Experimental and Numerical Studies on Preparation of Thin AZ31B/AA5052 Composite Plates Using Improved Explosive Welding Technique
Abstract
1. Introduction
2. Numerical Analysis
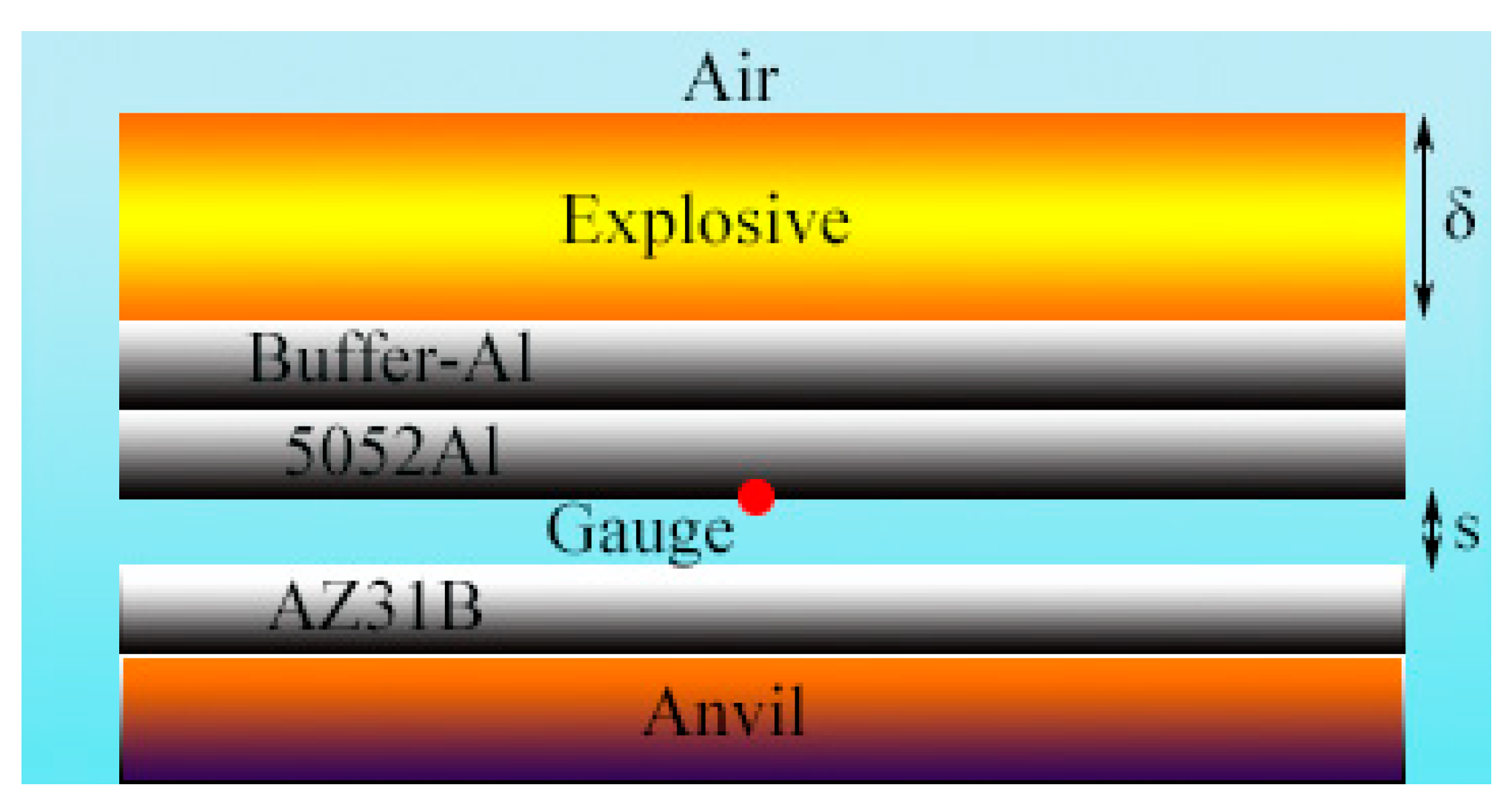
2.1. Numerical Arrangements for Explosive Welding
2.2. Determination of Welding Parameters
3. Experimental Procedure
3.1. Preparation of Materials
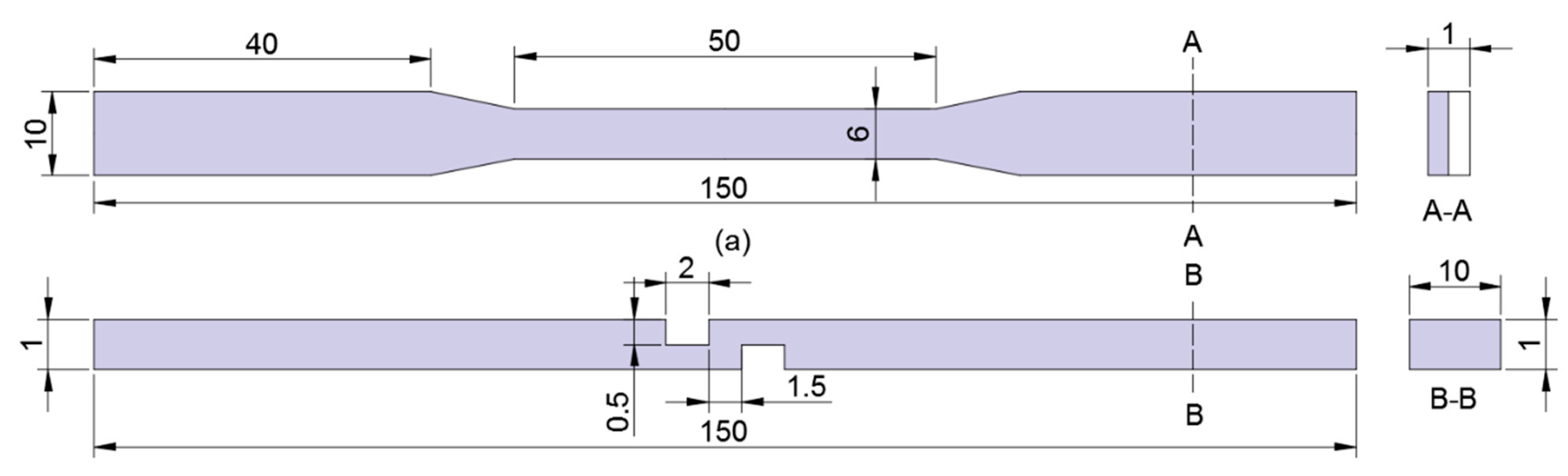
3.2. Characterization Methods
4. Results and Discussion
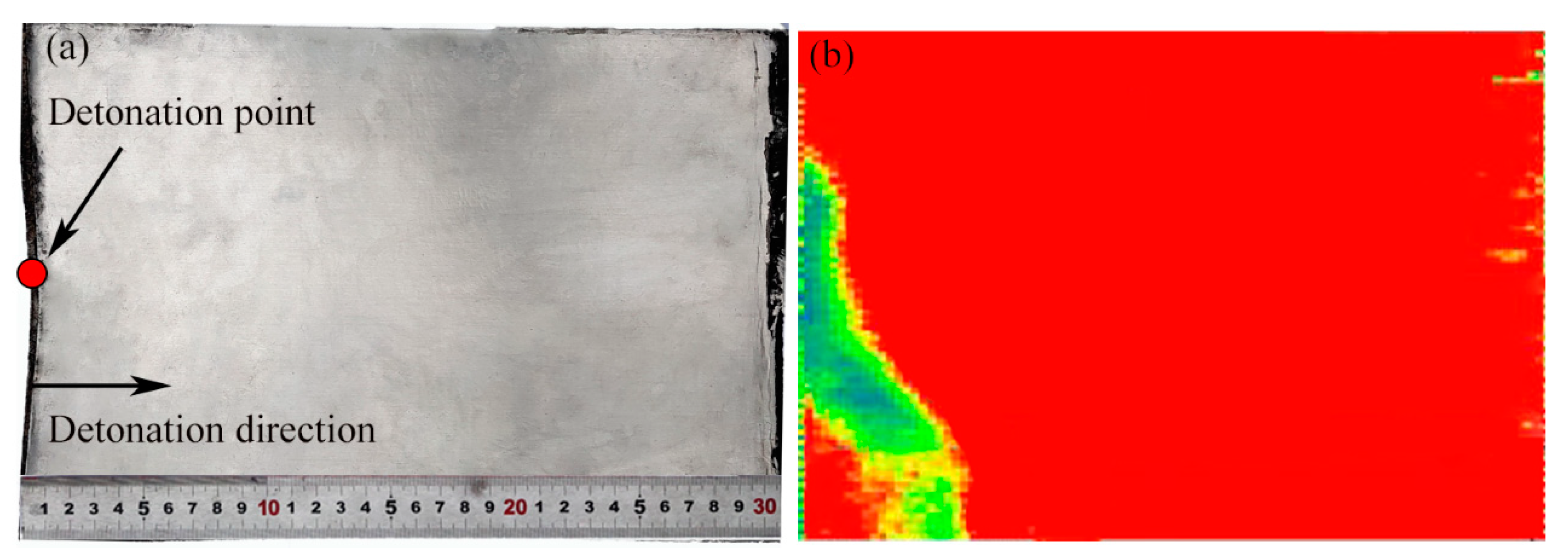
4.1. Macroscopic Feature of AA5052/AZ31B Plate
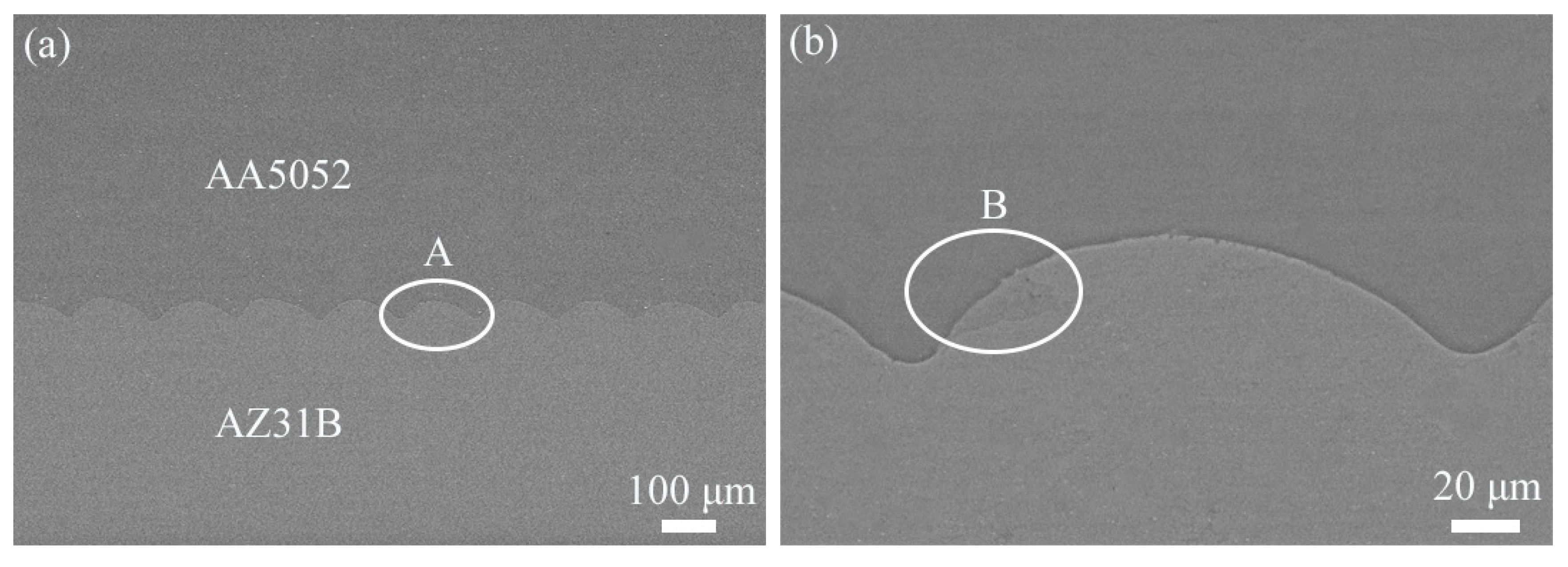
4.2. Microstructure of Bonding Interfaces
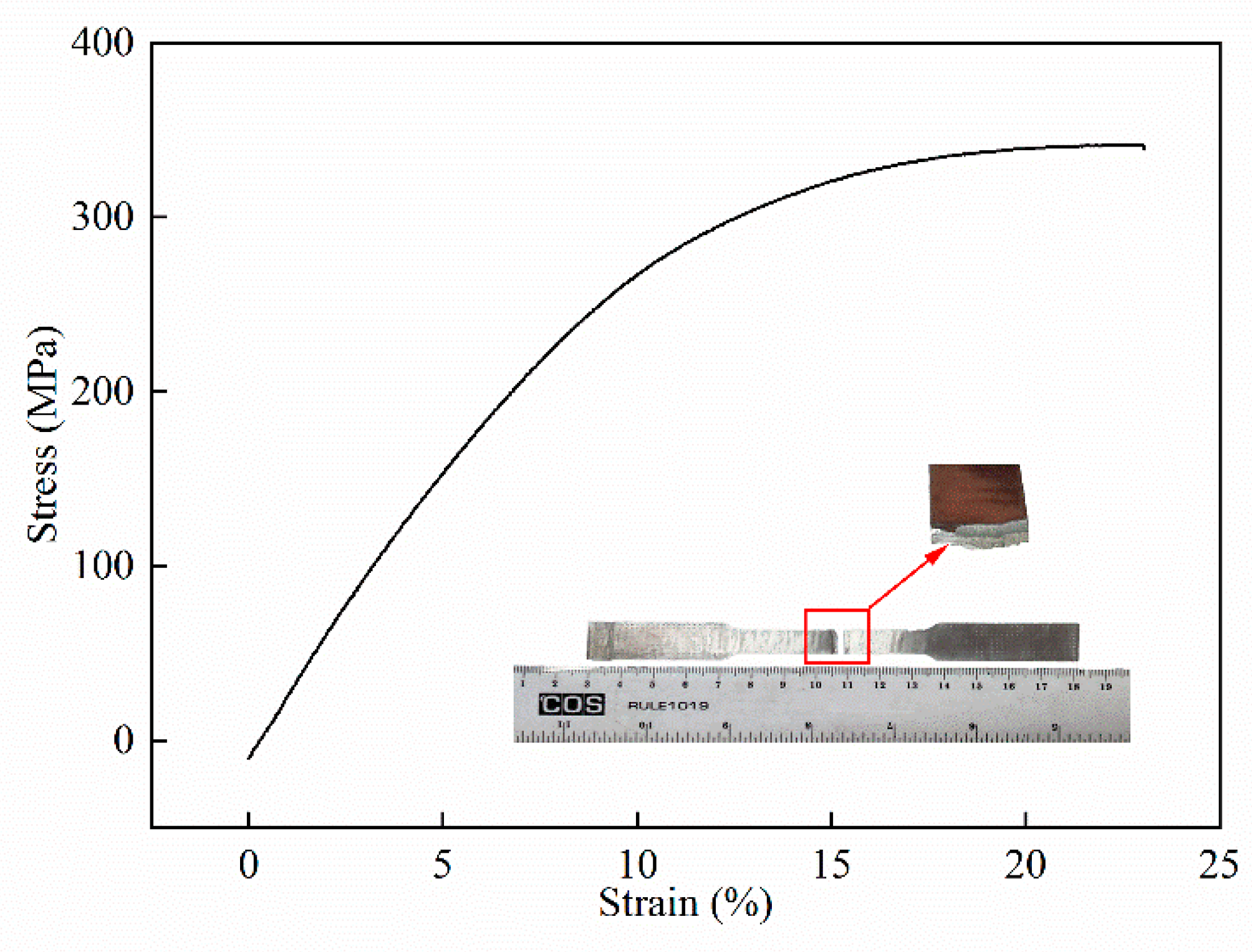
4.3. Tensile Tests
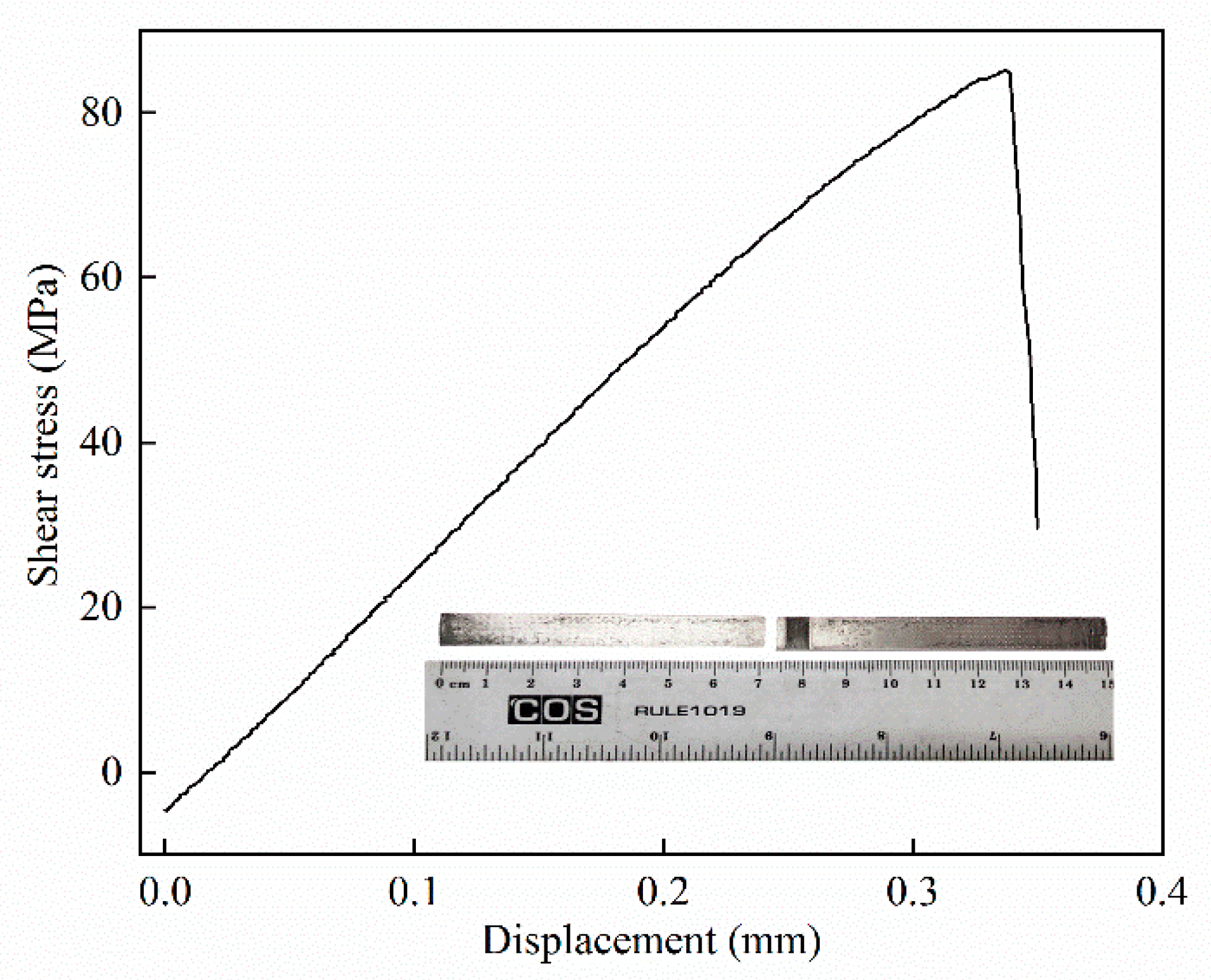
4.4. Tensile Shear Tests
5. Simulation of Welding Process with SPH Method
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashby, M.F.; Brechet, Y.J.M. Designing hybrid materials. Acta Mater. 2003, 51, 5801–5821. [Google Scholar] [CrossRef]
- Seetharaman, S.; Blawert, C.; Ng, B.M.; Wong, W.L.E.; Goh, C.S.; Hort, N.; Gupta, M. Effect of erbium modification on the microstructure, mechanical and corrosion characteristics of binary Mg–Al alloys. J. Alloy. Compd. 2015, 648, 759–770. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Zhang, W.; Wei, Y.; Cao, X.; Yan, Z. Microstructure evolution and mechanical properties of an aa6061/az31b alloy plate fabricated by explosive welding. J. Alloy. Compd. 2018, 735, 1759–1768. [Google Scholar] [CrossRef]
