Quasi-Static Compression Deformation and Energy Absorption Characteristics of Basalt Fiber-Containing Closed-Cell Aluminum Foam
Abstract
1. Introduction
2. Materials and Experimental
2.1. Specimens Preparation
2.2. Microstructure Observation and Theoretical Calculation
2.3. Mechanical Properties Test
3. Results
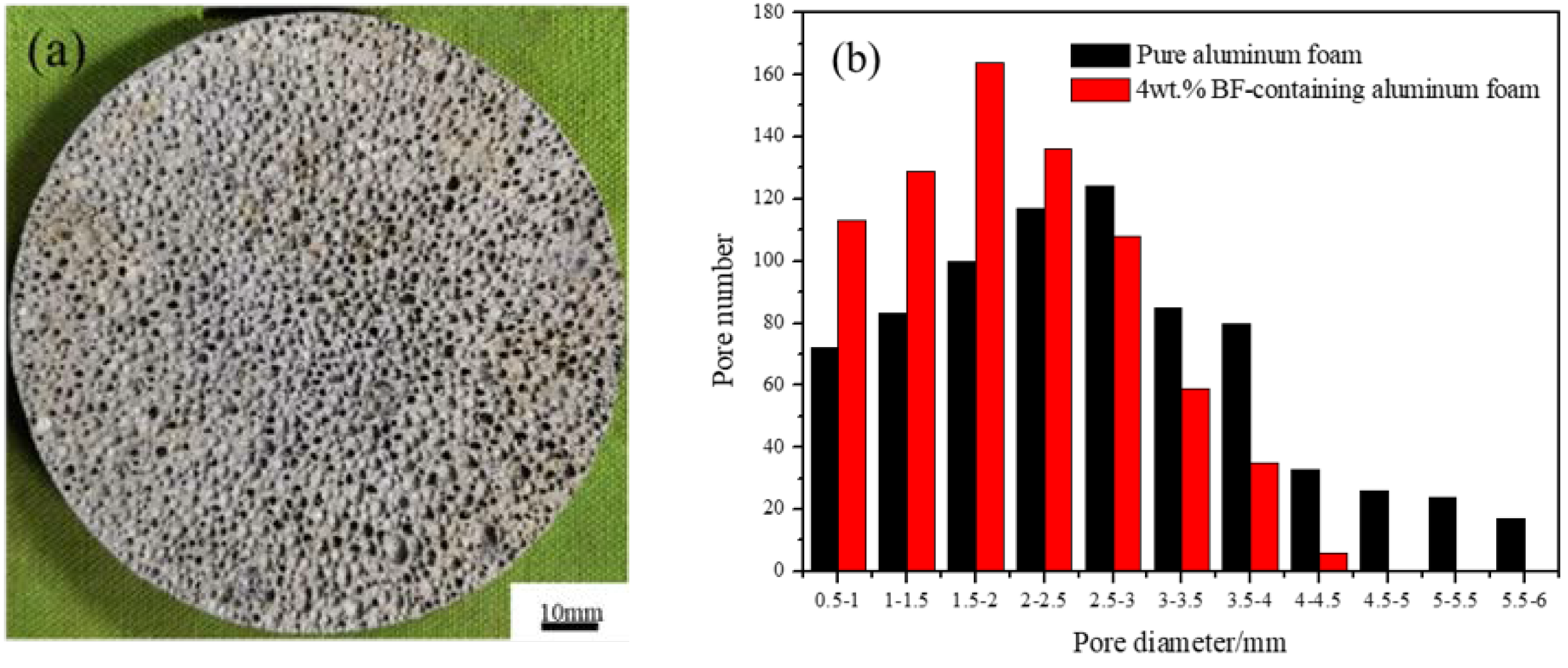
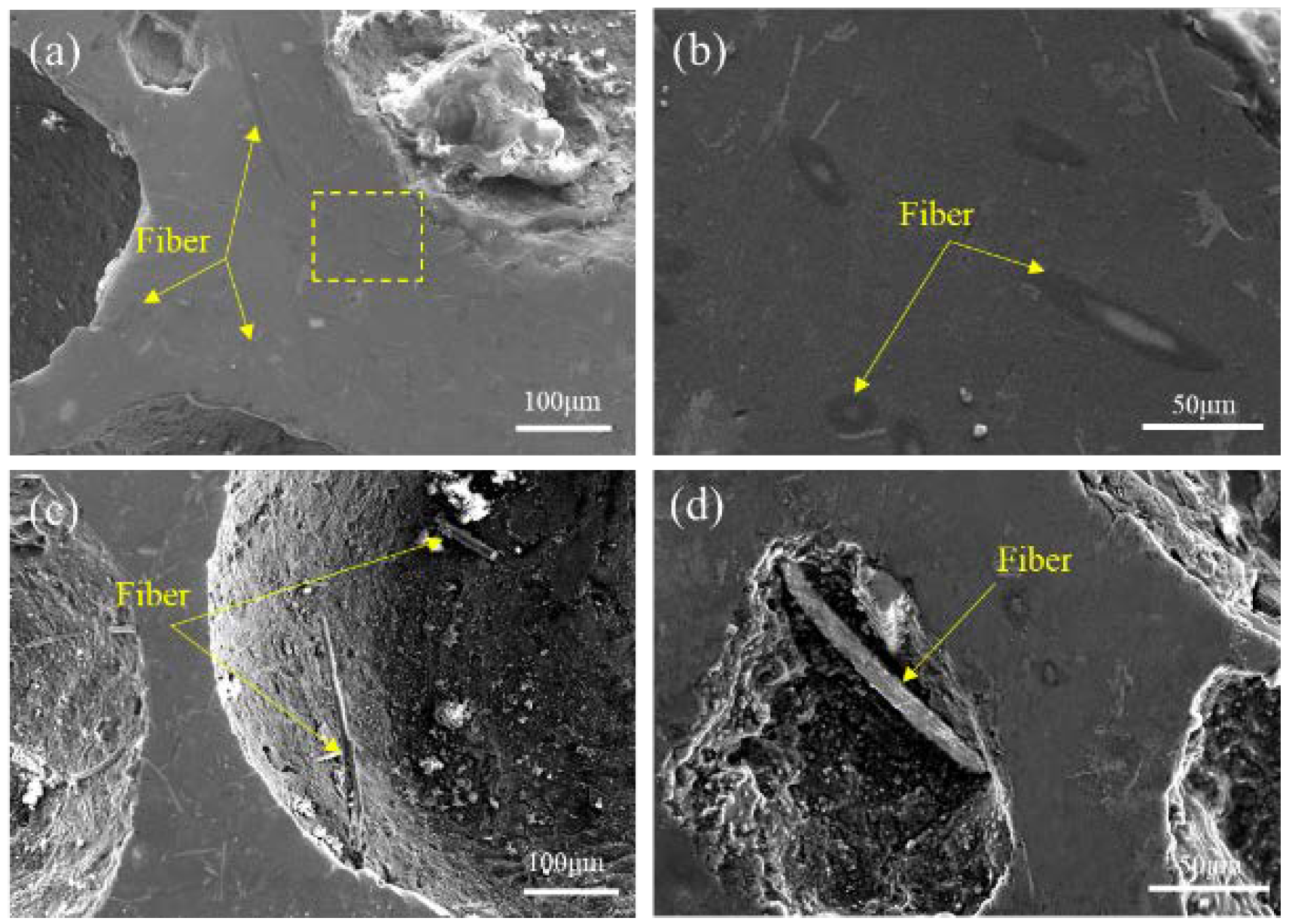
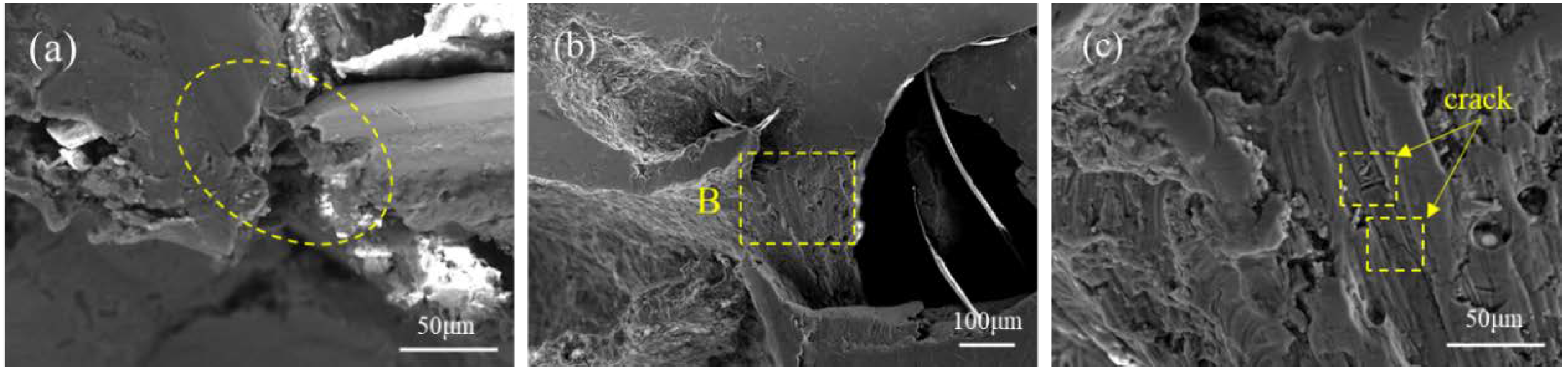
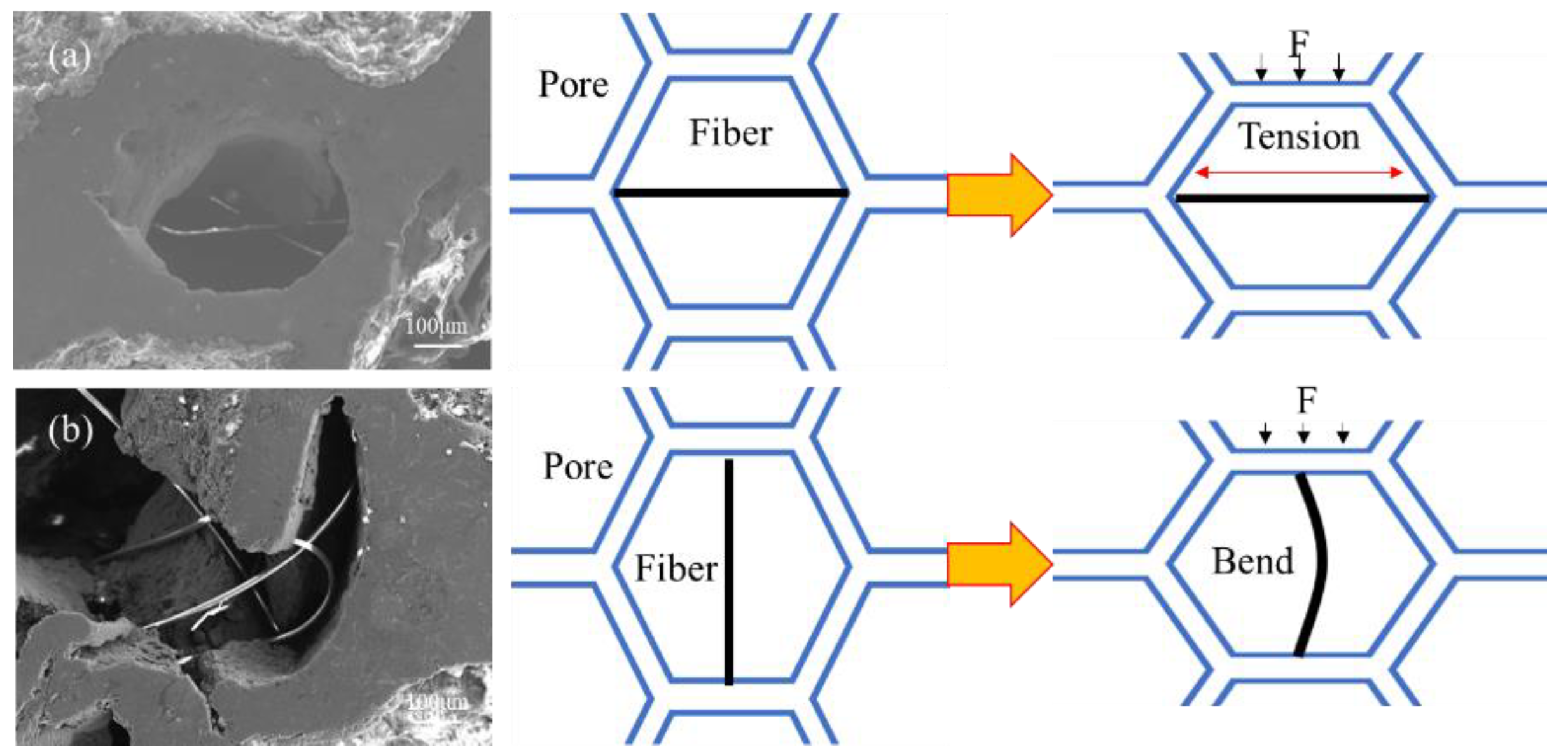
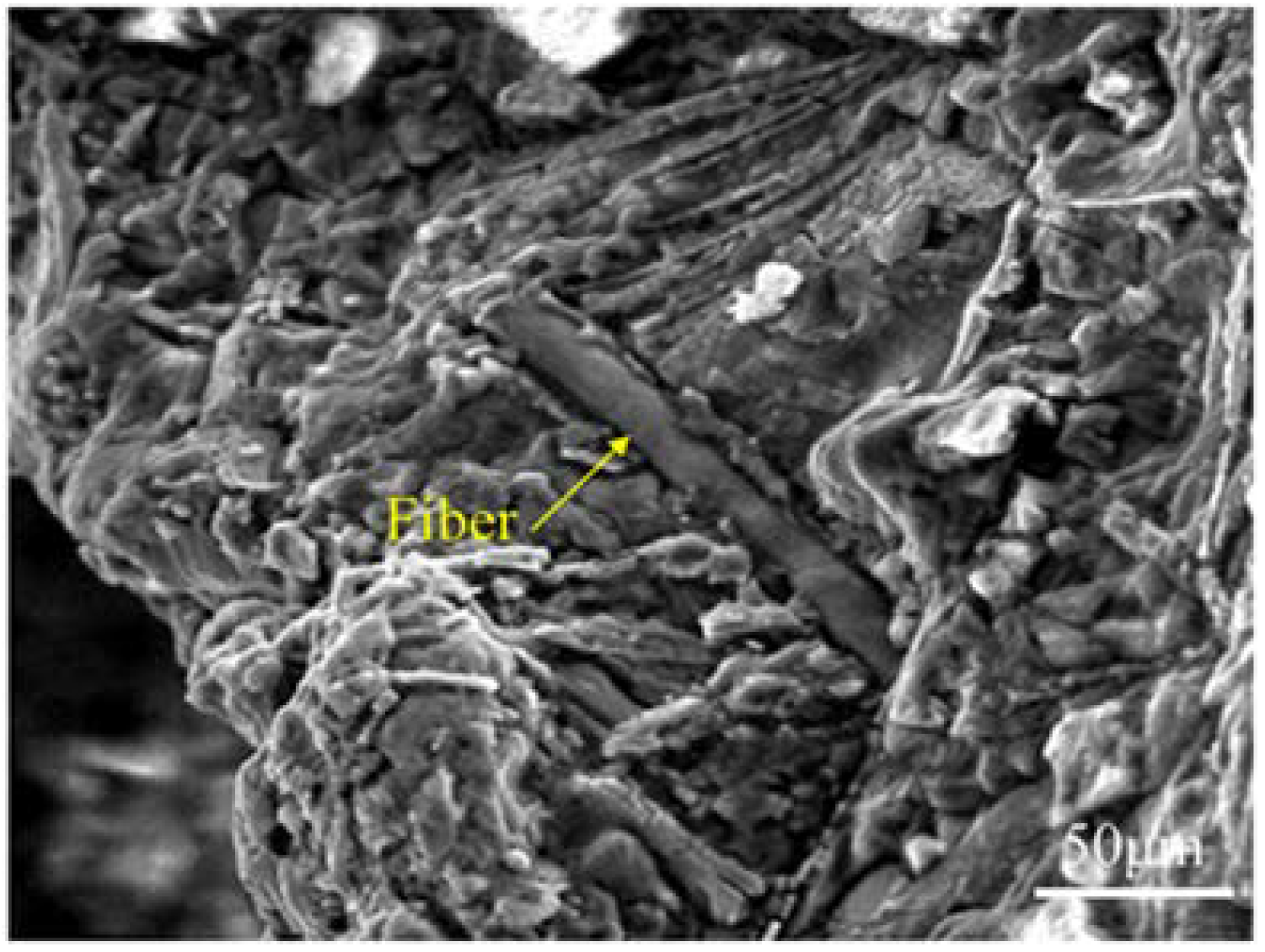
3.1. Structures of BF-Containing Aluminum Foam
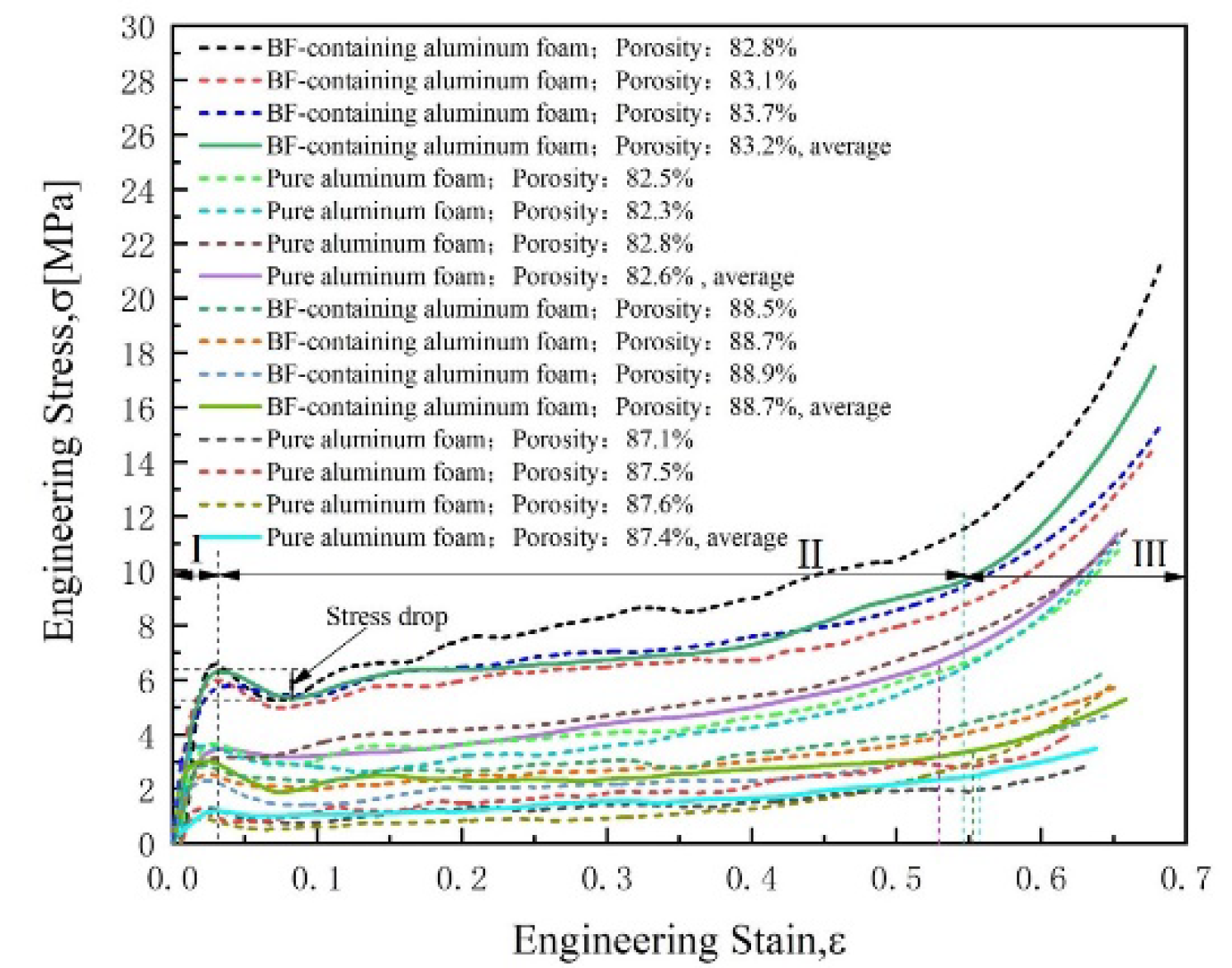
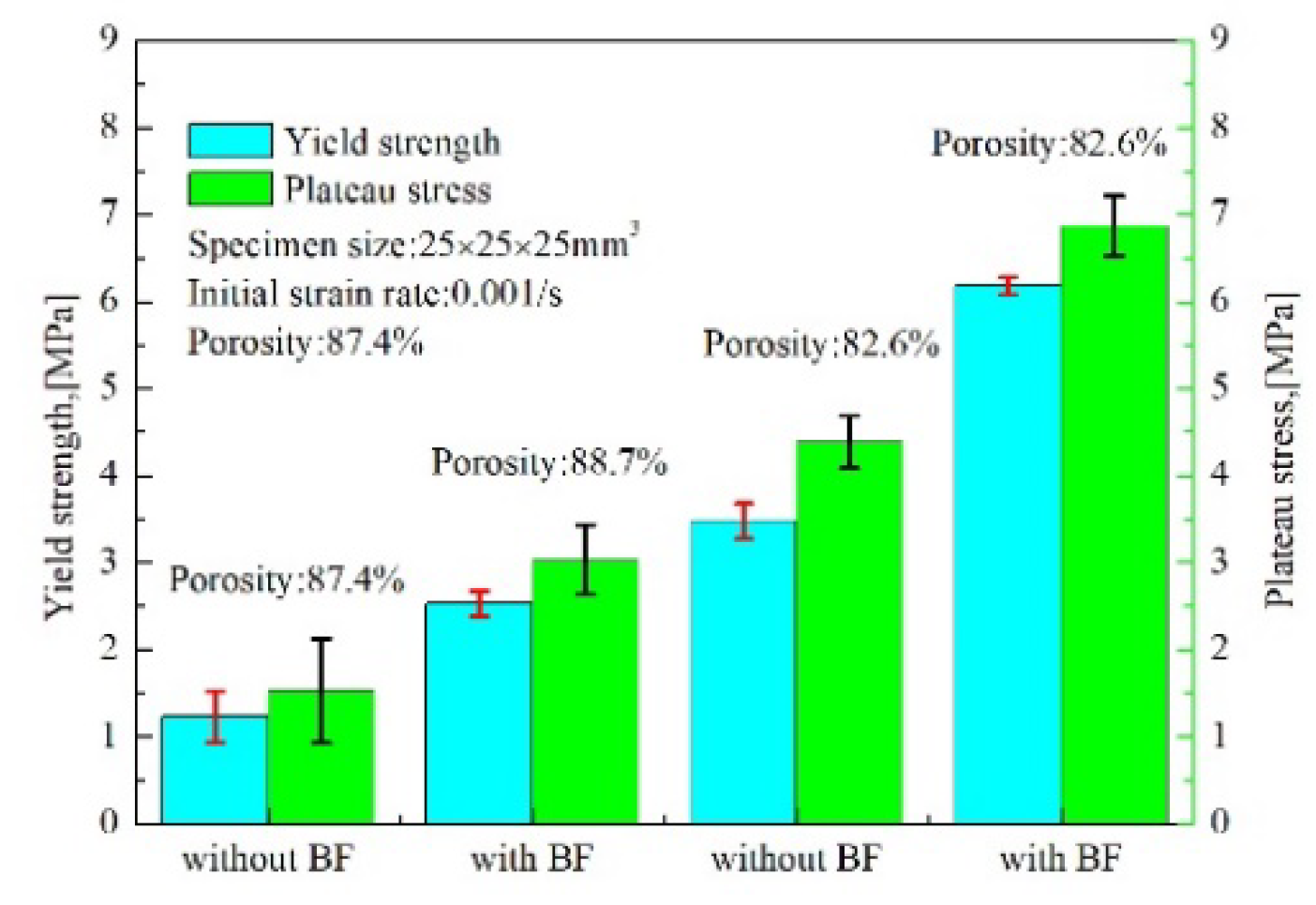
3.2. Compressive Stress–Strain Curves
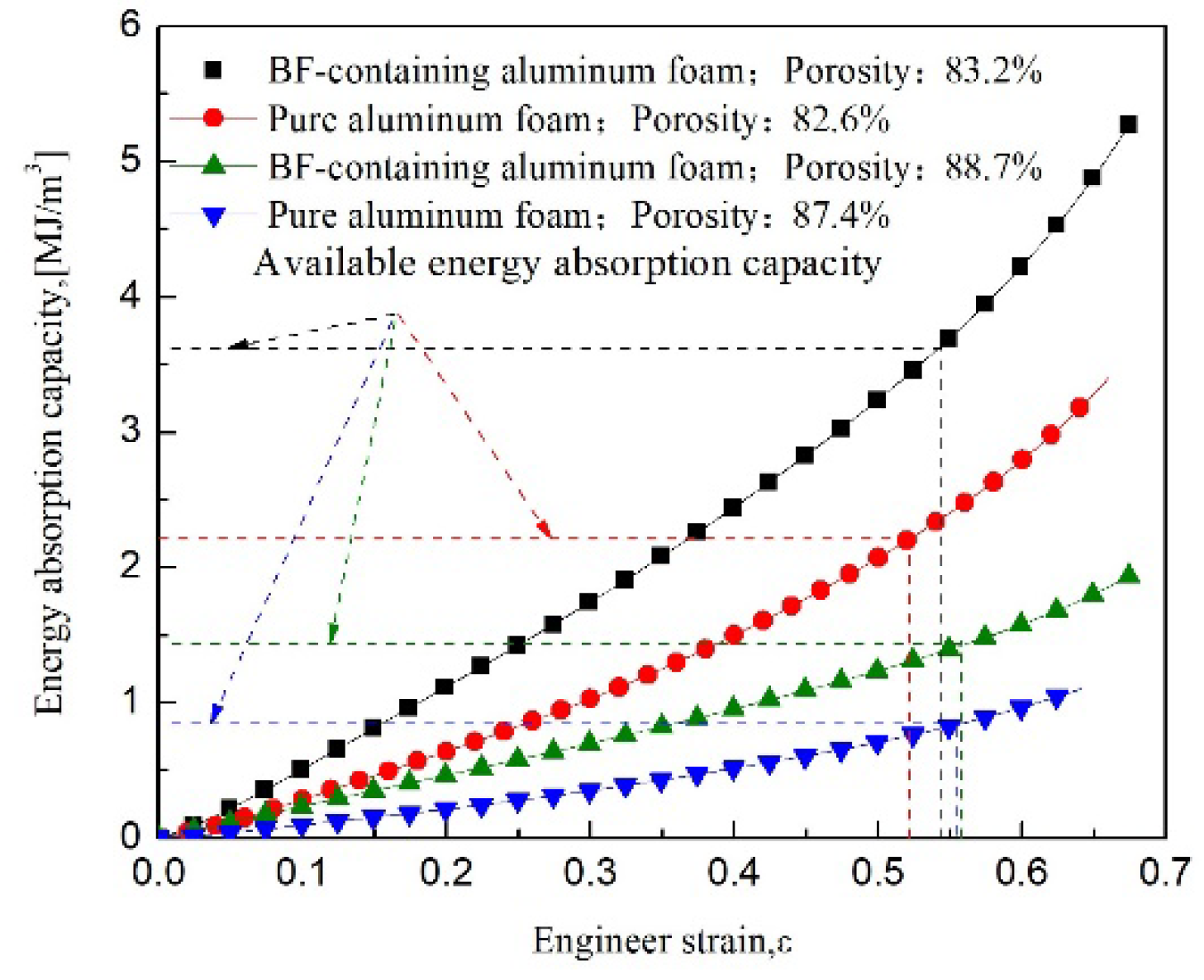
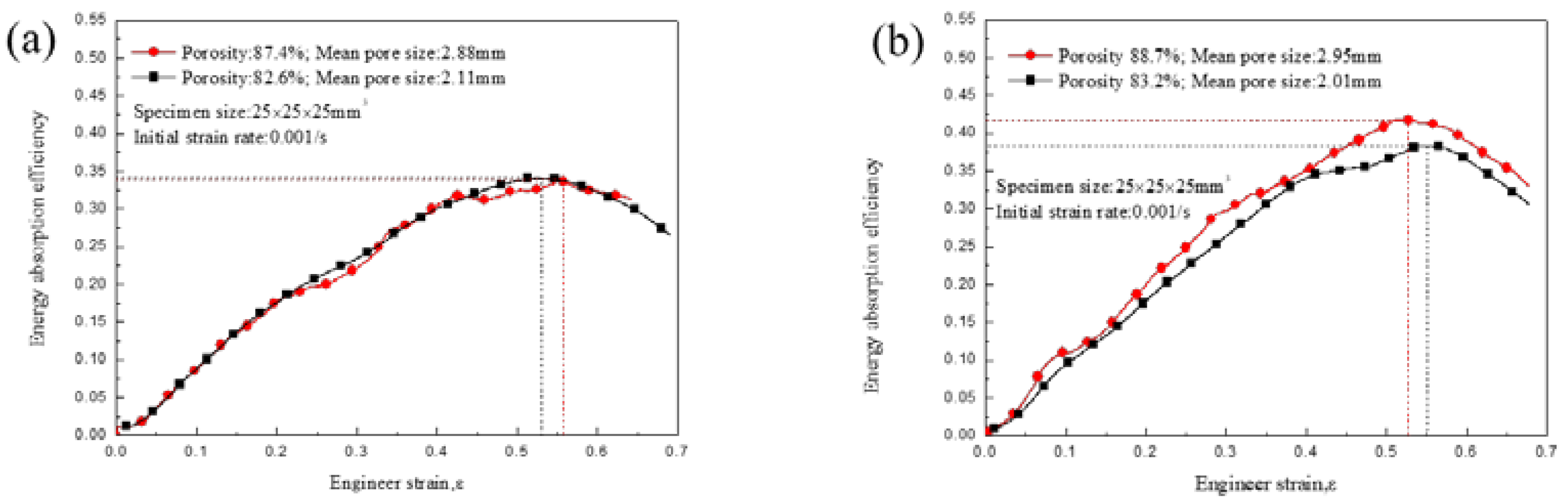
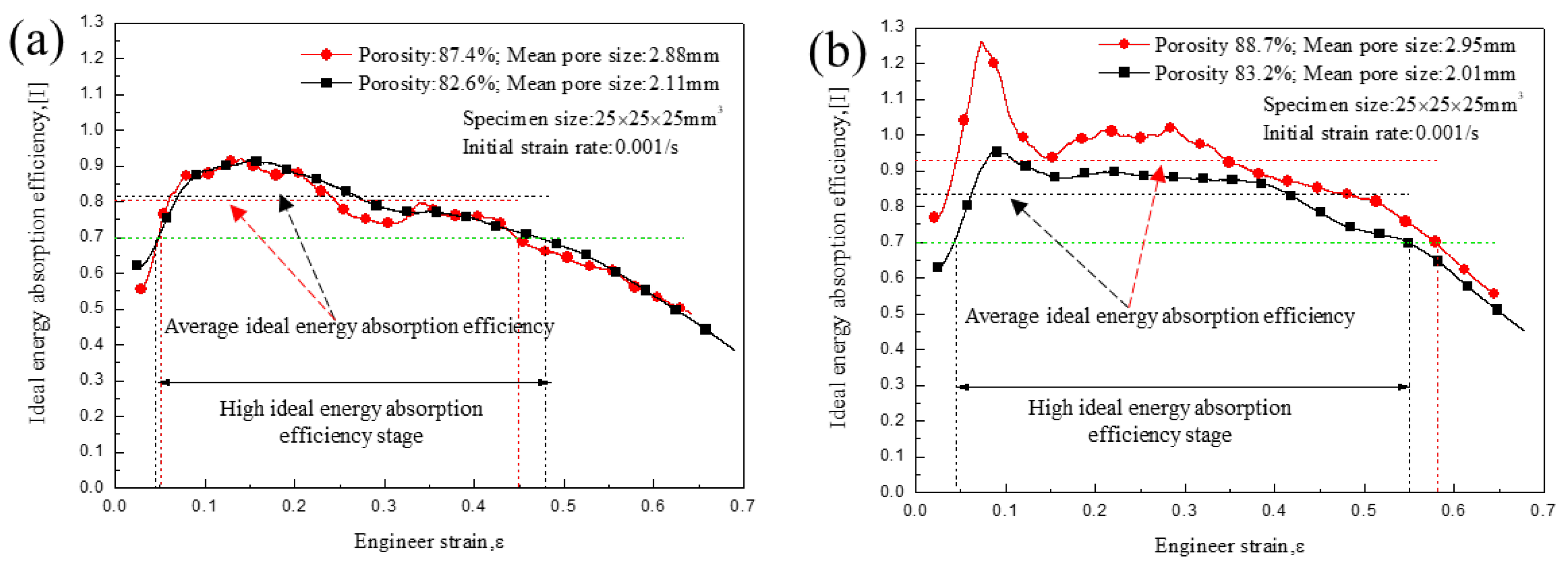
3.3. Energy Absorption Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xia, X.; Chen, X.; Zhang, Z.; Chen, X.; Zhao, W.; Liao, B.; Hur, B. Compressive properties of closed-cell aluminum foams with different contents of ceramic microspheres. Mater. Des. 2014, 56, 353–358. [Google Scholar] [CrossRef]
- Pinto, S.C.; Marques, P.A.A.P.; Vesenjak, M.V.; Vicente, R.; Luís, G.; Lovre, K.-O.; Isabel, D. Mechanical, Thermal, and acoustic properties of aluminum foams impregnated with epoxy/graphene oxide nanocomposites. Metals 2019, 9, 1214. [Google Scholar] [CrossRef]
