, K.; Hoehn, S. “Snow flake” structures in silicon nitride ceramics–Reasons for large scale optical inhomogeneities. J. Eur. Ceram. Soc. 2008, 28, 1049–1056. [Google Scholar] [CrossRef]
- Langer, J.; Hoffmann, M.J.; Guillon, O. Electric Field-Assisted Sintering in Comparison with the Hot Pressing of Yttria-Stabilized Zirconia. J. Am. Ceram. Soc. 2011, 94, 24–31. [Google Scholar] [CrossRef]
- Westbrook, J.H.; Fleischer, R.L. Crystal Structures of Intermetallic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Paufler, P.J.H. Westbrook, R.L. Intermetallic Compounds Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Cobbinah, P.V.; Matizamhuka, W.R. Solid-State Processing Route, Mechanical Behaviour, and Oxidation Resistance of TiAl Alloys. Adv. Mater. Sci. Eng. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Froes, F.H.; Suryanarayana, C.; Eliezer, D. Synthesis, properties and applications of titanium aluminides. J. Mater. Sci. 1992, 27, 5113–5140. [Google Scholar] [CrossRef]
- McCullough, C.; Valencia, J.J.; Levi, C.G.; Mehrabian, R. Phase equilibria and solidification in Ti-Al alloys. Acta Met. 1989, 37, 1321–1336. [Google Scholar] [CrossRef]
- Jones, S.A.; Shull, R.D.; McAlister, A.J.; Kaufman, M.J. Microstructural studies of Ti-Al alloys in the vicinity of the “eutectoid” reaction (α → α2 γ). Scr. Met. 1988, 22, 1235–1240. [Google Scholar] [CrossRef]
- JH, P. Phase Reactions and Processing in the Ti-Al Based Intermetallics. ISIJ Int. 1991, 31, 1080–1087. [Google Scholar]
- Djanarthany, S.; Viala, J.C.; Bouix, J. An overview of monolithic titanium aluminides based on Ti3Al and TiAl. Mater. Chem. Phys. 2001, 72, 301–319. [Google Scholar] [CrossRef]
- Sastry, S.M.L.; Lipsitt, H.A. Ordering transformations and mechanical properties of Ti 3 Ai and Ti 3 Al-Nb alloys. Met. Trans. A 1977, 8, 1543–1552. [Google Scholar] [CrossRef]