- Pramanik, A. Effects of reinforcement on wear resistance of aluminum matrix composites. Trans. Nonferr. Met. Soc. China 2016, 26, 348–358. [Google Scholar] [CrossRef]
- Wang, C.X.; Jiang, Y.B.; Xie, J.X.; Xu, S.; Zhou, D.J.; Zhang, X.J. Formation mechanism and control of aluminum layer thickness fluctuation in embedded aluminum–steel composite sheet produced by cold roll bonding process. Trans. Nonferr. Met. Soc. China 2017, 27, 1011–1018. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, H.-T.; Liu, J.; Feng, J. Microstructure and properties of Mg/Al joint welded by gas tungsten arc welding-assisted hybrid ultrasonic seam welding. Mater. Des. 2015, 77, 65–71. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Wang, Y.-X.; Li, X.-Q.; Li, X.-J.; Zhao, T.-J. Effect of inert gas-shielding on the interface and mechanical properties of Mg/Al explosive welding composite plate. J. Manuf. Process. 2019, 45, 166–175. [Google Scholar] [CrossRef]
- Suyuan, Y.; Bao, J. Microstructure and Properties of 5083 Al/1060 Al/AZ31 Composite Plate Fabricated by Explosive Welding. J. Mater. Eng. Perform. 2018, 27, 1177–1184. [Google Scholar] [CrossRef]
- Shao, X.; Guo, X.; Han, Y.; Lu, W.; Qin, J.; Zhang, D. Characterization of the diffusion bonding behavior of pure Ti and Ni with different surface roughness during hot pressing. Mater. Des. 2015, 65, 1001–1010. [Google Scholar] [CrossRef]
- Liu, W.S.; Long, L.P.; Ma, Y.Z.; Wu, L. Microstructure evolution and mechanical properties of Mg/Al diffusion bonded joints. J. Alloy. Compd. 2015, 643, 34–39. [Google Scholar] [CrossRef]
