Abstract
Marked degradation of tensile properties induced by plastic deformation after dynamic interactions between strain-induced martensite transformation and hydrogen has been investigated for type 316L stainless steel by hydrogen thermal desorption analysis. Upon modified hydrogen charging reported previously, the amount of hydrogen desorbed in the low temperature range increases; the degradation of tensile properties induced by interactions between plastic deformation and hydrogen at 25 °C or induced by interactions between martensite transformation and hydrogen at −196 °C occurs even for the stainless steel with high resistance to hydrogen embrittlement. The hydrogen thermal desorption behavior is changed by each interaction, suggesting changes in hydrogen states. For specimen fractured at 25 °C, the facet-like morphology and transgranular fracture are observed on the outer part of the fracture surface. At −196 °C, a quasi-cleave fracture is observed at the initiation area. Modified hydrogen charging significantly interacts both plastic deformation and martensite transformation, eventually enhancing the degradation of tensile properties. Upon plastic deformation at 25° C after the interactions between martensite transformation and hydrogen by straining to 0.2 at −196 °C, cracks nucleate in association with martensite formed by the interactions at −196 °C and marked degradation of tensile properties occurs. It is likely that the interactions between martensite transformation and hydrogen induce damage directly related to the degradation, thereby affecting subsequent deformation. Upon dehydrogenation after the interactions between the martensite transformation and hydrogen, no degradation of tensile properties is observed. The damage induced by the interactions between martensite transformation and hydrogen probably changes to harmless defects during dehydrogenation.
1. Introduction
Austenitic stainless steels are expected to be used as structural materials for hydrogen energy systems, including storage tanks, containers, and line pipes exposed to gaseous and liquid hydrogen for hydrogen service at low temperature, because of their high resistance to hydrogen embrittlement [1,2]. The resistance to hydrogen embrittlement for austenitic stainless steels depends on Ni equivalent or Md30 [3,4,5,6,7,8]. For type 316L stainless steel containing more than 12 mass% Ni, the resistance to hydrogen embrittlement is considered to be superior from the results obtained by conventional cathodic hydrogen charging or gas charging [4,5,6,7,8,9]. Upon hydrogen charging, tensile strain slightly decreases, but the fracture surface generally consists of dimples [3,4,5,6,9,10,11,12]. No significant hydrogen embrittlement occurs except at around −70 °C [4,13,14,15,16].
One of the reasons for the high resistance of type 316L stainless steel to hydrogen embrittlement may be its high austenite stability, although we omit the explanation of conventional mechanisms of hydrogen embrittlement here. The effects of the martensite phase on hydrogen embrittlement have been examined extensively for various austenitic stainless steels [17,18,19,20,21,22,23]. The martensite phase presumably enhances hydrogen embrittlement even for type 316L stainless steel in fatigue and tensile tests [22,23]. However, the martensite phase induced by pre-straining only slightly enhances hydrogen embrittlement [24,25]. In addition, although almost no martensite phase exists under tensile test at −40 °C, hydrogen embrittlement is enhanced [6]. There are studies discussions on whether the ε-martensite phase enhances hydrogen embrittlement [12,26,27,28,29,30]. Since the enhancement of hydrogen embrittlement is not necessarily related to the martensite phase, other factors should be considered. For example, it appears that damage and/or defects induced by interactions between plastic deformation and hydrogen or interactions between martensite transformation and hydrogen play important roles in hydrogen embrittlement. The damage and defects are directly and indirectly related to hydrogen embrittlement, respectively. Plastic deformation or the strain-induced martensite transformation readily interacts with unstable hydrogen such as diffusible hydrogen and hydrogen in solid solution [31,32,33,34,35]. As a result of the interactions, damage and defects are induced, thereby enhancing hydrogen embrittlement.
For the Ni–Ti superelastic alloy [34,35], since the stress-induced martensite transformation occurs before plastic deformation, hydrogen embrittlement essentially dominates the interactions between martensite transformation and hydrogen. In particular, the effects of the first interactions between martensite transformation and hydrogen in solid solution on hydrogen embrittlement are greatest [34]. The subsequent interactions between the transformation and hydrogen become gradually small, because hydrogen trapped at defects induced by the interactions slightly interacts with the subsequent martensite transformation [34]. The cyclic interactions induce the accumulation of substantial amounts of defects; accordingly, defects change to damage, i.e., the effects of hydrogen in solid solution on the interactions are stronger than those of hydrogen trapped at defects. Actually, upon training before hydrogen charging, hydrogen is preferentially trapped at defects induced by training; hence, hydrogen embrittlement is considerably suppressed [34]. Part of this mechanism may apply to other alloys with martensite transformation.
For austenitic stainless steels, because the strain-induced martensite transformation occurs after plastic deformation to some extent, most of hydrogen is trapped at defects induced by interactions between plastic deformation and hydrogen before the martensite transformation. As a result, hydrogen embrittlement appears to be suppressed. This amount of plastic deformation before martensite transformation decreases at a high strain rate and low temperature for thin plates insensitive to adiabatic heat [36,37,38]. For type 316L stainless steel, in particular, a large amount of plastic deformation is required before martensite transformation occurs because of the high stability of austenite. However, if a substantial amount of unstable hydrogen is charged, untrapped hydrogen will remain before martensite transformation. Accordingly, hydrogen embrittlement will be enhanced even for type 316L stainless steel. Under severe hydrogen charging conditions such as high current density [39], a slight brittle fracture has often been observed in a tensile test at room temperature, implying that some amount of unstable hydrogen is charged. Using modified hydrogen charging [24], we have demonstrated that the amount of hydrogen thermally desorbed in the low temperature range increases, eventually considerably enhancing the hydrogen embrittlement for type 304 stainless steel. The hydrogen desorbed in the low temperature range is presumably related to unstable states and strongly interacts with martensite transformation [21]. The modified charging possibly introduces substantial amount of unstable hydrogen even in type 316L stainless steel. If the interactions between the strain-induced martensite transformation and hydrogen induce damage, the tensile properties after the interactions will be degraded markedly.
The objective of the present study is to evaluate the hydrogen embrittlement behavior and deformation after interactions between strain-induced martensite transformation and hydrogen for type 316L stainless steel using modified hydrogen charging.
2. Materials and Methods
A commercially available type 316L stable austenitic stainless steel sheet subjected to solution heat treatment at 1120 °C after cold working with a thickness of 0.50 mm was used. The nominal chemical composition is shown in Table 1. The Ni equivalent (Nieq) and Md30 were calculated to be 27.8 mass% and −111 °C, respectively, using formulas reported previously [40,41],
and
where all elements are in mass fractions and ν is ASTM International grain size number. The gauge length and width of the specimens tested were 10 and 3 mm, respectively. The 0.2% proof and tensile strengths were 287 MPa and 577 MPa, respectively, at a strain rate of 8.33 × 10−4 s−1 and 25 ± 2 °C. The fracture strain was approximately 0.75. The strain for each specimen was calculated from the displacement of the cross head and the initial gauge length. An optical micrograph of the surface of a specimen etched with aqua regia (mixed solution of HCl and HNO3) is shown in Figure 1. The grains were polygonal, and the average grain size was approximately 20 μm measured by linear intercept method.
