Investigation on Solid-State Phase Transformations in a 2510 Duplex Stainless Steel Grade
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
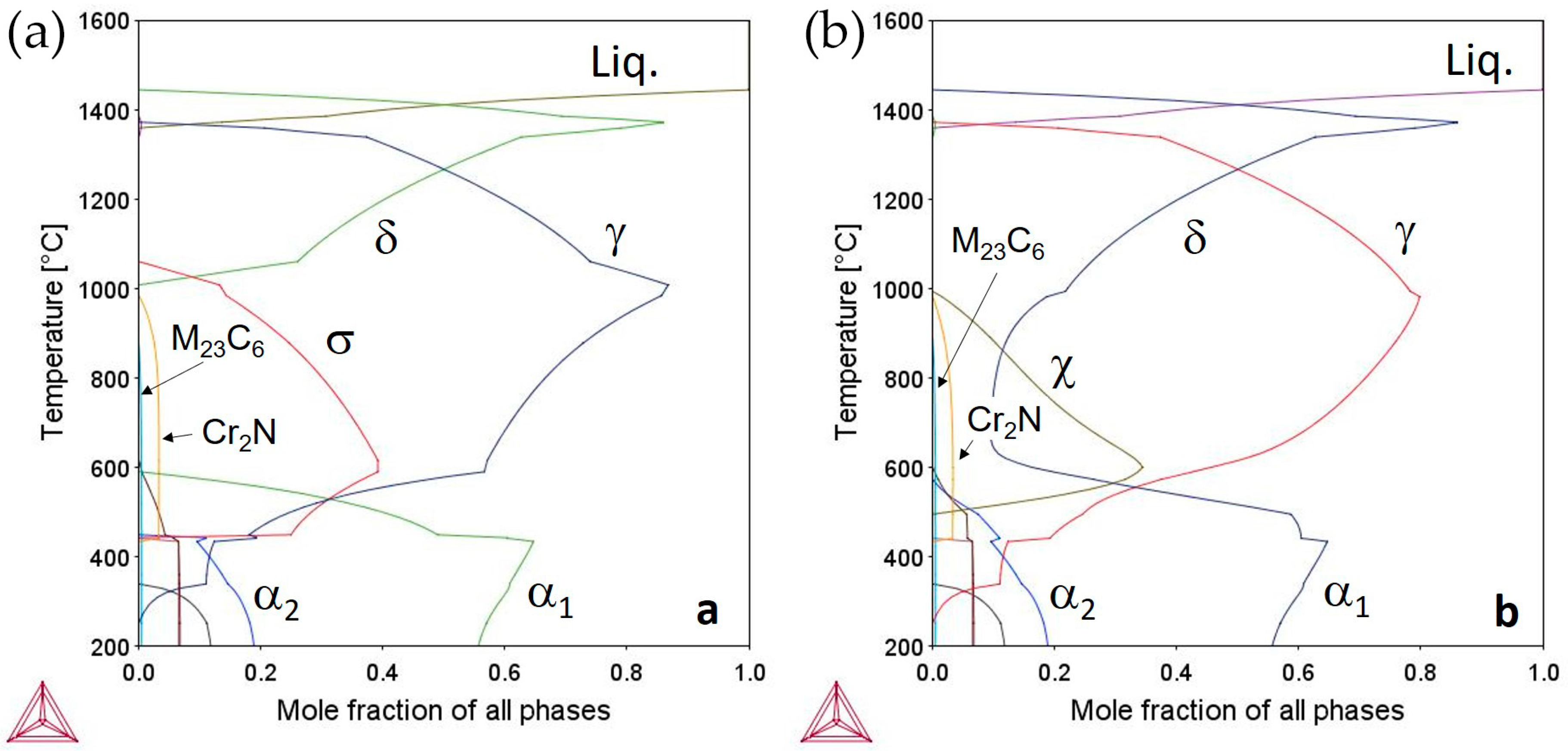
3.1. Thermodynamic Modeling
3.2. As-received Samples
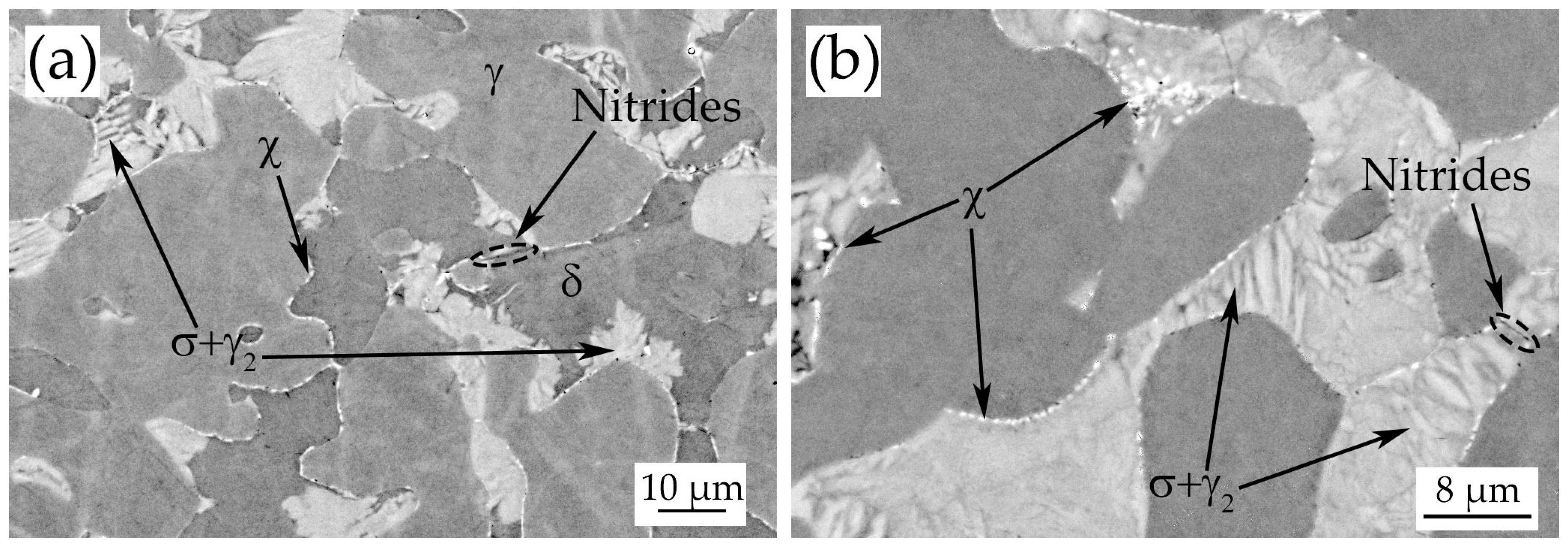
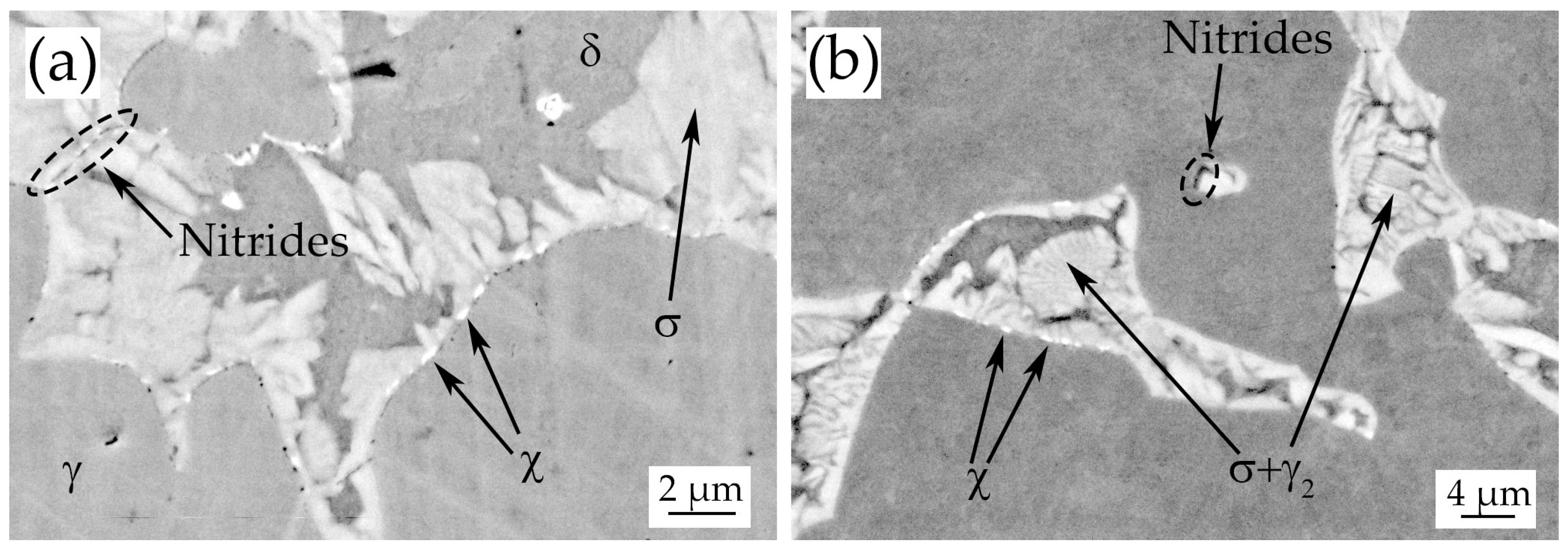
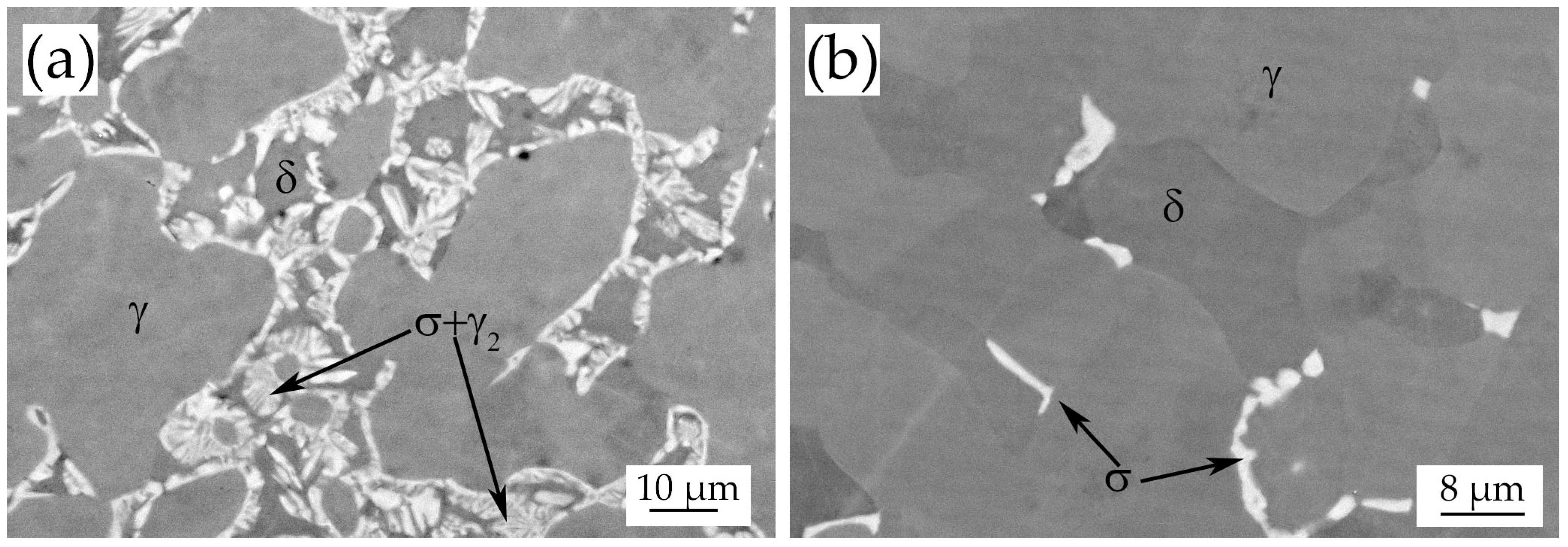