- Sarkar, S.; Datta, S.; Das, S.; Basu, D. Oxidation protection of gamma-titanium aluminide using glass–ceramic coatings. Surf. Coat. Technol. 2009, 203, 1797–1805. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Du, Y.; Yuan, J. Effects of Powder Preparation and Sintering Temperature on Properties of Spark Plasma Sintered Ti-48Al-2Cr-8Nb Alloy. Metals 2019, 9, 861. [Google Scholar] [CrossRef]
- Werwer, M.; Kabir, R.; Cornec, A.; Schwalbe, K.H. Fracture in lamellar TiAl simulated with the cohesive model. Eng. Fract. Mech. 2007, 74, 2615–2638. [Google Scholar] [CrossRef]
- Stoloff, N.S.; Sikka, V.K. Physical Metallurgy and Processing of Intermetallic Compounds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kothari, K.; Radhakrishnan, R.; Wereley, N.M. Advances in gamma titanium aluminides and their manufacturing techniques. Prog. Aerosp. Sci. 2012, 55, 1–16. [Google Scholar] [CrossRef]
- Kim, Y.W.; Dimiduk, D.M. Progress in the understanding of gamma titanium aluminides. JOM 1991, 43, 40–47. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Eur. Congr. Adv. Mater. Process. 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Hernandez, J.; Murr, L.E.; Gaytan, S.M.; Martinez, E.; Medina, F.; Wicker, R.B. Microstructures for two-phase gamma titanium aluminide fabricated by electron beam melting. Met. Microstruct Anal. 2012, 1, 14–27. [Google Scholar] [CrossRef]
- Recina, V. Mechanical Properties of Gamma Titanium Aluminides; Chalmers University of Technology: Gothenburg, Sweden, 2000. [Google Scholar]
- Wang, G.X.; Dahms, M. Influence of heat treatment on microstructure of Ti-35wt%Al prepared by elemental powder metallurgy. Scr. Met. Mater. 1992, 26, 717–722. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pant, B.; Sinha, P.P. Theory and practice of γ α 2 Ti aluminide: A review. Trans. Indian Inst. Met. 2014, 67, 143–165. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, H.; Qiang, J. The microstructure evolution, mechanical properties and densification mechanism of TiAl-based alloys prepared by spark plasma sintering. Metals 2017, 7, 201. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, H.; Zheng, W. Effect of temperature-related factors on densification, microstructure and mechanical properties of powder metallurgy TiAl-based alloys. Adv. Powder Technol. 2019, 30, 2555–2563. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Yan, H.; Jiang, K. Spark Plasma Sintering of Alumina Composites with Graphene Platelets and Silicon Carbide Nanoparticles. Adv. Eng. Mater. 2014, 16, 1111–1118. [Google Scholar] [CrossRef]
- Voisin, T.; Monchoux, J.P.; Hantcherli, M.; Mayer, S.; Clemens, H.; Couret, A. Microstructures and mechanical properties of a multi-phase β-solidifying TiAl alloy densified by spark plasma sintering. Acta Mater. 2014, 73, 107–115. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.; Wang, G. Dependence of heating rate in PCAS on microstructures and high temperature deformation properties of γ-TiAl intermetallic alloys. Intermetallics 2010, 18, 834–840. [Google Scholar] [CrossRef]
- Mphahlele, M.R.; Olevsky, E.A.; Olubambi, P.A. Chapter 12-Spark plasma sintering of near net shape titanium aluminide: A review. In Spark Plasma Sinter; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 281–299. [Google Scholar]
- Appel, F.; Clemens, H.; Fischer, F.D. Modeling concepts for intermetallic titanium aluminides. Prog. Mater. Sci. 2016, 81, 55–124. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Pflumm, R.; Friedle, S.; Schütze, M. Oxidation protection of γ-TiAl-based alloys–A review. Intermetallics 2015, 56, 1–14. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, J.; Chen, C.; Weng, F. High temperature oxidation behavior and research status of modifications on improving high temperature oxidation resistance of titanium alloys and titanium aluminides: A review. J. Alloy. Compd. 2016, 685, 784–798. [Google Scholar] [CrossRef]