Nieq (mass %) = Ni + 0.65Cr + 0.98Mo + 1.05Mn + 12.6C,
Md30 (°C) = 551 − 462(C + N) − 9.2Si − 8.1Mn − 13.7Cr − 29.0(Ni + Cu) − 18.5Mo − 68.0Nb − 1.42(ν − 8.0),

Table 1.
Nominal chemical composition of specimen used (mass %).
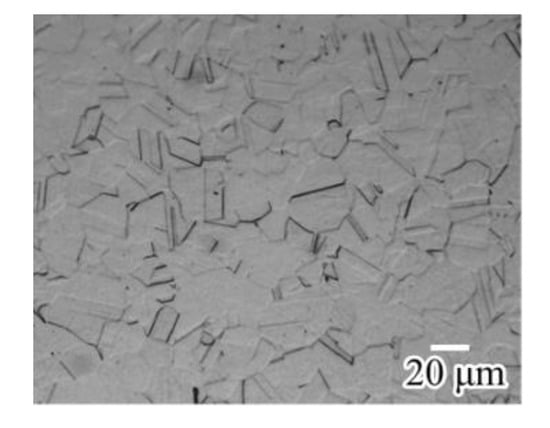
Figure 1.
Optical micrograph of surface of specimen.
Hydrogen was cathodically charged in 3.5% NaCl aqueous solution at 80 °C with a current density of 100 A/m2 under aerated condition. No catalytic poison was added in the solution to avoid contaminating the surface of the specimen. To diffuse hydrogen from the surface to the inside of a specimen as much as possible, the charging time was 96 h. A platinum wire was used as the counter electrode. This method is referred to as conventional hydrogen charging in the present study. As reported previously [24], for modified hydrogen charging, the counter electrode was enclosed in a glass tube with a porous bottom to prevent the dissolution of chlorine and oxygen gases, which evolved on the surface of counter electrode, into the solution (Figure 2). The other conditions of modified hydrogen charging were the same as those of conventional charging.
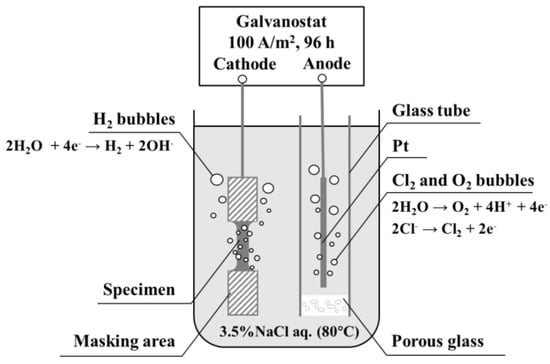
Figure 2.
Schematic diagram of modified hydrogen charging.
To evaluate the amount of charged hydrogen and hydrogen states, hydrogen thermal desorption analysis (TDA) was performed using a gas chromatograph at a constant heating rate of 100 °C/h from room temperature 25 ± 2 °C to 600 °C (J-SCIENCE LAB Co. Ltd., GC-MS, Kyoto, Japan), and a gas sample was analyzed at 5 min intervals using Ar as the carrier gas. TDA was started 15 min after the end of hydrogen charging. The amount of desorbed hydrogen (charged hydrogen) was defined as the integrated peak intensity. The amount of desorbed hydrogen for the uncharged specimen was less than the detection limit.
Tensile tests were carried out at 25 ± 2 °C in air and −196 °C in liquid nitrogen after 30 min from the end of hydrogen charging. The strain rate in tensile tests at 25 °C was 8.33 × 10−4 s−1. To reduce plastic deformation before martensite transformation as much as possible, the high strain rate of 8.33 × 10−2 s−1 was selected at −196 °C [36,37,38]. Moreover, to investigate the effects of interactions between the strain-induced martensite transformation and hydrogen on subsequent deformation, the charged specimens subjected to 0.2 straining at −196 °C were tensile-deformed until fracture at 25 °C in air (Figure 3). The fracture surfaces were examined by scanning electron microscopy (SEM: JEOL Ltd., JSM-6500F, Tokyo, Japan). The surface of a specimen finished with colloidal silica after grounding 60 μm from the surface was examined by electron backscatter diffraction pattern (EBSD) analysis using a program TSL OIM Data Collection (TexSEM Laboratories, Pegasus 2300 system, Tokyo, Japan).
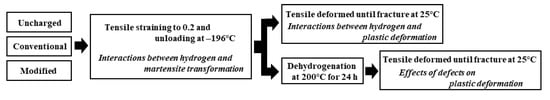
Figure 3.
Procedures for investigation effects of interactions between strain-induced martensite transformation and hydrogen on subsequent deformation.
To distinguish damage directly related to degradation from harmless defects for specimens subjected to modified hydrogen charging, dehydrogenation was carried out in air at 200 °C for 24 h immediately after straining to 0.2 at −196 °C. Immediately after dehydrogenation, the specimen was tensile-deformed until fracture at 25 °C in air, as shown in Figure 3. The amount of hydrogen desorbed from the specimen subjected to dehydrogenation was less than the detection limit.
3. Results and Discussion
3.1. Interactions between Martensite Transformation and Hydrogen
Hydrogen thermal desorption curves for specimens subjected to conventional or modified charging are shown in Figure 4. The amount of desorbed hydrogen for the modified charging varied widely (approximately 170–290 mass ppm), whereas that for the conventional charging was at most 35 mass ppm. Both charging methods probably provide adequate amounts of hydrogen for evaluating hydrogen embrittlement. For type 304 stainless steel [24], the amount of hydrogen charged by the modified method (324 ± 24 mass ppm) is larger than that of conventionally charged hydrogen (approximately 260 mass ppm). The amount of charged hydrogen was smaller for type 316L stainless steel than for type 304 stainless steel. Moreover, the extent of scattering of the amount of hydrogen was somewhat large for type 316L stainless steel. The reasons for these observations may be the differences in material factors such as the stability of austenite and microstructure, which should be further investigated in the future. It should be emphasized that the modified hydrogen charging method enhances hydrogen absorption irrespective of the type of stainless steel. For type 316L stainless steel subjected to modified hydrogen charging, the hydrogen desorbed from the specimen starting at room temperature to 400 °C with a single peak at 200 °C. For type 304 stainless steel subjected to modified hydrogen charging [24], the amount of hydrogen desorbed in the low temperature range, i.e., from room temperature to 200 °C, increases associated with potential shifting to the less noble direction and with increasing pH. The modified charging presumably leads to the increases in the amounts of absorbed hydrogen and hydrogen desorbed in the low temperature range related to unstable states of hydrogen, such as diffusible hydrogen for type 316L stainless steel as well as type 304 stainless steel.