- Wang, N.Z.; Maire, E.; Chen, X.; Adrien, J.; Li, Y.X.; Amani, Y. Compressive performance and deformation mechanism of the dynamic gas injection aluminum foams. Mater. Charact. 2019, 147, 11–20. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Le Barbenchon, L.; Kopp, J.B.; Girardot, J.; Viot, P. Reinforcement of cellular materials with short fibres: Application to a biobased cork multi-scale foam. Mech. Mater. 2020, 142, 103271. [Google Scholar] [CrossRef]
- Huang, L.; Wang, H.; Yang, D.H.; Ye, F.; Lu, Z.P. Effects of scandium additions on mechanical properties of cellular Al-based foams. Intermetallics 2012, 28, 71–76. [Google Scholar] [CrossRef]
- Xia, X.C.; Feng, H.; Zhang, X.; Zhao, W.M. The compressive properties of closed-cell aluminum foams with different Mn additions. Mater. Des. 2013, 51, 797–802. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Z.; Wang, J.; Zhang, X.; Zhao, W.; Liao, B.; Hur, B. Compressive characteristics of closed-cell aluminum foams after immersion in simulated seawater. Mater. Des. 2015, 67, 330–336. [Google Scholar] [CrossRef]
- Orbulov, I.N. Compressive properties of aluminium matrix syntactic foams. Mater. Sci. Eng. A 2012, 555, 52–56. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, J.; Xia, X.C.; Sun, X.H.; Song, K.H.; Zhao, W.M.; Liao, B. Fabrication and characterization of closed-cell aluminum foams with different contents of multi-walled carbon nanotubes. Mater. Des. 2015, 88, 359–365. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.Q.; Hao, H.; Liu, Z.X.; Yang, Y.K. Basalt scale-reinforced aluminum foam under static and dynamic loads. Compos. Struct. 2018, 203, 599–613. [Google Scholar] [CrossRef]