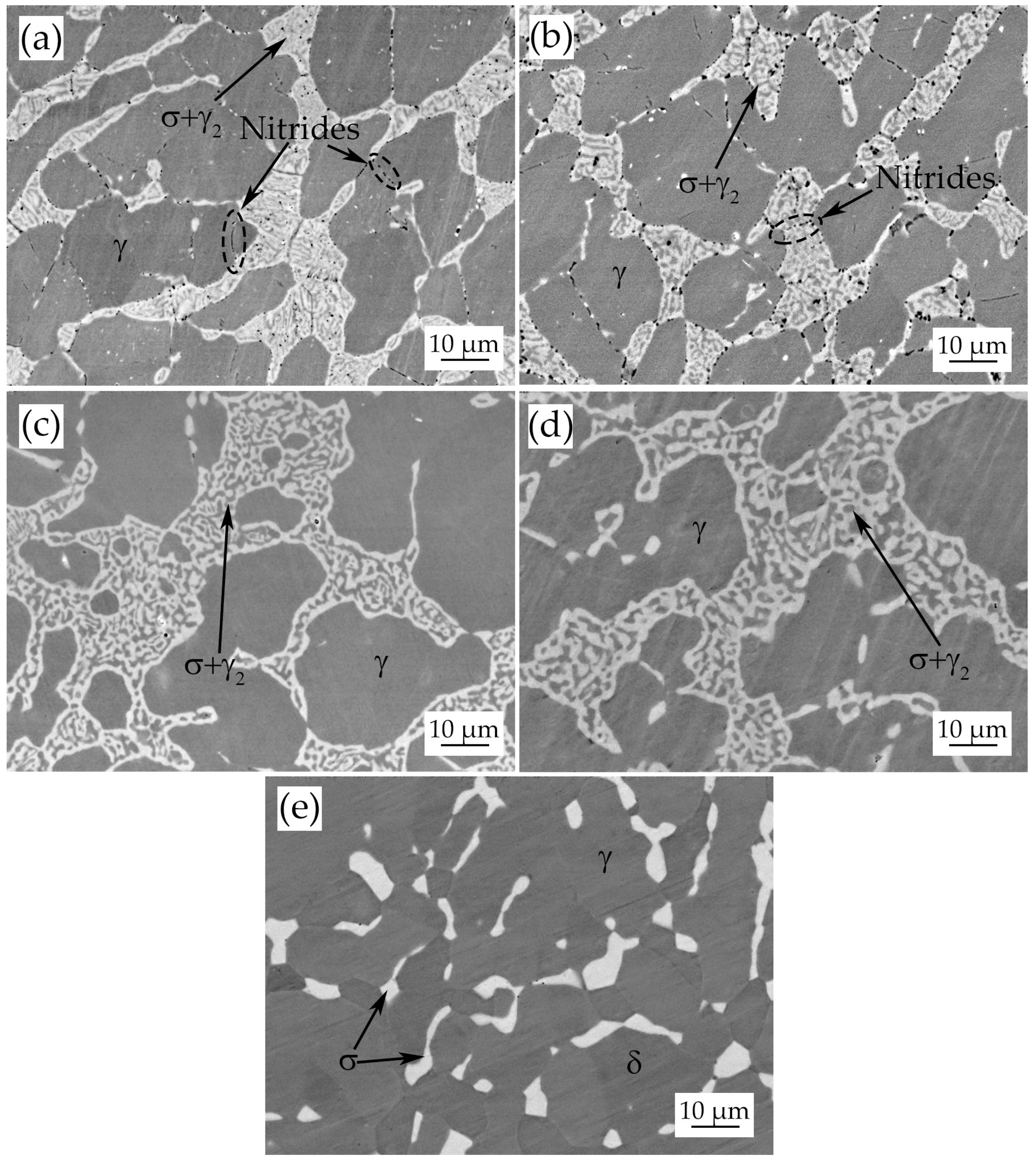
3.3. Heat-Treated Samples
3.4. Chemical Composition of the Phases
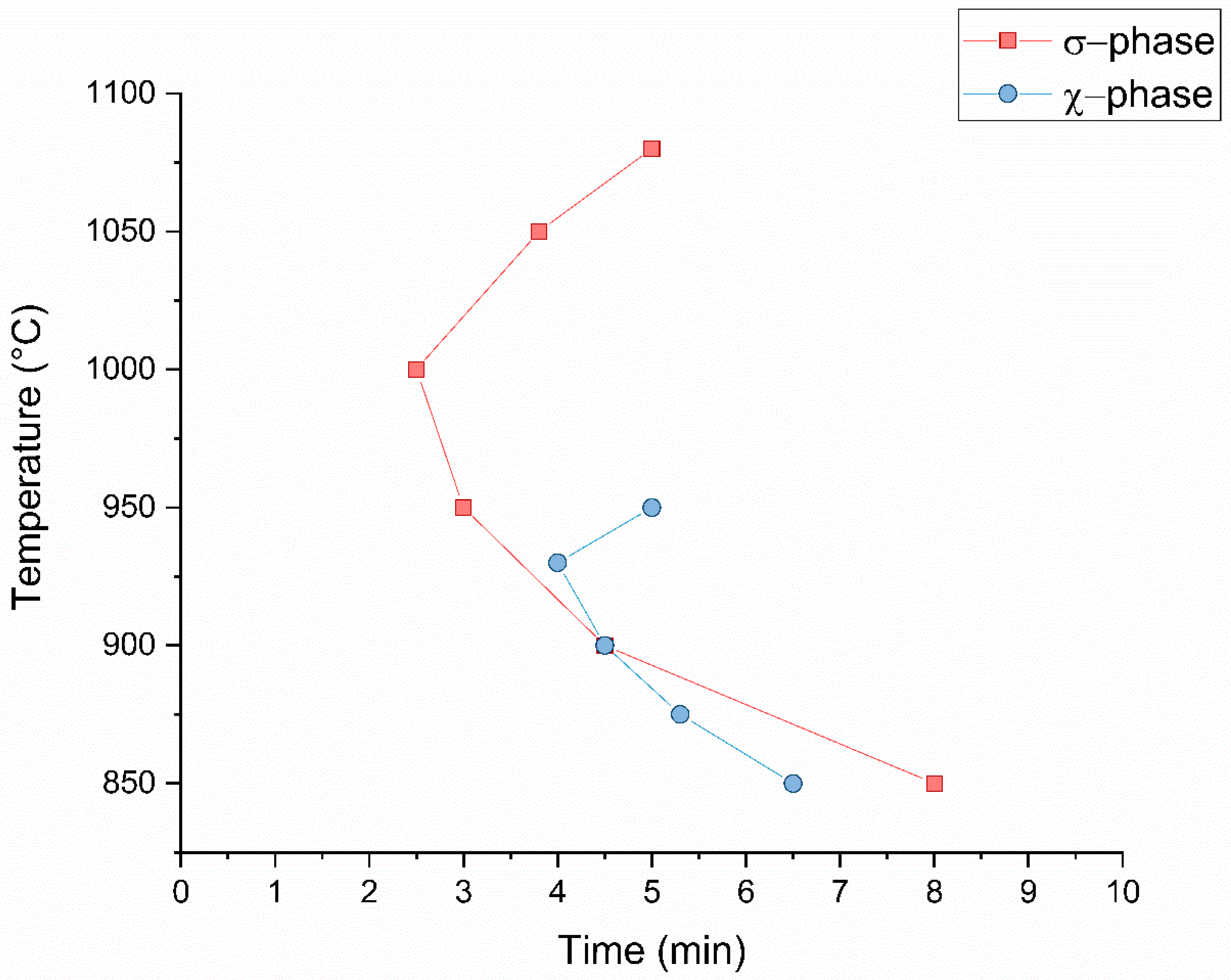
3.5. Precipitation Kinetics
4. Conclusions
- During the isothermal treatments at 850 °C–1080 °C σ-phase and χ-phase precipitate at the δ/γ grain boundaries after 4–10 min.
- The noses of TTP curves for σ-phase and χ-phase are at 1000 °C (2.5 min) and at 925 °C (4 min), respectively. These points are at a higher temperature, if compared to those of 2205 and 2507 grades, and the precipitation is more copious: these can be ascribed to the higher Cr and Mo content, which favors ferrite transformation.
- Ferrite completely transforms after a relatively short holding time (5–30 min) at every temperature.
- Chromium nitrides (probably Cr2N) were detected at grain boundaries in every treated sample up to 900 °C.
- Experimental results are in good agreement with those predicted by thermodynamic modeling provided by Thermo-Calc software and TCFe6 database: the temperature range of phase stability is predicted with reasonable accuracy, as well as the volume fraction and phase composition. Most discrepancies were ascribed to kinetic effects, i.e., the slowdown of eutectic decomposition leading to σ-phase formation and the reduced elements partitioning below 900 °C.
- The present study is a starting point to understand and model continuous cooling transformations of the welding process to improve welding quality in terms of mechanical, metallurgical and corrosion properties.