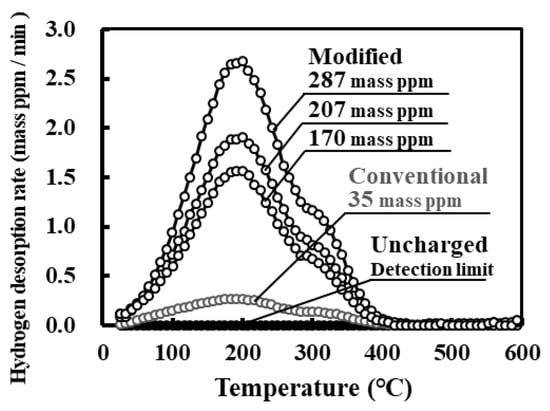
Figure 4.
Hydrogen thermal desorption curves for uncharged specimen and specimens subjected to conventional and modified hydrogen charging. Values are the amounts of desorbed hydrogen.
Charged hydrogen was concentrated at the surface layer of the specimen. Using the hydrogen diffusion coefficient at 80 °C (D = 7.2 × 10−15 m2·s−1) reported previously [42] and the amount of hydrogen charged by the modified method for 96 h (200 mass ppm), we estimated that hydrogen diffused from the surface of the specimen to a distance of approximately 80 µm. The hydrogen contents in the vicinity of the surface and at 80 µm from the surface of the specimen were higher than 1000 mass ppm and at least 200 mass ppm, respectively.
Figure 5 shows the typical tensile stress–strain curves at 25 °C and −196 °C for specimens subjected to conventional and modified charging. For conventional charging, the fracture strain at 25 °C slightly increased as compared with that for the uncharged specimen, because hardly any necking of the specimen occurs owing to the hardening of the specimen surface by hydrogen enrichment, as reported previously [24]. At −196 °C, the tensile behavior scarcely changed, although the proof and tensile strengths slightly increased. For modified charging, the proof and tensile strengths at 25 °C slightly increased, and the fracture strain decreased to around 0.4–0.5. At −196 °C, the proof and tensile strengths markedly increased, whereas the fracture strain decreased. In addition, almost no necking occurred. The increase in strength appears to be associated with the suppression of martensite transformation [43,44,45]. For type 316L stainless steel subjected to tensile test at −196 °C without hydrogen charging, the increase in flow stress is observed from the strain of 0.3 related to the strain-induced martensite transformation [46,47]. Similarly, for the uncharged and conventionally charged specimens, the increase in flow stress was observed in the present study. However, for the specimen subjected to modified hydrogen charging, the increase in flow stress from the strain at 0.3 was small, suggesting that martensite transformation is suppressed by hydrogen charged by the modified method. The suppression of the transformation due to the interactions between martensite transformation and hydrogen possibly enhances the formation of defects.
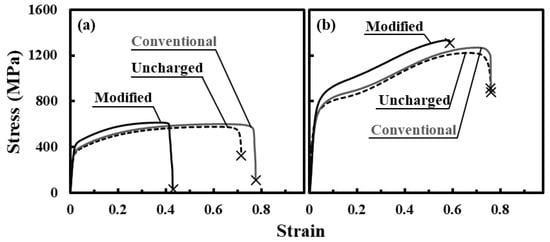
Figure 5.
Representative tensile stress–strain curves obtained at (a) 25 °C and (b) −196 °C for uncharged specimen and specimen subjected to conventional and modified hydrogen charging.
Figure 6 shows the thermal desorption curves for specimens fractured at 25 °C and −196 °C after modified hydrogen charging. The desorption peak shifted after fracture to the low temperature range, indicating that the hydrogen states are changed by plastic deformation and/or martensite transformation. The amount of peak shift for the specimen fractured at −196 °C was significantly larger than that at 25 °C. The primary cause of this is that upon heating, hydrogen readily diffuses in the martensite phase formed at −196 °C [22,48,49].
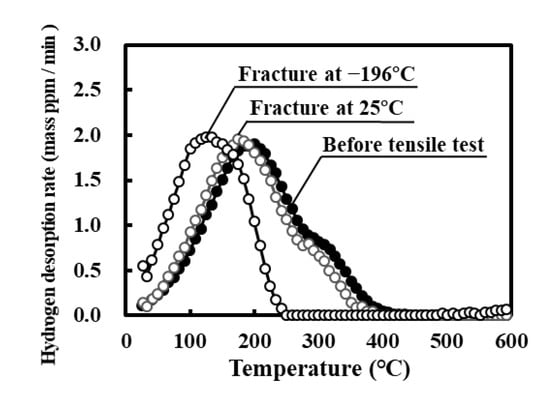
Figure 6.
Hydrogen thermal desorption curves for specimens subjected to tensile fracture at 25 °C or −196 °C after modified hydrogen charging. The curve for the specimen before the tensile test is reproduced from Figure 4.
Figure 7 shows the general and magnified views of fracture surfaces corresponding to the stress–strain curves shown in Figure 3. Upon tensile fracturing at 25 °C, for the uncharged specimen (Figure 7a), the reduction in area was approximately 82%, and the fracture surface was microscopically composed of primary and secondary dimples. For the conventional charging (Figure 7b), the reduction in area decreased to 68%, and small dimples of a few μm in size were observed. No marked embrittlement occurred, although the ductility slightly decreased. This result is consistent with that previously reported [42]. However, for the specimen subjected to modified charging (Figure 7c), almost no reduction in area was observed, and the facet-like morphology and transgranular fracture were observed at the rim of the fracture surface. The width of the rim almost corresponded to the hydrogen diffusion distance. This suggests that hydrogen desorbed in the low temperature range induced by modified charging plays important roles in interactions between plastic deformation of the austenite phase and hydrogen. It is likely that the interactions induce a substantial amount of damage. At −196 °C, for the uncharged specimen (Figure 7d), shear fracture macroscopically occurred; almost no reduction in area was observed, but the microscopic fracture surface was composed mainly of shear dimples. For conventional charging (Figure 7e), a similar shear fracture occurred. Effects of conventional charging on fracture morphology were scarcely observed except for small dimples at the rim of the fracture surface. This result agrees with that previously reported [42]. However, for modified charging (Figure 7f), no reduction in area was observed, and a quasi-cleave fracture was observed at the fracture initiation area and at the edges of both sides of the fracture surface. A slight transgranular fracture was observed. In comparison with type 304 stainless steel [24], the degree of degradation is small. Nevertheless, note that the dynamic interactions between the strain-induced martensite transformation and hydrogen desorbed in the low temperature range induced by modified charging lead to the enhancement of hydrogen embrittlement even for type 316L stainless steel.
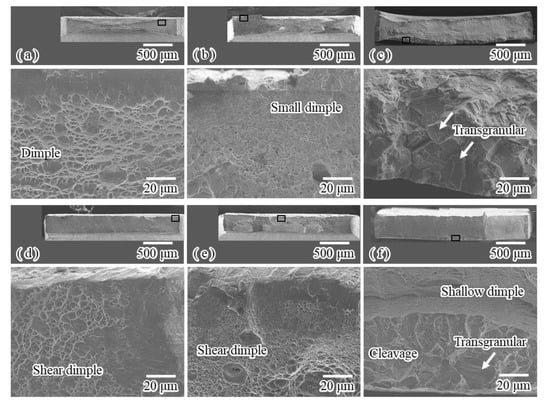
Figure 7.