- Çam, G.; Mistikoglu, S. Recent Developments in Friction Stir Welding of Al-alloys. J. Mater. Eng. Perform. 2014, 23, 1936–1953. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, J.; Jo, Y.; Lee, S.; Heo, J.; Chang, Y.; Lee, Y. Interface-correlated deformation behavior of a stainless steel-Al–Mg 3-ply composite. Mater. Charact. 2013, 75, 138–149. [Google Scholar] [CrossRef]
- Zhang, X.P.; Yang, T.H.; Castagne, S.; Wang, J.T. Microstructure; bonding strength and thickness ratio of Al/Mg/Al alloy laminated composites prepared by hot rolling. Mater. Sci. Eng. A 2011, 528, 1954–1960. [Google Scholar] [CrossRef]
- Lee, K.S.; Yoon, D.H.; Kim, H.K.; Kwon, Y.N.; Lee, Y.S. Effect of annealing on the interface microstructure and mechanical properties of a STS–Al–Mg 3-ply clad sheet. Mater. Sci. Eng. A 2012, 556, 319–330. [Google Scholar] [CrossRef]
- Borrisutthekul, R.; Miyashita, Y.; Mutoh, Y. Dissimilar material laser welding between magnesium alloy AZ31B and aluminum alloy A5052-O. Sci. Technol. Adv. Mater. 2005, 6, 199–204. [Google Scholar] [CrossRef]
- Yang, M.; Ma, H.H.; Yao, D.; Shen, Z.W. Experimental study for manufacturing 316L/CuCrZr hollow structural component. Fusion Eng. Des. 2019, 144, 107–118. [Google Scholar] [CrossRef]
- Blazynski, T.Z. Explosive Welding, Forming and Compaction; Elsevier: New York, NY, USA, 1983. [Google Scholar]
- Lysak, V.; Kuzmin, S. Energy balance during explosive welding. J. Mater. Process. Technol. 2015, 222, 356–364. [Google Scholar] [CrossRef]