Author Contributions
Funding
Conflicts of Interest
References
- Nilsson, J.-O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Kangas, P.; Karlsson, T.; Wilson, A. Microstructural stability and mechanical properties of a high nitrogen super duplex stainless steel. Mater. Sci. Forum 1999, 318, 751–756. [Google Scholar] [CrossRef]
- Chen, T.H.; Weng, K.L.; Yang, J.R. The effect of high-temperature exposure on the microstructural stability and toughness property in a 2205 duplex stainless steel. Mater. Sci. Eng. A 2002, 338, 259–270. [Google Scholar] [CrossRef]
- Karlsson, L.; Bengtsson, L.; Rolander, U.; Pak, S. The Kinetics of Intermetallic Phase Formation and is Effect on Corrosion Resistance of Duplex Stainless Steels SS 2377 (UNS 31803). In Application of Stainless Steels; The Institute of Metaks: Stockholm, Sweden, 1992; p. 335. [Google Scholar]
- Kashiwar, A.; Vennela, N.P.; Kamath, S.L.; Khatirkar, R.K. Effect of solution annealing temperature on precipitation in 2205 duplex stainless steel. Mater. Charact. 2012, 74, 55–63. [Google Scholar] [CrossRef]
- Leiva-García, R.; Fernandes, J.C.S.; Muñoz-Portero, M.J.; García-Antón, J. Study of the sensitisation process of a duplex stainless steel (UNS 1.4462) by means of confocal microscopy and localised electrochemical techniques. Corros. Sci. 2015, 94, 327–341. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, T.; Hu, J.; Jiang, Y.; Jiang, L.; Li, J. Microstructure evolution and pitting corrosion resistance of the Gleeble-simulated heat-affected zone of a newly developed lean duplex stainless steel 2002. J. Alloys Compd. 2016, 658, 1031–1040. [Google Scholar] [CrossRef]
- Melo, E.A.; Magnabosco, R. Influence of the Heterogeneous Nucleation Sites on the Kinetics of Intermetallic Phase Formation in Aged Duplex Stainless Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 5273–5284. [Google Scholar] [CrossRef]
- Yang, S.M.; Chen, Y.C.; Chen, C.H.; Huang, W.P.; Lin, D.Y. Microstructural characterization of δ/γ/σ/γ2/χ phases in silver-doped 2205 duplex stainless steel under 800 °C aging. J. Alloys Compd. 2015, 633, 48–53. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; López-Luque, H.; López-Jiménez, I.; Biezma, M.V. Identification of sigma and chi phases in duplex stainless steels. Mater. Charact. 2016, 112, 20–29. [Google Scholar] [CrossRef]
- Breda, M.; Pellizzari, M.; Frigo, M. σ-Phase in lean duplex stainless steel sheets. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 331–337. [Google Scholar] [CrossRef]
- Johnson, E.; Kim, Y.J.; Scott Chumbley, L.; Gleeson, B. Initial phase transformation diagram determination for the CD3MN cast duplex stainless steel. Scr. Mater. 2004, 50, 1351–1354. [Google Scholar] [CrossRef]
- Calliari, I.; Zanesco, M.; Ramous, E. Influence of isothermal aging on secondary phases precipitation and toughness of a duplex stainless steel SAF 2205. J. Mater. Sci. 2006, 41, 7643–7649. [Google Scholar] [CrossRef]
- Domínguez-Aguilar, M.A.; Newman, R.C. Detection of deleterious phases in duplex stainless steel by weak galvanostatic polarization in alkaline solution. Corros. Sci. 2006, 48, 2560–2576. [Google Scholar] [CrossRef]
- da Fonseca, G.S.; Mendes, P.S.N.; Silva, A.C.M. Sigma Phase: Nucleation and Growth. Metals 2019, 9, 34. [Google Scholar] [CrossRef]
- Straffelini, G.; Baldo, S.; Calliari, I.; Ramous, E. Effect of Aging on the Fracture Behavior of Lean Duplex Stainless Steels. Metall. Mater. Trans. A 2009, 40, 2616–2621. [Google Scholar] [CrossRef]
- Calliari, I.; Ramous, E.; Bassani, P. Phase Transformation in Duplex Stainless Steels after Isothermal Treatments, Continuous Cooling and Cold Working. Mater. Sci. Forum 2010, 638–642, 2986–2991. [Google Scholar] [CrossRef]