SEM images of general and magnified views of representative fracture surfaces for (a) uncharged specimen, specimens subjected to (b) conventional and (c) modified hydrogen charging fractured at 25 °C, (d) uncharged specimen, and specimens subjected to (e) conventional and (f) modified hydrogen charging fractured at −196 °C.
One important finding of the present study is that by using modified charging, the resistance to hydrogen embrittlement at 25 °C and −196 °C decreases even for type 316L stainless steel as well as type 304 stainless steel reported previously [24]. However, the decrease in resistance to hydrogen embrittlement is smaller for type 316L stainless steel than for type 304 stainless steel. The origin of this can be explained by considering the interactions between the strain-induced martensite transformation and hydrogen. Under the tensile deformation at 25 °C, the strain-induced martensite transformation occurs for type 304 stainless steel, but not for type 316L stainless steel. Thus, the martensite transformation considerably enhances hydrogen embrittlement of type 304 stainless steel [24]. In contrast, the possible reason for the decrease in resistance to hydrogen embrittlement at −196 °C is as follows: Because the stability of austenite is higher for type 316L stainless steel than for type 304 stainless steel, the amount of martensite transformation is smaller for type 316L stainless steel than for type 304 stainless steel under the same applied strain. Consequently, the effects of interactions between martensite transformation and hydrogen on the enhancement of hydrogen embrittlement are probably smaller for type 316L stainless steel than for type 304 stainless steel. Although the resistance of type 316L stainless steel to hydrogen embrittlement is considered to be superior [6,14], on the basis of the results of the present study, the conditions of hydrogen charging in previous studies may not be necessarily sufficient to evaluate it.
3.2. Deformation after Interactions
The stress–strain curves for specimens subjected to tensile fracture at 25 °C after straining to 0.2 and unloading at −196 °C are shown in Figure 8. For the uncharged specimen, the fracture occurred after a large amount of necking formed. For conventional charging, the curve only slightly changed compared with that for the uncharged specimen. The fracture strength was small, because main crack nucleated at the edge of the specimen and propagated with crack tip opening. In contrast, for modified charging, the fracture strain significantly decreased, although similar main crack nucleated at the edge of specimen and propagated with crack tip opening. These results clearly indicate that the first interactions between the strain-induced martensite transformation and hydrogen desorbed in the low temperature range have significant effects on the subsequent plastic deformation. As the tensile test continues at −196 °C, as shown in Figure 5, the effects of the first interactions perhaps diminish gradually, because most of the hydrogen is trapped at defects induced by the interactions. The early stage of the interactions has the largest effect on the enhancement of hydrogen embrittlement; hence, the degradation of tensile properties appears to be small. This is consistent with the findings obtained by the interactions between the stress-induced martensite transformation and hydrogen for the Ni–Ti superelastic alloy [34]. Nonetheless, upon plastic deformation at 25 °C after the first interactions at −196 °C, subsequent second interactions, i.e., the interactions between plastic deformation and hydrogen, occurs. In the second interactions, hydrogen trapped at defects induced by the first interactions newly interacts with plastic deformation at 25 °C. The mechanisms of interactions are different between the first and second interactions, since the dynamic process including changes in the crystalline structure of plastic deformation is different from that of martensite transformation. Although discussion of the detailed mechanisms is beyond the scope of the present study, this combination of the first and second interactions greatly enhances hydrogen embrittlement.
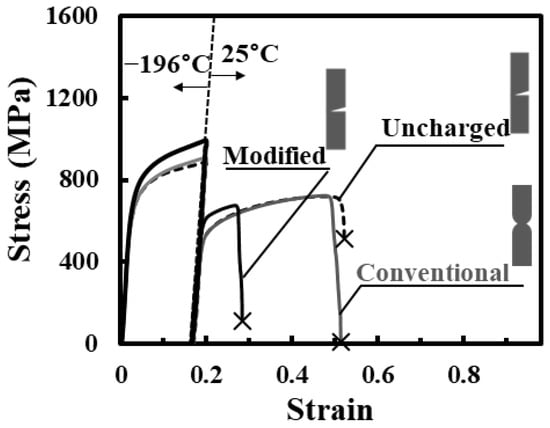
Figure 8.
Tensile stress–strain curves of uncharged and charged specimens subjected to tensile straining to 0.2 and unloading at −196 °C followed by tensile test until fracture at 25 °C. The schematic diagrams show the fracture mode of the specimen.
The hydrogen thermally desorbed in the high temperature range disappeared after the specimen was subjected to straining to 0.2 and unloading at −196 °C, as shown in Figure 9. Upon tensile straining to 0.1 at 25 °C after straining to 0.2 at −196 °C (total strain 0.3), the hydrogen desorbed in the high temperature range further disappeared. One possible reason is that the martensite phase slightly formed by straining to 0.2 enhances hydrogen diffusion. Although details of hydrogen desorption should be investigated, hydrogen states are probably changed in the early stage of first interactions under straining to 0.2 at −196 °C and the subsequent second interactions under straining to 0.1 at 25 °C.
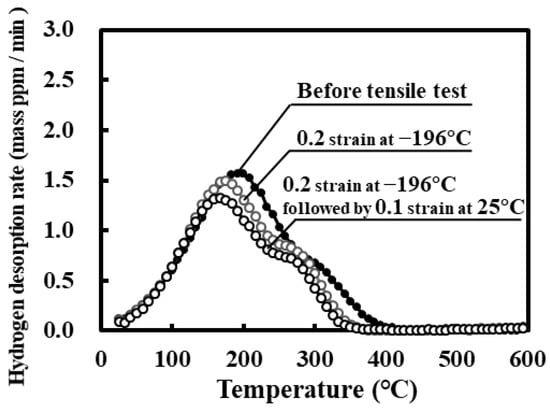
Figure 9.
Hydrogen thermal desorption curves for specimens subjected to modified hydrogen charging, tensile straining to 0.2 and unloading at −196 °C followed by tensile straining to 0.1 and unloading at 25 °C. The curve for the specimen before the tensile test is reproduced from Figure 4.
Figure 10 shows the general and magnified views of fracture surfaces corresponding to the stress–strain curves shown in Figure 8. For the uncharged specimen, the reduction in area was approximately 72% (Figure 10a) and the fracture surface was microscopically composed of dimples. This result suggests that the strain-induced martensite transformation under straining to 0.2 at −196 °C results in the slight decrease in the ductility at 25 °C. For conventional charging, no reduction in area was observed (Figure 10b) and facets-like morphology and transgranular fracture were observed locally at the corner edge of the fracture surface. This clearly indicates that even for conventional charging, the first interactions between martensite transformation and hydrogen followed by the subsequent interactions between the plastic deformation of martensite and/or austenite phases and hydrogen, i.e., dual interactions, enhance hydrogen embrittlement. For modified charging, no reduction in area was observed; intergranular and transgranular fractures (Figure 10c) were observed at the entire rim of the fracture surface. Microscopic streaks on the facet were often observed. The area fraction of the brittle area was largest under the present experimental conditions. In the case of modified charging, it is likely that the hydrogen desorbed in the low temperature range strongly interacts with the strain-induced martensite transformation, thereby inducing damage. Subsequently, the second interactions between plastic deformation and hydrogen lead to a marked degradation of tensile properties.