- Mu, Y.L.; Yao, G.C. Effect of fly ash particles on the compressive properties of closed-cell aluminum foams. J. Mater. Eng. Perform. 2010, 19, 995–997. [Google Scholar] [CrossRef]
- Mu, Y.L.; Zu, G.Y.; Cao, Z.K.; Yao, G.C.; Wang, Q.D. Metal foam stabilization by copper-coated carbon fibers. Scr. Mater. 2013, 68, 459–462. [Google Scholar] [CrossRef]
- Cao, Z.K.; Li, B.; Yao, G.C.; Wang, Y. Fabrication of aluminum foam stabilized by copper-coated carbon fibers. Mater. Sci. Eng. A 2008, 486, 350–356. [Google Scholar] [CrossRef]
- Jamshaid, H. Basalt fber and its applications. J. Textile. Eng. Fashion. Technol. 2017, 1, 254–255. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Bella, G.D.; Valenza, A. A review on basalt fibre and its composites. Compos. Part B 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Ward, P.J.; Atkinson, H.V.; Anderson, P.R.G.; Elias, L.G.; Garcia, B.; Kahlen, L.; Rodriguez-Ibabe, J.M. Semi-solid processing of novel MMCs based on hypereutectic aluminium-basal Short fiber alloys. Acta Mater. 1996, 44, 1717–1727. [Google Scholar] [CrossRef]
- Ezhil, V.S.; Paul, V.S. Microstructure and mechanical properties of as cast aluminium alloy 7075/basalt dispersed metal matrix composites. J. Miner. Mater. Charact. Eng. 2014, 2, 182–193. [Google Scholar]