- Calliari, I.; Breda, M.; Ramous, E.; Brunelli, K.; Pizzo, M.; Menapace, C. Impact toughness of an isothermally treated Zeron® 100 SDSS. J. Mater. Eng. Perform. 2012, 21, 2117–2123. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Karlsson, L.; Wessman, S.; Fuertes, N. Effect of sigma phase morphology on the degradation of properties in a super duplex stainless steel. Materials 2018, 1, 933. [Google Scholar] [CrossRef]
- Kaçar, R.; Baylan, O. An investigation of microstructure/property relationships in dissimilar welds between martensitic and austenitic stainless steels. Mater. Des. 2004, 25, 317–329. [Google Scholar] [CrossRef]
- Mallik, A.K. Computer calculations of phase diagrams. Bull. Mater. Sci. 1986, 8, 107–121. [Google Scholar] [CrossRef]
- Sundman, B.; Jansson, B.; Andersson, J.O. The Thermo-Calc databank system. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Wessman, S.; Pettersson, R.; Hertzman, S. On phase equilibria in duplex stainless steels. Steel Res. Int. 2010, 81, 337–346. [Google Scholar] [CrossRef]
- Calliari, I.; Pellizzari, M.; Ramous, E. Precipitation of secondary phases in super duplex stainless steel ZERON100 isothermally aged. Mater. Sci. Technol. 2011, 27, 928–932. [Google Scholar] [CrossRef]
- Calliari, I.; Pellizzari, M.; Zanellato, M.; Ramous, E. The phase stability in Cr-Ni and Cr-Mn duplex stainless steels. J. Mater. Sci. 2011, 46, 6916–6924. [Google Scholar] [CrossRef]
- Sato, Y.S.; Kokawa, H. Preferential precipitation site of sigma phase in duplex stainless steel weld metal. Scr. Mater. 1999, 40, 659–663. [Google Scholar] [CrossRef]
- Kasper, J.S. The ordering of atoms in the chi-phase of the iron-chromium-molybdenum system. Acta Metall. 1954, 2, 456–461. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. σ-phase morphologies and their effect on mechanical properties of duplex stainless steels. Int. J. Mater. Res. 2008, 99, 1163–1170. [Google Scholar] [CrossRef]
- Magnabosco, R. Kinetics of sigma phase formation in a duplex stainless steel. Mater. Res. 2009, 12, 321–327. [Google Scholar] [CrossRef]
- Elmer, J.; Palmer, T.; Specht, E. Direct Observations of Sigma Phase Formation in Duplex Stainless Steels using In Situ Synchrotron X-Ray Diffraction. Metall. Trans. A 2007, 38, 464–475. [Google Scholar] [CrossRef]
- Byun, S.H.; Kang, N.; Lee, T.H.; Ahn, S.K.; Lee, H.W.; Chang, W.S.; Cho, K.M. Kinetics of Cr/Mo-rich precipitates formation for 25Cr-6.9Ni-3.8Mo-0.3N super duplex stainless steel. Met. Mater. Int. 2012, 18, 201–207. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ugurlu, O.; Jiang, C.; Gleeson, B.; Chumbley, L.S. Microstructural evolution of secondary phases in the cast duplex stainless steels CD3MN and CD3MWCuN. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2007, 38, 203–211. [Google Scholar] [CrossRef][Green Version]
- Calliari, I.; Brunelli, K.; Dabalà, M.; Ramous, E. Measuring secondary phases in duplex stainless steels. J. Miner. Met. Mater. Soc. 2009, 61, 80–83. [Google Scholar] [CrossRef]
- Deng, B.; Jiang, Y.M.; Gao, J.; Li, J. Effect of annealing treatment on microstructure evolution and the associated corrosion behavior of a super-duplex stainless steel. J. Alloys Compd. 2010, 493, 461–464. [Google Scholar] [CrossRef]
- Calliari, I.; Dabalà, M.; Ramous, E.; Straffelini, G. New Lean Duplex Stainless Steels for Structural Applications. Mater. Sci. Forum 2008, 604–605, 419–426. [Google Scholar] [CrossRef]
| Steel Grade | C | Si | Mn | Cr | Ni | Mo | P | S | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 2510 SDSS | 0.020 | 0.44 | 0.37 | 25.30 | 9.9 | 4.0 | 0.018 | 0.001 | 0.275 | Bal. |