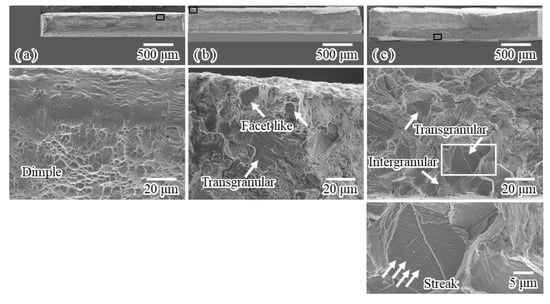
Figure 10.
SEM images of general and magnified views of representative fracture surfaces of specimens subjected to tensile fracture at 25 °C after tensile straining to 0.2 at −196 °C; (a) uncharged specimen, (b) conventionally charged specimen and (c) specimen charged by modified method.
Figure 11 shows general views of the image quality (IQ), inverse pole figure (IPF) and phase maps of specimens subjected to tensile deformation with strain to 0.2 and unloading at −196 °C. For the uncharged specimen without deformation (Figure 11a), no martensite phase was detected from the phase map. Upon tensile straining (Figure 11b), the deformation twins were often observed approximately 45° to the tensile axis, as shown in the IPF map. From the phase map, martensite transformation occurred at intersections of shear bands, as similarly reported previously [50,51]. The volume fractions of α′ and ε martensite phases were approximately 12% and 10%, respectively. For conventional charging (Figure 11c), upon tensile straining, the shear bands and deformation twins were observed, but martensite transformation was suppressed. The volume fraction of the α′ martensite phase was markedly decreased to approximately 5%, although the volume fraction of the ε martensite phase was approximately 9%. The EBSD results also indicate that the strain-induced martensite transformation is suppressed by hydrogen. For modified charging (Figure 11d), upon tensile straining, shear bands including deformation twins were observed, but only a slight amount of the martensite phase was observed. Even ε martensite transformation was suppressed, and the volume fraction of this phase was approximately 6%. This transformation suppression is in accord with the flow stress behavior observed from the stress–strain curve shown in Figure 5. In general, hydrogen suppresses α′ martensite transformation [48,52,53,54] and enhances ε martensite transformation [55,56,57,58,59,60]. However, for iron-based alloys [61], hydrogen suppresses the thermally induced ε martensite transformation. In the present study, the hydrogen charged by the modified method suppresses both α′ and ε martensite transformations. The suppression of stress-induced martensite transformations due to unstable hydrogen such as diffusible hydrogen has also been demonstrated for the Ni–Ti superelastic alloy [35]. Instead of suppressing transformation, a substantial amount of damage appears to be induced by the first interactions between martensite transformation and hydrogen.
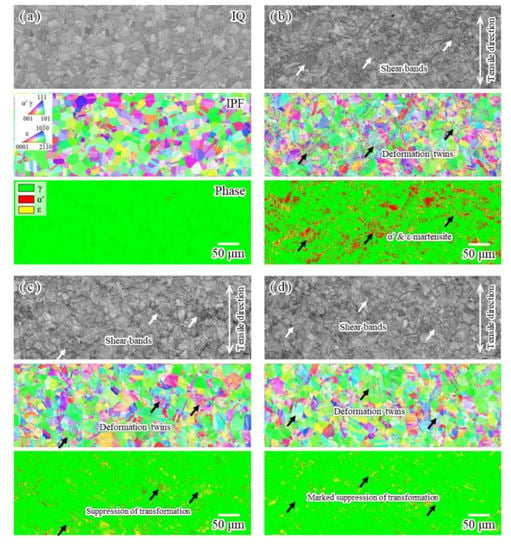
Figure 11.
EBSD maps (IQ, IPF and phase maps) of surface of (a) uncharged specimen before tensile test and specimens subjected to tensile straining to 0.2 and unloading at −196 °C; (b) uncharged specimen, (c) conventionally charged specimen and (d) specimen charged by modified method.
Figure 12 shows magnified views of IPF, IQ and phase maps for the specimen subjected to modified charging and tensile deformation at 25 °C until near fracture after tensile straining to 0.2 at −196 °C. The IPF and IQ maps clearly indicate the formation of shear bands including deformation twins. The width of shear bands (approximately 200–600 nm) well corresponded to that of streaks on the facet on the fracture surface shown in Figure 10c. In the IQ map, the cracks were nucleated near shear bands. The shear bands accompanied by the suppression of transformation are presumably evidence of the formation of damage. Damage is induced in not only the martensite phase but also the austenite phase owing to the suppression of transformation. The phase map indicates that the cracks were nucleated near the martensite phase formed at −196 °C. These cracks were formed by only plastic deformation after the first interactions. Therefore, the crack nucleation is probably associated with the subsequent second interactions between hydrogen and deformation around the martensite phase induced by the first interactions between martensite transformation and hydrogen. This crack nucleation is the direct cause of the marked degradation of tensile properties. hydrogen. Upon straining after gas charging for SUS 304 stainless steel [27], a thin ε martensite phase (5–10 nm) forms with substantial amounts of stacking faults at twin boundaries and is related to hydrogen embrittlement. Similarly, in the present study, the thin ε martensite phase may exist at shear bands including deformation twins and enhance hydrogen embrittlement.
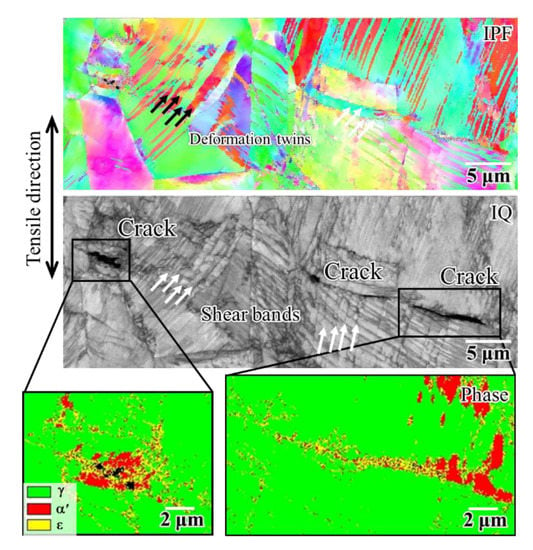
Figure 12.
EBSD maps (IPF, IQ and phase maps) of surface of specimen subjected to modified charging around cracks, tensile straining to 0.2 and unloading at −196 °C followed by tensile deformation until near fracture and unloading at 25 °C.