- Yang, Z.M.; Liu, J.X.; Feng, X.Y.; Xu Li, S.K.; Ren Jie, Y.X. Investigation on mechanical properties and failure mechanisms of basalt fiber reinforced aluminum matrix composites under different loading conditions. J. Compos. Mater. 2017, 52, 1907–1914. [Google Scholar]
- Mc-Danels, D.L. Analysis of Stress-Strain, Fracture, and Ductility Behavior of Aluminum Matrix Composites Containing Discontinuous Basalt Short Fiber Carbide Reinforcement. Metall. Mater. Trans. A 1985, 16, 1105–1115. [Google Scholar] [CrossRef]
- Akhlaghi, F.; Eslami-Farsani, R.; Sabet, S.M.M. Synthesis and characteristics of continuous basalt fiber reinforced aluminum matrix composites. J. Compos. Mater. 2013, 47, 3379–3388. [Google Scholar] [CrossRef]
- Adole, O.; Barekar, N.; Anguilano, L.; Minton, T.; Novytskyi, A.; McKay, B. Fibre/matrix intermetallic phase formation in novel aluminium-basalt composites. Mater. Lett. 2019, 239, 128–131. [Google Scholar] [CrossRef]
- Wang, N.Z.; Chen, X.; Li, Y.X.; Liu, Z.Y.; Zhao, Z.P.; Cheng, Y.; Liu, Y.; Zhang, H.W. The cell size reduction of aluminum foam with dynamic gas injection based on the improved foamable melt. Colloid. Surface. A 2017, 527, 123–131. [Google Scholar] [CrossRef]
- Yang, K.M.; Yang, X.D.; Liu, E.Z.; Shi, C.S.; Ma, L.Y.; He, C.N.; Li, Q.Y.; Li, J.J.; Zhao, N.Q. Elevated temperature compressive properties and energy absorption response of in-situ grown CNT-reinforced Al composite foams. Mater. Sci. Eng. A 2017, 690, 294–302. [Google Scholar] [CrossRef]