- Bataev, I.A.; Tanaka, S.; Zhou, Q.; Lazurenko, D.V.; Junior, A.J.; Bataev, A.A.; Hokamoto, K.; Mori, A.; Chen, P. Towards better understanding of explosive welding by combination of numerical simulation and experimental study. Mater. Des. 2019, 169, 107649. [Google Scholar] [CrossRef]
- Fronczek, D.M.; Chulist, R.; Litynska-Dobrzynska, L.; Kac, S.; Schell, N.; Kania, Z. Microstructure and Kinetics of Intermetallic Phase Growth of Three-Layered A1050/AZ31/A1050 Clads Prepared by Explosive Welding Combined with Subsequent Annealing. Mater. Des. 2017, 130, 120–130. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.; Cao, X.; Wu, J. The effect of annealing on the interface microstructure and mechanical characteristics of AZ31B/AA6061 composite plates fabricated by explosive welding. Mater. Des. 2015, 65, 1100–1109. [Google Scholar] [CrossRef]
- Inao, D.; Mori, A.; Tanaka, S.; Hokamoto, K. Explosive welding of thin aluminum plate onto magnesium alloy plate using a gelatin layer as a pressure-transmitting medium. Metals 2020, 10, 106. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Dong, Y.; Cao, X.Q.; Yang, W.W.; Wang, W.X. Influence of multi-pass rolling and subsequent annealing on the interface microstructure and mechanical properties of the explosive welding Mg/Al composite plates. Mater. Sci. Eng. Struct. Mater. Prop. Misrostruct. Process. 2018, 723, 97–108. [Google Scholar] [CrossRef]
- Findik, F. Recent developments in explosive welding. Mater. Des. 2011, 32, 1081–1093. [Google Scholar] [CrossRef]