3.3. Recovering by Dehydrogenation
Figure 13 shows the typical stress–strain curves at 25 °C for the uncharged specimen and the specimen subjected to modified charging, dehydrogenation at 200 °C for 24 h after the tensile straining to 0.2 and unloading at −196 °C. The fracture strain of the uncharged specimen was smaller than that of the specimen subjected to modified charging. The probable cause of this is that the amount of the martensite phase induced by the tensile straining at −196 °C is larger for the uncharged specimen than for the modified charged specimen, as shown in Figure 11. No marked degradation of tensile properties of the modified charged specimen was observed after dehydrogenation. Thus, the degradation of tensile properties induced by the first interactions between martensite transformation and hydrogen is recovered by dehydrogenation. Under dehydrogenation at 200 °C, it appears that the martensite phase and defects such as dislocations and shear bands remain, although vacancies start to be annihilated [62,63,64]. The probable origin of recovery by dehydrogenation is that the damage induced by the first interactions changes to harmless defects. It is unlikely that the martensite phase and defects significantly affect the subsequent plastic deformation. They act as damage only in the presence of hydrogen. The damage induced by the first interactions may exist as a complex of hydrogen and defects including dislocations and vacancies.
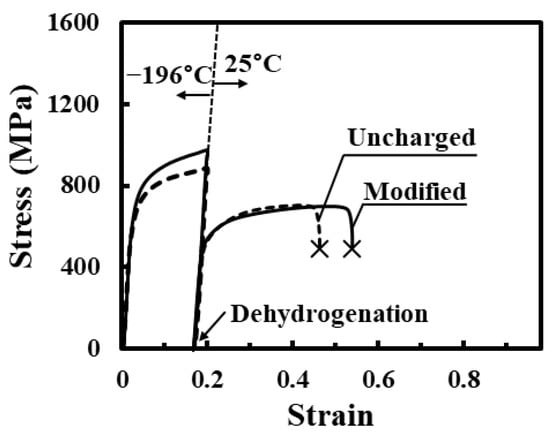
Figure 13.
Tensile stress–strain curves at 25 °C for uncharged specimen and specimen subjected to modified charging and dehydrogenation at 200 °C for 24 h after the tensile straining to 0.2 at −196 °C.
4. Conclusions
We have demonstrated that by the modified charging, hydrogen embrittlement occurs even for type 316L stainless steel at 25 °C and −196 °C. The main conclusions are the following:
(1) Not only the interactions between plastic deformation and unstable hydrogen but also the interactions between the strain-induced martensite transformation and unstable hydrogen induce damage, thereby enhancing hydrogen embrittlement.
(2) The effects of the interactions between the strain-induced martensite transformation and hydrogen on tensile properties are smaller for the type 316L stainless steel than for the type 304 stainless steel.
(3) The first interactions between martensite transformation and hydrogen followed by the subsequent second interactions between plastic deformation and hydrogen, i.e., dual interactions, substantially enhance hydrogen embrittlement. In this case, the crack nucleation around the martensite phase associated with the suppression of martensite transformation is the direct cause of the marked degradation of tensile properties.
(4) The damage induced by the first interactions is recovered by dehydrogenation. The marked degradation of tensile properties of type 316L stainless steel occurs in some cases; hence, the re-evaluation of resistance to hydrogen embrittlement is necessary.
Author Contributions
Conceptualization, K.N. and K.Y.; methodology, K.Y.; formal analysis, K.N. and K.Y.; investigation, K.N. and K.Y.; resources, K.Y.; data curation, K.N. and K.Y.; writing—original draft preparation, K.N. and K.Y.; visualization, K.Y.; project administration, K.Y.; funding acquisition, K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI Grant Number 18K04780.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Michler, T.; Naumann, J.; Sattler, E. Influence of high pressure gaseous hydrogen on S–N fatigue in two austenitic stainless steels. Int. J. Fatigue 2013, 51, 1–7. [Google Scholar] [CrossRef]
- Tsuchiyama, T.; Tsuboi, K.; Iwanaga, S.; Masumura, T.; Macadre, A.; Nakada, N.; Takaki, S. Suppression of hydrogen embrittlement by formation of a stable austenite layer in metastable austenitic stainless steel. Scripta Mater. 2014, 90, 14–16. [Google Scholar] [CrossRef]
- Takaki, S.; Nanba, S.; Imakawa, K.; Macadre, A.; Yamabe, J.; Matsunaga, H.; Matsuoka, S. Determination of hydrogen compatibility for solution-treated austenitic stainless steels based on a newly proposed nickel-equivalent equation. Int. J. Hydrogen Energy 2016, 41, 15095–15100. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, M.; Imade, M.; Fukuyama, S.; Yokogawa, K. Effect of nickel equivalent on hydrogen gas embrittlement of austenitic stainless steels based on type 316 at low temperatures. Acta Mater. 2008, 56, 3414–3421. [Google Scholar] [CrossRef]
- San Marchi, C.; Somerday, B.P.; Tang, X.; Schiroky, G.H. Effects of alloy composition and strain hardening on tensile fracture of hydrogen-precharged type 316 stainless steels. Int. J. Hydrogen Energy 2008, 33, 889–904. [Google Scholar] [CrossRef]
- Omura, T.; Miyahara, M.; Semba, H.; Igarashi, M.; Hirata, H. Evaluation of hydrogen embrittlement properties of stainless steels by SSRT and external pressure fatigue tests. J. Jpn. Petrol. Inst. 2008, 46, 205–213. [Google Scholar]
- Ohmiya, S.; Fujii, H. Effects of Ni and Cr contents on fatigue crack growth properties of SUS316-based stainless steels in high-pressure gaseous hydrogen. ISIJ Int. 2012, 52, 247–254. [Google Scholar] [CrossRef]
- Izawa, C.; Wagner, S.; Deutges, M.; Weber, S.; Pargeter, R.; Michler, T.; Uchida, H.H.; Gemma, R.; Pundt, A. Relationship between hydrogen embrittlement and Md30 temperature, Prediction of low-nickel austenitic stainless steel’s resistance. Int. J. Hydrogen Energy 2019, 44, 25064–25075. [Google Scholar] [CrossRef]
- Michler, T.; San Marchi, C.; Naumann, J.; Weber, S.; Martin, M. Hydrogen environment embrittlement of stable austenitic steels. Int. J. Hydrogen Energy 2012, 37, 16231–16246. [Google Scholar] [CrossRef]
- Murakami, K.; Yabe, N.; Suzuki, H.; Takai, K.; Hagihara, Y.; Wada, Y. Substitution of High-pressure Charge by Electrolysis Charge and Hydrogen Environment Embrittlement Susceptibilities for Inconel 625 and SUS 316L; American Society of Mechanical Engineers. Pressure Vessels and Piping Division: Vancouver, BC, Canada, 2006; pp. 563–570, PVP2006-ICPVT-11-93397. [Google Scholar]