- Mondal, D.P.; Goel, M.D.; Das, S. Compressive deformation and energy absorption characteristics of closed cell aluminum-fly ash particle composite foam. Mater. Sci. Eng. A 2009, 507, 102–109. [Google Scholar] [CrossRef]
- Goel, M.D.; Peroni, M.; Solomos, G.; Mondal, D.P.; Matsagar, V.A.; Gupta, A.K.; Larcher, M.; Marburg, S. Dynamic compression behavior of cenosphere aluminum alloy syntactic foam. Mater. Des. 2012, 42, 418–423. [Google Scholar] [CrossRef]
- Goel, M.D.; Matsagar, V.A.; Gupta, A.K.; Marburg, S. Strain rate sensitivity of closed cell aluminum fly ash foam. Trans. Nonferrous. Met. Soc. 2013, 23, 1080–1089. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, T.; Li, S.Q.; Ruan, D.; Wang, Z.H.; Lu, G.X. Sample size effect on the mechanical behavior of aluminum foam. Int. J. Mech. Sci. 2019, 15, 622–638. [Google Scholar] [CrossRef]
- Wang, Z.H.; Shen, J.H.; Lu, G.X.; Zhao, L.M. Compressive behavior of closed-cell aluminum alloy foams at medium strain rates. Mater. Sci. Eng. A 2011, 528, 2326–2330. [Google Scholar] [CrossRef]
- Paul, A.; Ramamurty, U. Strain rate sensitivity of a closed-cell aluminum foam. Mater. Sci. Eng. A 2000, 1, 1–7. [Google Scholar] [CrossRef]
- Su, M.; Wang, H.; Hao, H.; Fiedler, T. Compressive properties of expanded glass and alumina hollowspheres hybrid reinforced aluminum matrix syntactic foams. J. Alloys Compd. 2020, 821, 153233. [Google Scholar] [CrossRef]