- Cowan, G.R.; Bergmann, O.R.; Holtzman, A.H. Mechanism of bond zone wave formation in explosion-clad metals. Met. Mater. Trans. A 1971, 2, 3145–3155. [Google Scholar] [CrossRef]
- Vaidyanathan, P.; Ramanathan, A. Computer-aided design of explosive welding systems. J. Mater. Process. Technol. 1993, 38, 501–516. [Google Scholar] [CrossRef]
- Lee, E.L.; Hornig, H.C.; Kury, J.W. Adiabatic Expansion of High Explosive Detonation Products. In Adiabatic Expansion of High Explosive Detonation Products; California University, Livermore. Lawrence Radiation Lab.: Livermore, CA, USA, 1968. [Google Scholar]
- Xu, J.; Yang, M.; Ma, H.; Shen, Z.; Chen, Z. Fabrication and performance studies on explosively welded CuCrZr/316L bimetallic plate applied in extreme environments. J. Mater. Res. Technol. 2020, 9, 8971–8984. [Google Scholar] [CrossRef]
- Hokamoto, K.; Shimomiya, K.; Nishi, M.; Krstulović-opara, L.; Vesenjak, M.; Ren, Z. Fabrication of unidirectional porous-structured aluminum through explosive compaction using cylindrical geometry. J. Mater. Process. Technol. 2018, 251, 262–266. [Google Scholar] [CrossRef]
- Yuan, X.D.; Wang, W.X.; Cao, X.Q.; Zhang, T.T.; Xie, R.S.; Liu, R.F. Numerical study on the interfacial behavior of Mg/Al plate in explosive/impact welding. Sci. Eng. Compos. Mater. 2017, 24, 581–590. [Google Scholar] [CrossRef]
- Li, X.; Ma, H.; Shen, Z. Research on explosive welding of aluminum alloy to steel with dovetail grooves. Mater. Des. 2015, 87, 815–824. [Google Scholar] [CrossRef]