- Lu, X.; He, X.B.; Zhang, B.; Qu, X.H.; Zhang, L.; Guo, Z.X.; Tian, J.J. High-temperature oxidation behavior of TiAl-based alloys fabricated by spark plasma sintering. J. Alloy. Compd. 2009, 478, 220–225. [Google Scholar] [CrossRef]
- Kumpfert, J.; Kim, Y.W.; Dimiduk, D.M. Effect of microstructure on fatigue and tensile properties of the gamma TiAl alloy Ti-46.5Al-3.0Nb-2.1Cr-0.2W. Mater. Sci. Eng. A 1995, 192, 465–473. [Google Scholar] [CrossRef]
- Cobbinah, P.V.; Matizamhuka, W.; Machaka, R.; Shongwe, M.B.; Yamabe-Mitarai, Y. The effect of Ta additions on the oxidation resistance of SPS-produced TiAl alloys. Int. J. Adv. Manuf. Technol. 2020, 106, 3203–3215. [Google Scholar] [CrossRef]
- Kenel, C.; Lis, A.; Dawson, K.; Stiefel, M.; Pecnik, C.; Barras, J.; Colella, A.; Hauser, C.; Tatlock, G.J.; Leinenbach, C.; et al. Mechanical performance and oxidation resistance of an ODS γ-TiAl alloy processed by spark plasma sintering and laser additive manufacturing. Intermetallics 2017, 91, 169–180. [Google Scholar] [CrossRef]
- Kim, Y.W. Ordered intermetallic alloys, part III: Gamma titanium aluminides. JOM 1994, 46, 30–39. [Google Scholar] [CrossRef]
- Huang, S.C.; Hall, E.L. The effects of Cr additions to binary TiAl-base alloys. Met. Trans. A 1991, 22, 2619–2627. [Google Scholar] [CrossRef]
- Huang, S.C.; Hall, E.L. Characterization of the effect of vanadium additions to TiAl base alloys. Acta Met. Mater. 1991, 39, 1053–1060. [Google Scholar] [CrossRef]