- Xie, B.; Fan, Y.Z.; Mu, T.Z.; Deng, B. Fabrication and energy absorption properties of titanium foam with CaCl2 as a space holder. Mater. Sci. Eng. A 2017, 708, 419–423. [Google Scholar] [CrossRef]
- Liu, J.A.; Yu, S.R.; Hu, Z.Q.; Liu, Y.H.; Zhu, X.Y. Deformation and energy absorption characteristic of Al2O3f/Zn–Al composite foams during compression. J. Alloys Compd. 2010, 506, 620–625. [Google Scholar] [CrossRef]
- Mu, Y.L.; Yao, G.C.; Cao, Z.K.; Luo, H.J.; Zu, G.Y. Strain-rate effects on the compressive response of closed-cell copper-coated carbon fiber/aluminum composite foam. Scr. Mater. 2011, 64, 61–64. [Google Scholar] [CrossRef]
- Lin, Y.F.; Zhang, Q.; Chang, J.; Wang, H.Y.; Feng, X.W.; Wang, J. Microstructural characterization and compression mechanical response of glass hollow spheres/Al syntactic foams with different Mg additions. Mater. Sci. Eng. A 2019, 766, 138338. [Google Scholar] [CrossRef]
- Kadkhodapour, J.; Raeisi, S. Micro–macro investigation of deformation and failure in closed-cell aluminum foams. Comp. Mater. Sci. 2014, 83, 137–148. [Google Scholar] [CrossRef]
- Kannan, K.; Misra, A. Short glass fiber reinforced PA6 and ABS blends: Mechanical properties and morpholog. Int. Polym. Proeess. 1994, 9, 184–192. [Google Scholar] [CrossRef]