- Yang, M.; Ma, H.-H.; Shen, Z.; Huang, Z.; Tian, Q.; Tian, J. Dissimilar material welding of tantalum foil and Q235 steel plate using improved explosive welding technique. Mater. Des. 2020, 186, 108348. [Google Scholar] [CrossRef]
- China National Standardization Management Committee. GB/T 1031-2009 Surface Roughness Obtained by Surface Structure Contour Method; China National Standardization Management Committee: Beijing, China, 2009. [Google Scholar]
- China National Standardization Management Committee. GB/T 6396-2008. Clad Steel Plates-Mechanical and Technological Test; China National Standardization Management Committee: Beijing, China, 2008. [Google Scholar]
- Godunov, S.K.; Deribas, A.A.; Zabrodin, A.V.; Kozin, N.S. Hydrodynamic effects in colliding solids. J. Comput. Phys. 1970, 5, 517–539. [Google Scholar] [CrossRef]
- Yang, M.; Ma, H.; Shen, Z. Study on self-restrained explosive welding with high energy efficiency. Int. J. Adv. Manuf. Technol. 2018, 99, 3123–3132. [Google Scholar] [CrossRef]
- Yang, M.; Ma, H.-H.; Shen, Z.; Sun, Y. Study on explosive welding for manufacturing meshing bonding interface of CuCrZr to 316L stainless steel. Fusion Eng. Des. 2019, 143, 106–114. [Google Scholar] [CrossRef]
- Paul, H.; Miszczyk, M.; Chulist, R.; Prażmowski, M.; Morgiel, J.; Gałka, A.; Faryna, M.; Brisset, F. Microstructure and phase constitution in the bonding zone of explosively welded tantalum and stainless steel sheets. Mater. Des. 2018, 153, 177–189. [Google Scholar] [CrossRef]