- Hashimoto, K. High-Temperature Tensile Properties of Ti-Al-X (X=Cr,W) Consisting of Î ± 2, Î2 and Î3 in Three Phases. Mater. Sci. Forum 2012, 706–709, 1066–1070. [Google Scholar] [CrossRef]
- Zhu, D.D.; Wang, H.W.; Qi, J.Q.; Zou, C.M.; Wei, Z.J. Effect of Cr addition on microstructures and nanohardness of rapidly solidified Ti–48Al alloy. Mater. Sci. Technol. 2012, 28, 1385–1390. [Google Scholar] [CrossRef]
- Kawabata, T.; Fukai, H.; Izumi, O. Effect of ternary additions on mechanical properties of TiAl. Acta Mater. 1998, 46, 2185–2194. [Google Scholar] [CrossRef]
- Pelachová, T.; Lapin, J. Cyclic oxidation behaviour of intermetallic Ti-46Al-8Ta alloy in air. Kov. Mater 2015, 53, 415–422. [Google Scholar] [CrossRef]
- Yuanyuan, L.; Weidong, Z.; Zhengping, X.; Xiaonan, M.; Yingli, Y.; Jinping, W.; Hangbiao, S. Microstructure, mechanical properties and oxidation behavior of a hot-extruded TiAl containing Ta. Rare Met. Mater. Eng. 2015, 44, 282–287. [Google Scholar] [CrossRef]
- Mitoraj, M.; Godlewska, E.M. Oxidation of Ti–46Al–8Ta in air at 700 °C and 800 °C under thermal cycling conditions. Intermetallics 2013, 34, 112–121. [Google Scholar] [CrossRef]
- Popela, T.; Vojtěch, D.; Novák, P.; Knotek, V.; Průša, F.; Michalcová, A.; Novák, M.; Šerák, J. High-temperature oxidation of Ti-Al-Ta and Ti-Al-Nb alloys. Metal 2010, 18, 1–4. [Google Scholar]
- Lapin, J.; Pelachová, T.; Dománková, M. Long-term creep behaviour of cast TiAl-Ta alloy. Intermetallics 2018, 95, 24–32. [Google Scholar] [CrossRef]
- Lapin, J.; Pelachová, T.; Dománková, M. Creep behaviour of a new air-hardenable intermetallic Ti–46Al–8Ta alloy. Intermetallics 2011, 19, 814–819. [Google Scholar] [CrossRef]
- Hodge, A.M.; Hsiung, L.M.; Nieh, T.G. Creep of nearly lamellar TiAl alloy containing W. Scr. Mater. 2004, 51, 411–415. [Google Scholar] [CrossRef]