- Xia, X.C.; Zhao, W.M.; Feng, X.Z.; Feng, H.; Zhang, X. Effect of homogenizing heat treatment on the compressive properties of closed-cell Mg alloy foams. Mater. Des. 2013, 49, 19–24. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Lian, J.S.; Yang, D.Z.; Dong, S.L. An analytical study of the influence of thermal residual stresses on the elastic and yield behaviors of short fiber-reinforced metal matrix composites. Mater. Sci. Eng. A 1998, 248, 256–275. [Google Scholar] [CrossRef]
- Ezhil Vannana, S.; Paul Vizhian, S.; Karthigeyan, R. Investigation on the influence of basalt fiber on thermal properties of Al7075/ basalt fiber metal matrix composites. Procedia Eng. 2014, 97, 432–438. [Google Scholar] [CrossRef]
- Noda, N.A.; Chen, D.; Zhang, G.W.; Sano, Y. Single-fiber pull-out analysis comparing the intensities of singular stress fields (ISSFs) at fiber end/entry points. Int. J. Mech. Sci. 2020, 165, 105196. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Abbandanak, S.N.; Eslami-Farsani, R.; Siadati, S.H. Effects of various aluminum surface treatments on the basalt fiber metal laminates interlaminar adhesion. Int. J. Adhes. Adhes. 2018, 84, 184–193. [Google Scholar] [CrossRef]
- Yua, S.; Oh, K.H.; Hong, S.H. Enhancement of the mechanical properties of basalt fiber-reinforced polyamide 6,6 composites by improving interfacial bonding strength through plasma-polymerization. Compos. Sci. Technol. 2019, 182, 107756. [Google Scholar] [CrossRef]

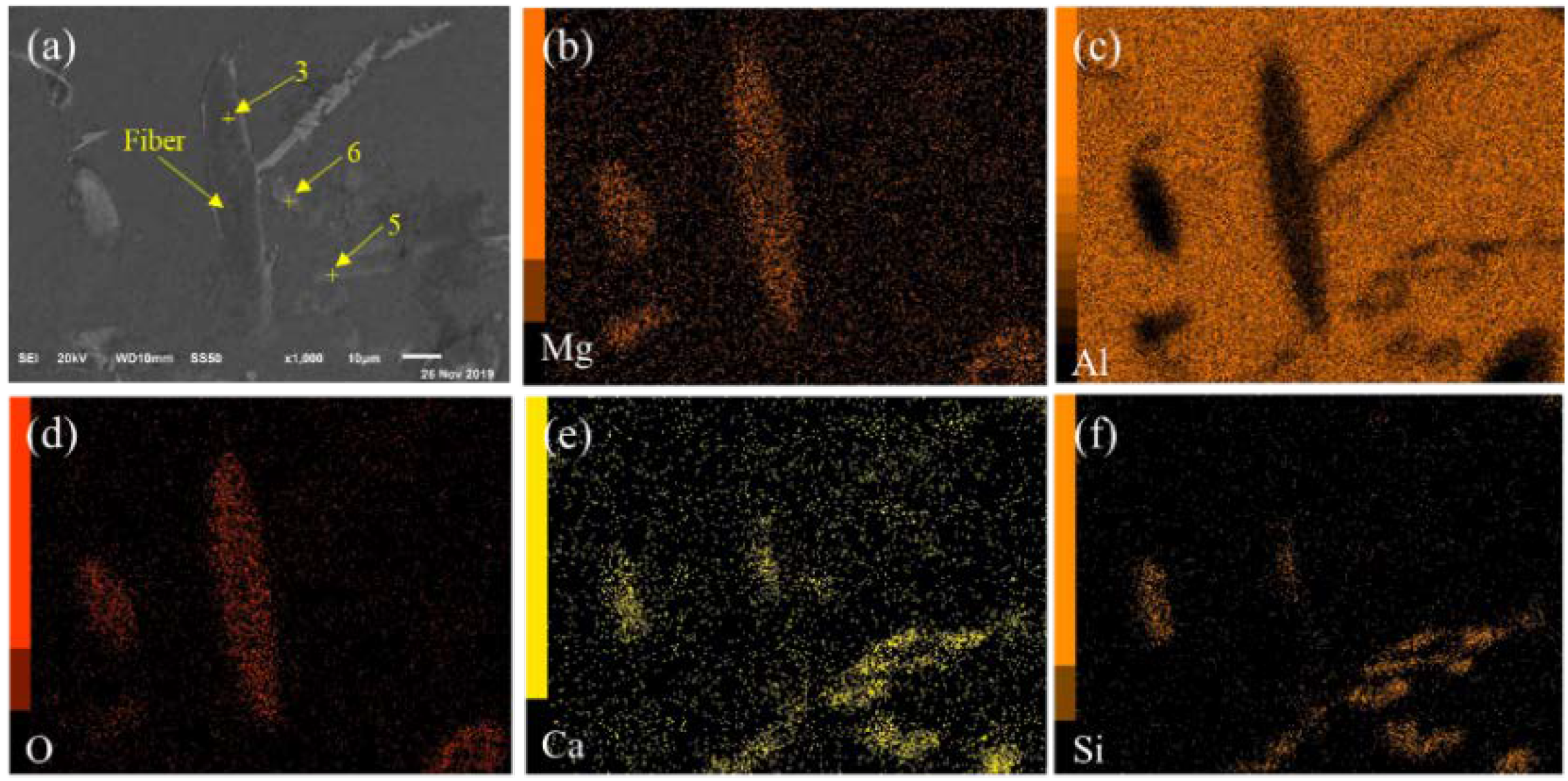
| No. | Composition (wt.%) | ||||
|---|---|---|---|---|---|
| Al | Mg | O | Si | Ca | |
| 1 | 37.82 | 10.32 | 51.58 | - | - |