- Luo, C.; Liang, W.; Chen, Z.; Zhang, J.; Chi, C.; Yang, F. Effect of high temperature annealing and subsequent hot rolling on microstructural evolution at the bond-interface of Al/Mg/Al alloy laminated composites. Mater. Charact. 2013, 84, 34–40. [Google Scholar] [CrossRef]
- Chen, P.; Feng, J.; Zhou, Q.; An, E.; Li, J.; Yuan, Y.; Ou, S. Investigation on the Explosive Welding of 1100 Aluminum Alloy and AZ31 Magnesium Alloy. J. Mater. Eng. Perform. 2016, 25, 2635–2641. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Z.; Shen, W.; Wang, J.; Zhang, L.; Chin, B. Microstructure and properties of magnesium AZ31B–aluminum 7075 explosively welded composite plate. Mater. Sci. Eng. A 2010, 527, 2241–2245. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, S. Microstructure and Properties of Bonding Interface in Explosive Welded AZ31/1060 Composite Plate. China. J. Rare Met. 2016, 40, 996–1001. (In Chinese) [Google Scholar]
- Hayhurst, C.J.; Clegg, R.A. Cylindrically symmetric SPH simulations of hypervelocity impacts on thin plates. Int. J. Impact Eng. 1997, 20, 337–348. [Google Scholar] [CrossRef]
- Bondar, M.P. Localisation of plastic deformation on contacts, determining the formation of a strong joint. Combust. Explos. Shock Waves 1995, 31, 612–616. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Liu, H.; Shen, Z.; Hu, Y.; Li, W.; Gao, Y.; Guo, C. Numerical study of the mechanism of explosive/impact welding using Smoothed Particle Hydrodynamics method. Mater. Des. 2012, 35, 210–219. [Google Scholar] [CrossRef]
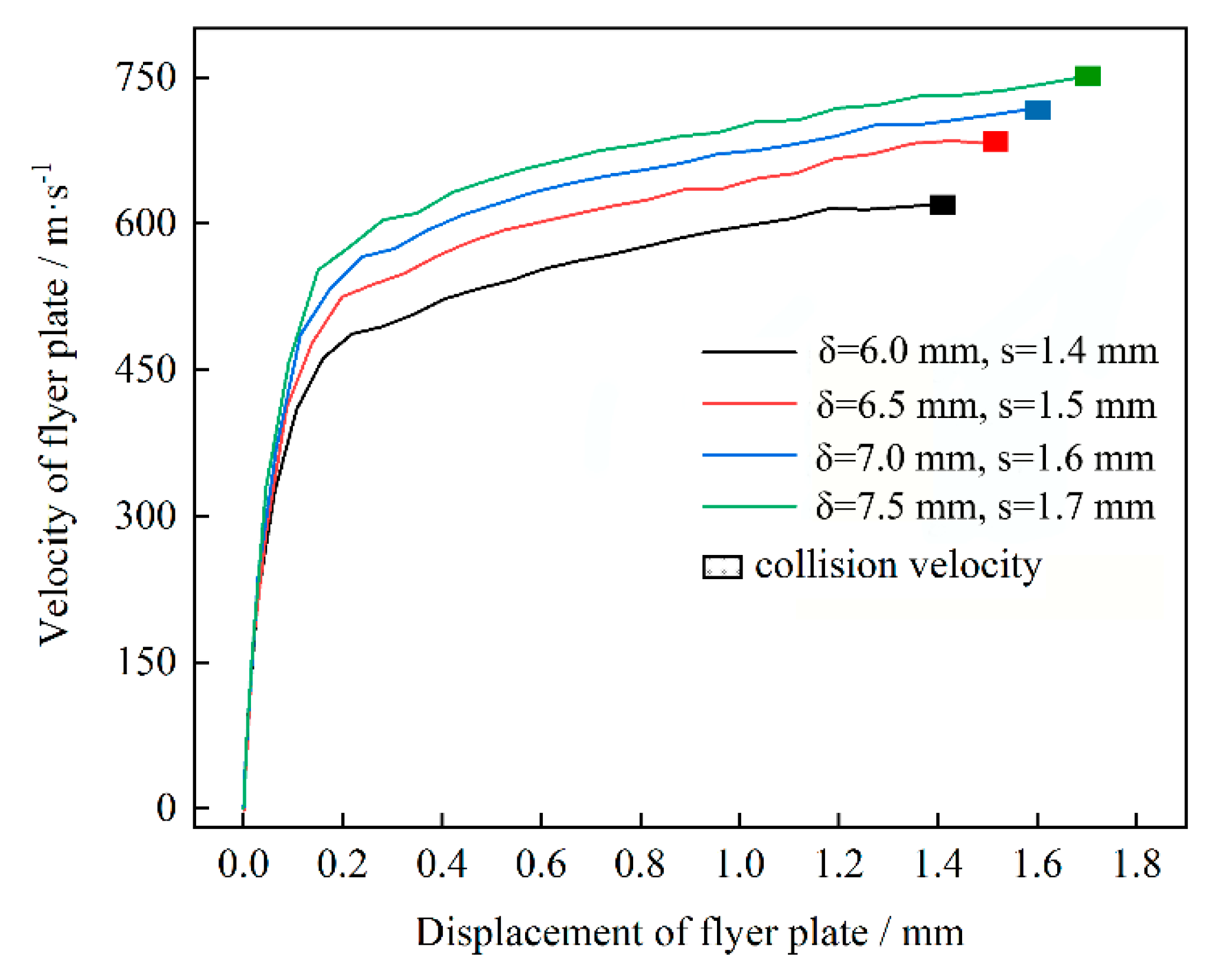