- Fang, H.; Chen, R.; Yang, Y.; Tan, Y.; Su, Y.; Ding, H.; Guo, J. Effects of Boron On Microstructure Evolution and Mechanical Properties in Ti46Al8Nb2.6C0.8Ta Alloys. Adv. Eng. Mater. 2019, 21, 1900143. [Google Scholar] [CrossRef]
- Li, M.; Xiao, S.; Xiao, L.; Xu, L.; Tian, J.; Chen, Y. Effects of carbon and boron addition on microstructure and mechanical properties of TiAl alloys. J. Alloy. Compd. 2017, 728, 206–221. [Google Scholar] [CrossRef]
- Čegan, T.; Szurman, I. Thermal stability and precipitation strengthening of fully lamellar Ti-45Al-5Nb-0.2 B-0.75 C alloy. Met. Mater. 2018, 55, 421–430. [Google Scholar]
- Wang, Q.; Ding, H.; Zhang, H.; Chen, R.; Guo, J.; Fu, H. Variations of microstructure and tensile property of γ-TiAl alloys with 0–0.5at% C additives. Mater. Sci. Eng. A 2017, 700, 198–208. [Google Scholar] [CrossRef]
- Li, M.; Xiao, S.; Chen, Y.; Xu, L.; Tian, J. The effect of boron addition on the high-temperature properties and microstructure evolution of high Nb containing TiAl alloys. Mater. Sci. Eng. A 2018, 733, 190–198. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Kuprienko, V.S.; Fadeev, A.V.; Bazlov, A.I.; Belov, V.D.; Titov, A.Y.; Koltygin, A.V.; Komissarov, A.A.; Plisetskaya, I.V.; Logachev, I.A. Influence of Y and Zr on TiAl43Nb4Mo1B0. 1 titanium aluminide microstructure and properties. Mater. Sci. Technol. 2020, 36, 548–555. [Google Scholar] [CrossRef]
- Xu, W.; Huang, K.; Wu, S.; Zong, Y.; Shan, D. Influence of Mo content on microstructure and mechanical properties of β-containing TiAl alloy. Trans. Nonferrous Met. Soc. China 2017, 27, 820–828. [Google Scholar] [CrossRef]
- Wu, Y.; Hwang, S.K. Microstructural refinement and improvement of mechanical properties and oxidation resistance in EPM TiAl-based intermetallics with yttrium addition. Acta Mater. 2002, 50, 1479–1493. [Google Scholar] [CrossRef]