- Matsuo, T.; Yamabe, J.; Matsuoka, S. Effects of hydrogen on tensile properties and fracture surface morphologies of Type 316L stainless steel. Int. J. Hydrogen Energy 2014, 39, 3542–3551. [Google Scholar] [CrossRef]
- Hatano, M.; Fujinami, M.; Arai, K.; Fujii, H.; Nagumo, M. Hydrogen embrittlement of austenitic stainless steels revealed by deformation microstructures and strain-induced creation of vacancies. Acta Mater. 2014, 67, 342–353. [Google Scholar] [CrossRef]
- Zhang, L.; Imade, M.; An, B.; Wen, M.; Iijima, T.; Fukuyama, S.; Yokogawa, K. Internal reversible hydrogen embrittlement of austenitic stainless steels based on type 316 at low temperatures. ISIJ Int. 2012, 52, 240–246. [Google Scholar] [CrossRef]
- Han, G.; He, J.; Fukuyama, S.; Yokogawa, K. Effect of strain-induced martensite on hydrogen environment embrittlement of sensitized austenitic stainless steels at low temperatures. Acta Mater. 1998, 46, 4559–4570. [Google Scholar] [CrossRef]
- Michler, T.; Lee, Y.; Gangloff, R.P.; Naumann, J. Influence of macro segregation on hydrogen environment embrittlement of SUS 316L stainless steel. Int. J. Hydrogen Energy 2009, 34, 3201–3209. [Google Scholar] [CrossRef]
- San Marchi, C.; Michler, T.; Nibur, K.A.; Somerday, B.P. On the physical differences between tensile testing of type 304 and 316 austenitic stainless steels with internal hydrogen and in external hydrogen. Int. J. Hydrogen Energy 2010, 35, 9736–9745. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Gong, J.; Shen, L.; Dong, W. Hydrogen embrittlement of catholically hydrogen-precharged 304L austenitic stainless steel: Effect of plastic pre-strain. Int. J. Hydrogen Energy 2014, 39, 13909–13918. [Google Scholar] [CrossRef]
- Koyama, M.; Ogawa, T.; Yan, D.; Matsumoto, Y.; Tasan, C.C.; Takai, K.; Tsuzaki, K. Hydrogen desorption and cracking associated with martensitic transformation in Fe-Cr-Ni-Based austenitic steels with different carbon contents. Int. J. Hydrogen Energy 2017, 42, 26423–26435. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, C.; Zheng, J.; Zhang, L. Effects of α′ martensite and deformation twin on hydrogen-assisted fatigue crack growth in cold/warm-rolled type 304 stainless steel. Int. J. Hydrogen Energy 2018, 43, 3342–3352. [Google Scholar] [CrossRef]
- Zhou, C.; Song, Y.; Shi, Q.; Hu, S.; Zheng, J.; Xu, P.; Zhang, L. Effect of pre-strain on hydrogen embrittlement of metastable austenitic stainless steel under different hydrogen conditions. Int. J. Hydrogen Energy 2019, 44, 26036–26048. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Wu, W. Effect of α′ martensite content induced by tensile plastic prestrain on hydrogen transport and hydrogen embrittlement of 304L austenitic stainless steel. Metals 2018, 8, 660. [Google Scholar] [CrossRef]
- Kanezaki, T.; Narazaki, C.; Mine, Y.; Matsuoka, S.; Murakami, Y. Effects of hydrogen on fatigue crack growth behavior of austenitic stainless steels. Int. J. Hydrogen Energy 2008, 33, 2604–2619. [Google Scholar] [CrossRef]
- Mine, Y.; Narazaki, C.; Murakami, K.; Matsuoka, S.; Murakami, Y. Hydrogen transport in solution-treated and pre-strained austenitic stainless steels and its role in hydrogen-enhanced fatigue crack growth. Int. J. Hydrogen Energy 2009, 34, 1097–1107. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Yokoyama, K.; Sakai, J. Role of dynamic interactions between hydrogen and strain-induced martensite transformation in hydrogen embrittlement of type 304 stainless steel. ISIJ Int. 2015, 55, 1772–1780. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Zheng, J.; Zhao, Y.; Xu, P.; Liu, X.; Zhou, C.; Li, X. Influence of low temperature prestrain on hydrogen gas embrittlement of metastable austenitic stainless steels. Int. J. Hydrogen Energy 2013, 38, 11181–11187. [Google Scholar] [CrossRef]
- Hatano, M.; Kubota, Y.; Shobu, T.; Mori, S. Presence of ε-martensite as an intermediate phase during the strain-induced transformation of SUS304 stainless steel. Philos. Mag. Lett. 2016, 96, 220–227. [Google Scholar] [CrossRef]
- Hatano, M.; Tsukasaki, H.; Kawaguchi, A.; Kawaguchi, S.; Kubota, Y.; Ishii, Y.; Mori, S. Strain-induced ε-martensitic transformation and hydrogen embrittlement of SUS304 stainless steel. Philos. Mag. Lett. 2019, 99, 404–413. [Google Scholar] [CrossRef]
- Park, I.J.; Jung, J.G.; Jo, S.Y.; Lee, S.M.; Lee, Y.K. The effect of pre-strain on the resistance to hydrogen embrittlement in 316L austenitic stainless steel. Mater. Trans. 2014, 55, 964–970. [Google Scholar] [CrossRef]
- Ogawa, T.; Koyama, M.; Nishikura, Y.; Tsuzaki, K.; Noguchi, H. Fatigue behavior of Fe-Cr-Ni-based metastable austenitic steels with an identical tensile strength and different solute carbon contents. ISIJ Int. 2018, 58, 1910–1919. [Google Scholar] [CrossRef]
- Teus, S.M.; Shyvanyuk, V.N.; Gavriljuk, V.G. Hydrogen-induced γ→ε transformation and the role of ε-martensite in hydrogen embrittlement of austenitic steels. Mater. Sci. Eng. A 2008, 497, 290–294. [Google Scholar] [CrossRef]
- Takai, K.; Shoda, H.; Suzuki, H.; Nagumo, M. Lattice defects dominating hydrogen-related failure of metals. Acta Mater. 2008, 56, 5158–5167. [Google Scholar] [CrossRef]
- Nagumo, M.; Takai, K. The predominant role of strain-induced vacancies in hydrogen embrittlement of steels: Overview. Acta Mater. 2019, 165, 722–733. [Google Scholar] [CrossRef]
- Takashima, K.; Han, R.; Yokoyama, K.; Funakawa, Y. Hydrogen embrittlement induced by hydrogen charging during deformation of ultra-high strength steel sheet consisting of ferrite and nanometer-sized precipitates. ISIJ Int. 2019, 59, 2327–2333. [Google Scholar] [CrossRef]
- Yokoyama, K.; Hirata, Y.; Inaba, T.; Mutoh, K.; Sakai, J. Strong interactions between hydrogen in solid solution and stress-induced martensite transformation of Ni-Ti superelastic alloy. Philos. Mag. Lett. 2017, 97, 11–18. [Google Scholar] [CrossRef]
- Yokoyama, K.; Tomita, M.; Sakai, J. Hydrogen embrittlement behavior induced by dynamic martensite transformation of Ni-Ti superelastic alloy. Acta Mater. 2009, 57, 1875–1885. [Google Scholar] [CrossRef]
- Hecker, S.S.; Stout, M.G.; Staudhammer, K.P.; Smith, J.L. Effects of strain state and strain rate on deformation-induced transformation in 304 stainless steel. Metall. Trans. A 1982, 13, 619–626. [Google Scholar] [CrossRef]