| T (°C)/t (min) | 3 | 4 | 5 | 7 | 10 | 15 | 25 | 30 | 24 h |
|---|---|---|---|---|---|---|---|---|---|
| 850 | × | × | × | × | × | × | |||
| 900 | × | × | × | × | × | ||||
| 950 | × | × | × | ||||||
| 1000 | × | × | × | ||||||
| 1050 | × | × | × | ||||||
| 1080 | × | × | × |
| Element | Ferrite | Austenite | Partition Coefficient (Ferrite/Austenite) | |||
|---|---|---|---|---|---|---|
| EDS | Thermo-Calc | EDS | Thermo-Calc | EDS | Thermo-Calc | |
| Cr | 28.1 | 28.6 | 24.0 | 23.9 | 1.17 | 1.19 |
| Mo | 6.0 | 5.3 | 3.9 | 3.4 | 1.54 | 1.56 |
| Ni | 6.6 | 6.6 | 10.7 | 11.3 | 0.61 | 0.58 |
| Temperature | Ferrite | Austenite | Sigma | Cr2N | ||||
|---|---|---|---|---|---|---|---|---|
| TC | Exp. | TC | Exp. | TC | Exp. | TC | Exp. | |
| 850 °C | - | - | 70.4 | 79.2 | 26.7 | 20.8 | 2.8 | yes |
| 900 °C | - | - | 75.0 | 79.5 | 22.8 | 20.5 | 2.2 | yes |
| 950 °C | - | - | 80.9 | 78.9 | 18.0 | 21.1 | 1.1 | - |
| 1000 °C | - | - | 86.4 | 79.9 | 13.6 | 20.1 | - | - |
| 1050 °C | 20.8 | 18.6 | 76.6 | 71.7 | 2.5 | 9.7 | - | - |
| 1100 °C | 30.0 | 29.4 | 70.0 | 70.6 | - | - | - | - |
| Temperature | Time | Ferrite | Austenite | Sigma | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Ni | Mo | Cr | Ni | Mo | Cr | Ni | Mo | ||
| 850 °C | 30′ | - | - | - | 23.8 | 11.0 | 3.8 | 28.3 | 6.4 | 6.5 |
| 24 h | - | - | - | 24.3 (19.7) | 11.1 (12.4) | 3.7 (1.7) | 30.1 (35.4) | 5.6 (4.3) | 8.2 (9.8) | |
| 900 °C | 30′ | - | - | - | 23.9 | 11.0 | 3.5 | 28.3 | 6.6 | 6.5 |
| 24 h | - | - | - | 24.2 (21.1) | 11.0 (11.8) | 3.7 (1.9) | 30.3 (34.9) | 5.9 (4.4) | 9.0 (10.5) | |
| 950 °C | 5′ | - | - | - | 23.7 | 10.9 | 3.9 | 28.2 | 7.1 | 7.4 |
| 24 h | - | - | - | 23.9 (22.6) | 11.1 (11.2) | 3.8 (2.3) | 28.9 (34.3) | 5.9 (4.5) | 10.5 (11.4) | |
| 1000 °C | 5′ | - | - | - | 23.7 | 11.2 | 3.7 | 28.00 | 6.9 | 7.5 |
| 24 h | - | - | - | 24.1 (23.9) | 11.3 (10.8) | 3.5 (2.6) | 31.3 (33.7) | 5.5 (4.6) | 10.1 (12.2) | |
| 1050 °C | 10′ | 28.4 | 6.3 | 5.7 | 24.2 | 11.4 | 3.8 | 30.1 | 5.7 | 11.0 |
| 24 h | 28.9 (29.4) | 6.1 (6.1) | 5.9 (5.2) | 23.7 (23.9) | 10.9 (11.1) | 3.4 (3.3) | 31.1 (32.3) | 5.4 (4.8) | 11.8 (14.1) | |
| Temperature | Si | Mn | Cr | Ni | Mo | C | N | Fe |
|---|---|---|---|---|---|---|---|---|
| 850 °C | 0 | 0.06 | 81.73 | 0.08 | 5.95 | 0.37 | 10.51 | Balance |
| 900 °C | 0 | 0.06 | 82.06 | 0.11 | 5.11 | 0.40 | 10.40 | Balance |
| 950 °C | 0 | 0.06 | 82.20 | 0.14 | 4.43 | 0.34 | 10.40 | Balance |
| 1000 °C | - | - | - | - | - | - | - | Balance |
| 1050 °C | - | - | - | - | - | - | - | Balance |
| 1100 °C | - | - | - | - | - | - | - | Balance |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calliari, I.; Breda, M.; Gennari, C.; Pezzato, L.; Pellizzari, M.; Zambon, A. Investigation on Solid-State Phase Transformations in a 2510 Duplex Stainless Steel Grade. Metals 2020, 10, 967. https://doi.org/10.3390/met10070967
Calliari I, Breda M, Gennari C, Pezzato L, Pellizzari M, Zambon A. Investigation on Solid-State Phase Transformations in a 2510 Duplex Stainless Steel Grade. Metals. 2020; 10(7):967. https://doi.org/10.3390/met10070967
Chicago/Turabian StyleCalliari, Irene, Marco Breda, Claudio Gennari, Luca Pezzato, Massimo Pellizzari, and Andrea Zambon. 2020. "Investigation on Solid-State Phase Transformations in a 2510 Duplex Stainless Steel Grade" Metals 10, no. 7: 967. https://doi.org/10.3390/met10070967
APA StyleCalliari, I., Breda, M., Gennari, C., Pezzato, L., Pellizzari, M., & Zambon, A. (2020). Investigation on Solid-State Phase Transformations in a 2510 Duplex Stainless Steel Grade. Metals, 10(7), 967. https://doi.org/10.3390/met10070967