| 2 | 35.31 | 11.13 | 51.38 | - | - |
| 3 | 39.85 | 10.66 | 49.32 | - | - |
| 4 | 62.88 | - | - | 15.98 | 8.37 |
| 5 | 60.63 | - | - | 18.54 | 9.65 |
| 6 | 78.63 | - | - | 5.69 | 5.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Zhang, Z.; Chen, X.; Han, X.; Xu, T.; Li, X.; Ding, J.; Liu, H.; Xia, X.; Gao, Y.; et al. Quasi-Static Compression Deformation and Energy Absorption Characteristics of Basalt Fiber-Containing Closed-Cell Aluminum Foam. Metals 2020, 10, 921. https://doi.org/10.3390/met10070921
Yang D, Zhang Z, Chen X, Han X, Xu T, Li X, Ding J, Liu H, Xia X, Gao Y, et al. Quasi-Static Compression Deformation and Energy Absorption Characteristics of Basalt Fiber-Containing Closed-Cell Aluminum Foam. Metals. 2020; 10(7):921. https://doi.org/10.3390/met10070921
Chicago/Turabian StyleYang, Donghui, Zichen Zhang, Xueguang Chen, Xing Han, Tao Xu, Xinglei Li, Jian Ding, Haifeng Liu, Xingchuan Xia, Yugang Gao, and et al. 2020. "Quasi-Static Compression Deformation and Energy Absorption Characteristics of Basalt Fiber-Containing Closed-Cell Aluminum Foam" Metals 10, no. 7: 921. https://doi.org/10.3390/met10070921
APA StyleYang, D., Zhang, Z., Chen, X., Han, X., Xu, T., Li, X., Ding, J., Liu, H., Xia, X., Gao, Y., Wang, Y., & Sun, Y. (2020). Quasi-Static Compression Deformation and Energy Absorption Characteristics of Basalt Fiber-Containing Closed-Cell Aluminum Foam. Metals, 10(7), 921. https://doi.org/10.3390/met10070921