| Explosive Thickness δ/mm | Stand-Off Distance s/mm | Collision Velocity Vp/m·s−1 | Collision Angle β/° |
|---|---|---|---|
| 6.0 | 1.4 | 620 | 12.7 |
| Element | Si | Cu | Mg | Zn | Mn | Cr | Fe | Al |
|---|---|---|---|---|---|---|---|---|
| Content | ≤0.25 | ≤0.10 | 2.20–2.80 | ≤0.10 | ≤0.10 | 0.15–0.35 | ≤0.40 | Bal. |
| Element | Al | Si | Mn | Ca | Zn | Mn | Fe | Cu | Mg |
|---|---|---|---|---|---|---|---|---|---|
| Content | 2.50–3.50 | 0.08 | 2.00 | 0.04 | 0.6–1.4 | 0.2–1.0 | 0.003 | 0.01 | Bal. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Li, X.; Shi, B.; Wu, Y. Experimental and Numerical Studies on Preparation of Thin AZ31B/AA5052 Composite Plates Using Improved Explosive Welding Technique. Metals 2020, 10, 1023. https://doi.org/10.3390/met10081023
Wang Q, Li X, Shi B, Wu Y. Experimental and Numerical Studies on Preparation of Thin AZ31B/AA5052 Composite Plates Using Improved Explosive Welding Technique. Metals. 2020; 10(8):1023. https://doi.org/10.3390/met10081023
Chicago/Turabian StyleWang, Qi, Xuejiao Li, Biming Shi, and Yong Wu. 2020. "Experimental and Numerical Studies on Preparation of Thin AZ31B/AA5052 Composite Plates Using Improved Explosive Welding Technique" Metals 10, no. 8: 1023. https://doi.org/10.3390/met10081023
APA StyleWang, Q., Li, X., Shi, B., & Wu, Y. (2020). Experimental and Numerical Studies on Preparation of Thin AZ31B/AA5052 Composite Plates Using Improved Explosive Welding Technique. Metals, 10(8), 1023. https://doi.org/10.3390/met10081023