- Talonen, J.; Hänninen, H.; Nenonen, P.; Pape, G. Effect of strain rate on the strain-induced γ→α′-martensite transformation and mechanical properties of austenitic stainless steels. Metall. Mater. Trans. A 2005, 36, 421–432. [Google Scholar] [CrossRef]
- Takagi, Y.; Ueji, R.; Mizuguchi, T.; Tsuchida, N. Influence of strain rate on TRIP effect in SUS301L metastable austenite steel. Tetsu Hagané 2011, 97, 44–50. [Google Scholar] [CrossRef]
- Herms, E.; Olive, J.M.; Puiggali, M. Hydrogen embrittlement of 316L type stainless steel. Mater. Sci. Eng. A 1999, 272, 279–283. [Google Scholar] [CrossRef]
- Hirayama, T.; Ogirima, M. Influence of chemical composition on martensitic transformation in Fe-Cr-Ni stainless steel. J. Jpn. Inst. Met. 1970, 34, 507–510. [Google Scholar] [CrossRef]
- Nohara, K.; Ono, Y.; Ohashi, N. Composition and grain size dependencies of strain-induced martensitic transformation in metastable Austenitic stainless steels. Tetsu Hagané 1977, 63, 772–782. [Google Scholar] [CrossRef]
- Brass, A.M.; Chêne, J. Hydrogen uptake in 316L stainless steel: Consequences on the tensile properties. Corros. Sci. 2006, 48, 3222–3242. [Google Scholar] [CrossRef]
- Garion, C.; Skoczeń, B.; Sgobba, S. Constitutive modelling and identification of parameters of the plastic strain-induced martensitic transformation in 316L stainless steel at cryogenic temperatures. Int. J. Plast. 2006, 22, 1234–1264. [Google Scholar] [CrossRef]
- Tabin, J.; Skoczen, B.; Bielski, J. Discontinuous plastic flow coupled with strain induced fcc–bcc phase transformation at extremely low temperatures. Mech. Mater. 2019, 129, 23–40. [Google Scholar] [CrossRef]
- Seetharaman, V.; Krishnan, R. Influence of the martensitic transformation on the deformation behaviour of an AISI 316 stainless steel at low temperatures. J. Mater. Sci. 1981, 16, 523–530. [Google Scholar] [CrossRef]
- Ueki, S.; Koga, K.; Mine, Y.; Takashima, K. Crystallographic characterisation of hydrogen-induced twin boundary separation in type 304 stainless steel using micro-tensile testing. ISIJ Int. 2019, 59, 927–934. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Park, J.; Nahm, S.H.; Baek, U.B. Effect of hydrogen on tensile flow and failure mechanism of low nickel-type 316L austenitic stainless steel. J. Mech. Sci. Technol. 2019, 33, 5843–5849. [Google Scholar] [CrossRef]
- Mine, Y.; Horita, Z.; Murakami, Y. Effect of hydrogen on martensite formation in austenitic stainless steels in high-pressure torsion. Acta Mater. 2009, 57, 2993–3002. [Google Scholar] [CrossRef]
- Pu, S.D.; Turk, A.; Lenka, S.; Ooi, S.W. Study of hydrogen release resulting from the transformation of austenite into martensite. Mater. Sci. Eng. A 2019, 754, 628–635. [Google Scholar] [CrossRef]
- Talonen, J.; Hänninen, H. Formation of shear bands and strain-induced martensite during plastic deformation of metastable austenitic stainless steels. Acta Mater. 2007, 55, 6108–6118. [Google Scholar] [CrossRef]
- Galindo-Nava, E.I.; Rivera-Díaz-del-Castillo, P.E.J. Understanding martensite and twin formation in austenitic steels: A model describing TRIP and TWIP effects. Acta Mater. 2017, 128, 120–134. [Google Scholar] [CrossRef]
- Bak, S.H.; Kim, S.S.; Lee, D.B. Effect of hydrogen on dislocation structure and strain-induced martensite transformation in 316L stainless steel. RSC Adv. 2017, 7, 27840–27845. [Google Scholar] [CrossRef]
- El-Tahawy, M.; Um, T.; Nam, H.-S.; Choe, H.; Gubicza, J. The effect of hydrogen charging on the evolution of lattice defects and phase composition during tension in 316L stainless steel. Mater. Sci. Eng. A 2019, 739, 31–36. [Google Scholar] [CrossRef]
- Lu, X.; Ma, Y.; Zamanzade, M.; Deng, Y.; Wang, D.; Bleck, W.; Song, W.W.; Barnoush, A. Insight into hydrogen effect on a duplex medium-Mn steel revealed by in-situ nanoindentation test. Int. J. Hydrogen Energy 2019, 44, 20545–20551. [Google Scholar] [CrossRef]
- Hosoya, Y.; Inoue, A.; Masumoto, T. Effect of hydrogen on crack propagation behavior and microstructures around cracks in austenitic stainless steels. Tetsu Hagané 1978, 64, 769–778. [Google Scholar] [CrossRef]
- Narita, N.; Altstetter, C.J.; Birnbaum, H.K. Hydrogen-related phase transformations in austenitic stainless steels. Metall. Trans. A 1982, 13, 1355–1365. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Hänninen, H.; Tarasenko, A.V.; Tereshchenko, A.S.; Ullakko, K. Phase transformations and relaxation phenomena caused by hydrogen in stable austenitic stainless steels. Acta Metall. Mater. 1995, 43, 559–568. [Google Scholar] [CrossRef]
- Hermida, J.D.; Roviglione, A. Stacking fault energy decrease in austenitic stainless steels induced by hydrogen pairs formation. Scripta Mater. 1998, 39, 1145–1149. [Google Scholar] [CrossRef]
- Sugiyama, S.; Ohkubo, H.; Takenaka, M.; Ohsawa, K.; Ansari, M.I.; Tsukuda, N.; Kuramoto, E. The effect of electrical hydrogen charging on the strength of 316 stainless steel. J. Nucl. Mater. 2000, 283, 863–867. [Google Scholar] [CrossRef]
- Koyama, M.; Terao, N.; Tsuzaki, K. Revisiting the effects of hydrogen on deformation-induced γ-ε martensitic transformation. Mater. Lett. 2019, 249, 197–200. [Google Scholar] [CrossRef]
- Koyama, M.; Hirata, K.; Abe, Y.; Mitsuda, A.; Iikubo, S.; Tsuzaki, K. An unconventional hydrogen effect that suppresses thermal formation of the hcp phase in fcc steels. Sci. Rep. 2018, 8, 16136. [Google Scholar] [CrossRef]
- Arunkumar, J.; Abhaya, S.; Rajaraman, R.; Amarendra, G.; Nair, K.G.M.; Sundar, C.S.; Raj, B. Defect recovery in proton irradiated Ti-modified stainless steel probed by positron annihilation. J. Nucl. Mater. 2009, 384, 245–248. [Google Scholar] [CrossRef]
- Dryzek, E.; Sarnek, M.; Wróbel, M. Reverse transformation of deformation-induced martensite in austenitic stainless steel studied by positron annihilation. J. Mater. Sci. 2014, 49, 8449–8458. [Google Scholar] [CrossRef]
- Dryzek, E.; Sarnek, M.; Wróbel, M. Thermal stability of rolled metastable austenitic stainless steel 1.4307 studied using positron annihilation. Metall. Mater. Trans. A 2019, 50, 581–589. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).