- Kong, F.T.; Chen, Y.Y.; Li, B.H. Influence of yttrium on the high temperature deformability of TiAl alloys. Mater. Sci. Eng. A 2009, 499, 53–57. [Google Scholar] [CrossRef]
- Stringer, J. The reactive element effect in high-temperature corrosion. Mater. Sci. Eng. A 1989, 120, 129–137. [Google Scholar] [CrossRef]
- Hadi, M.; Bayat, O.; Meratian, M.; Shafyei, A.; Ebrahimzadeh, I. Oxidation Properties of a Beta-Stabilized TiAl Alloy Modified by Rare Earth Elements. Oxid. Met. 2018, 90, 421–434. [Google Scholar] [CrossRef]
- Wimler, D.; Lindemann, J.; Clemens, H.; Mayer, S. Microstructural Evolution and Mechanical Properties of an Advanced γ-TiAl Based Alloy Processed by Spark Plasma Sintering. Materials 2019, 12, 1523. [Google Scholar] [CrossRef]
- Saage, H.; Huang, A.J.; Hu, D.; Loretto, M.H.; Wu, X. Microstructures and tensile properties of massively transformed and aged Ti46Al8Nb and Ti46Al8Ta alloys. Intermetallics 2009, 17, 32–38. [Google Scholar] [CrossRef]
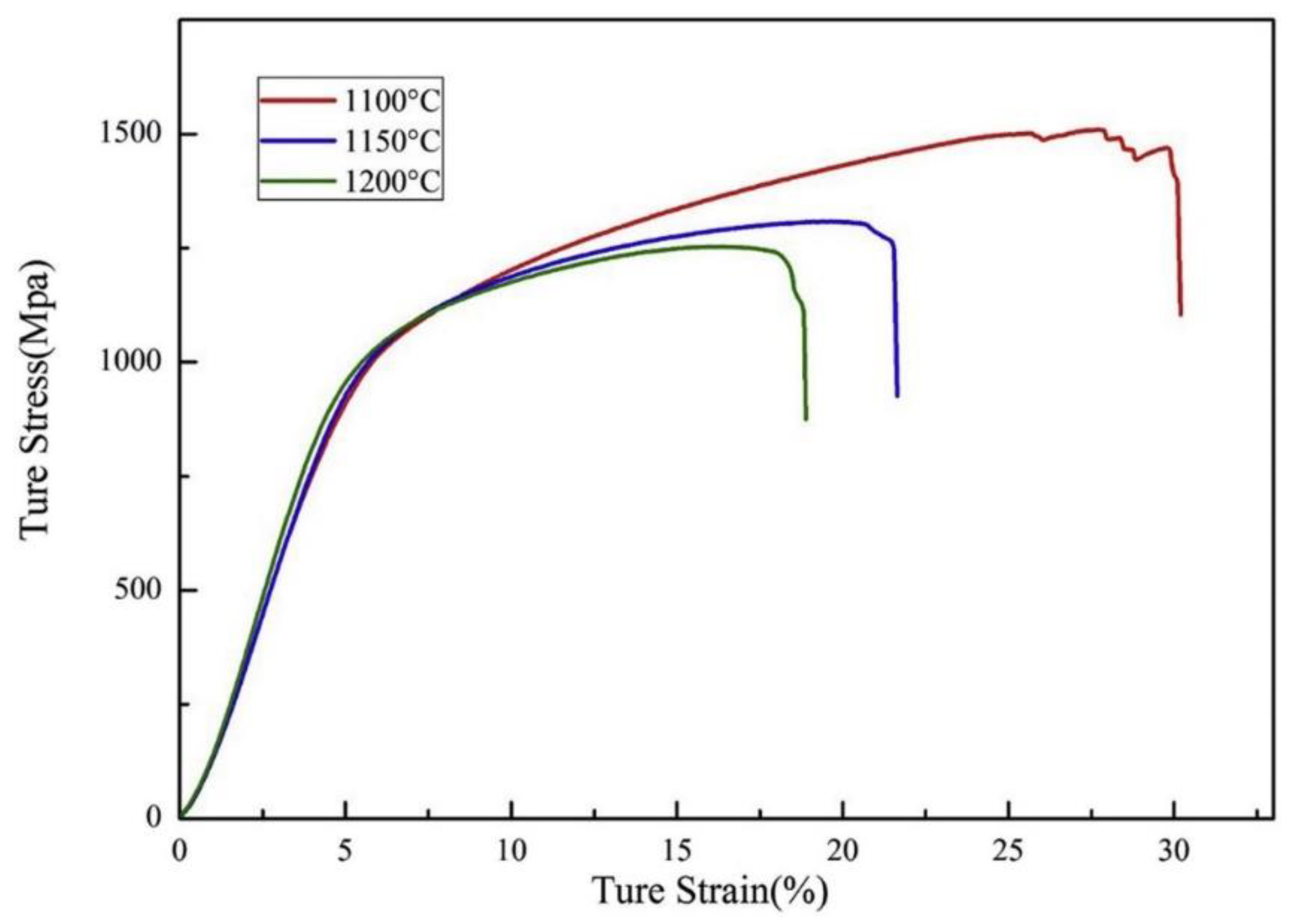

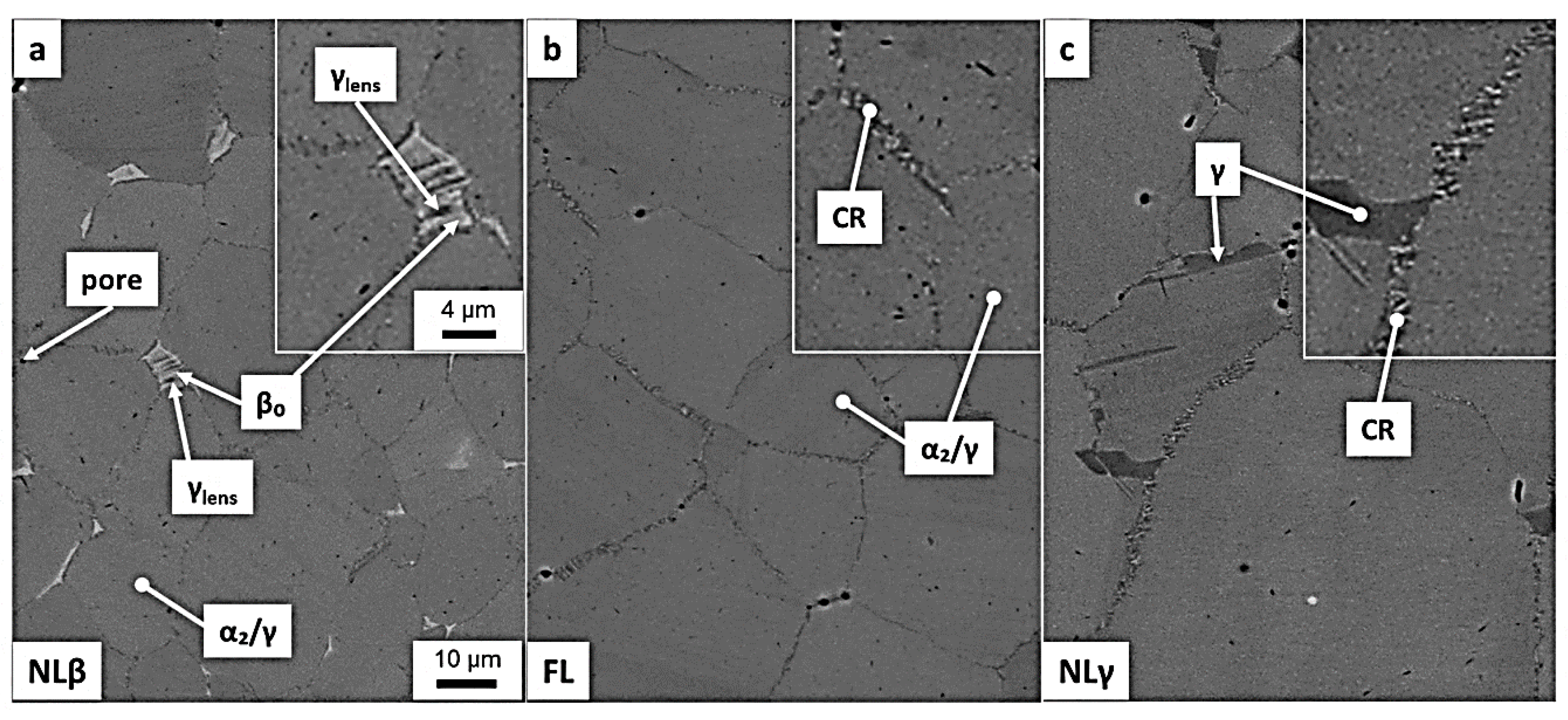
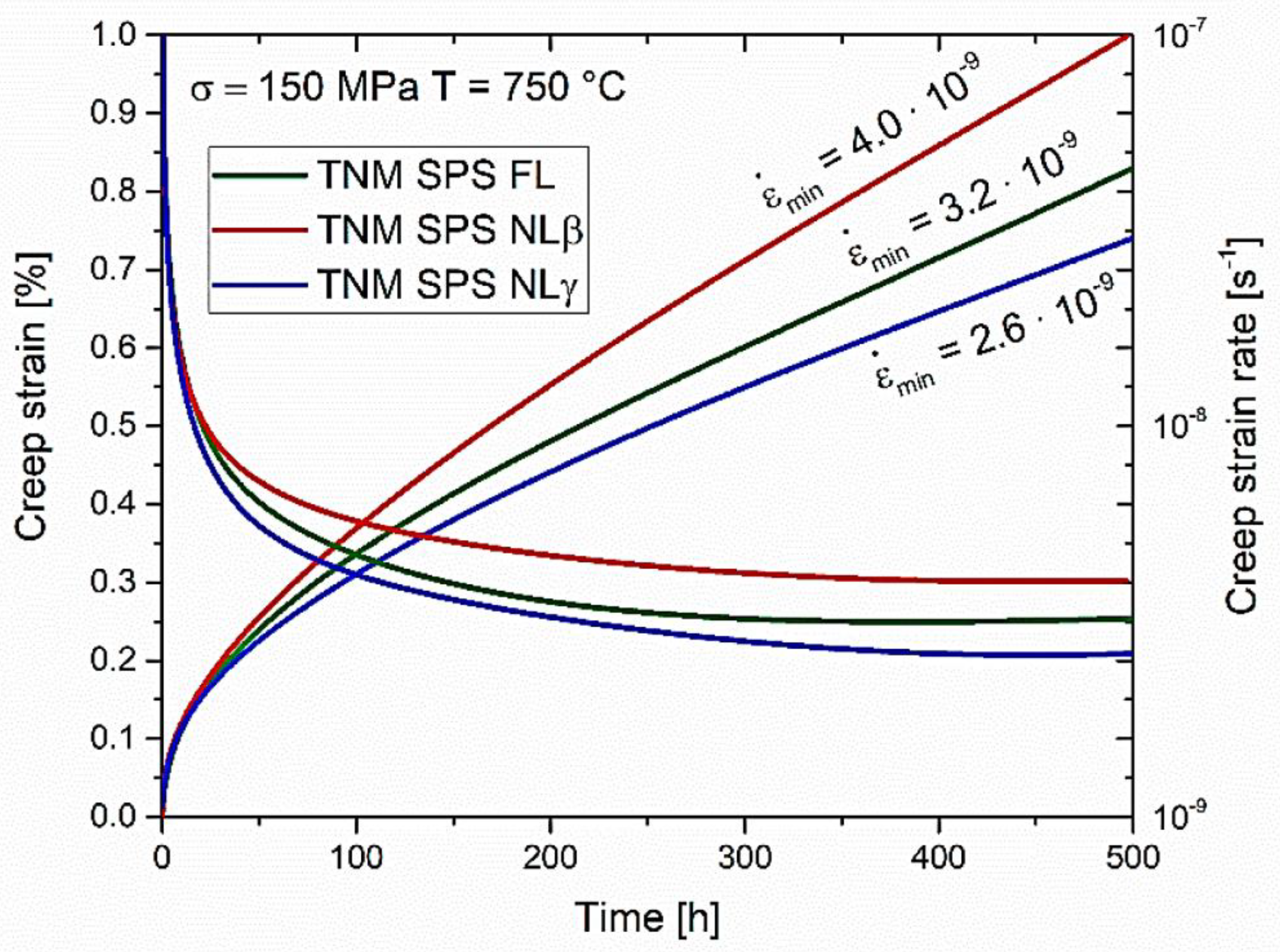
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogale, N.F.; Matizamhuka, W.R. Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges. Metals 2020, 10, 1080. https://doi.org/10.3390/met10081080
Mogale NF, Matizamhuka WR. Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges. Metals. 2020; 10(8):1080. https://doi.org/10.3390/met10081080
Chicago/Turabian StyleMogale, Ntebogeng F., and Wallace R. Matizamhuka. 2020. "Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges" Metals 10, no. 8: 1080. https://doi.org/10.3390/met10081080
APA StyleMogale, N. F., & Matizamhuka, W. R. (2020). Spark Plasma Sintering of Titanium Aluminides: A Progress Review on Processing, Structure-Property Relations, Alloy Development and Challenges. Metals, 10(8), 1080. https://doi.org/10.3390/met10081080





