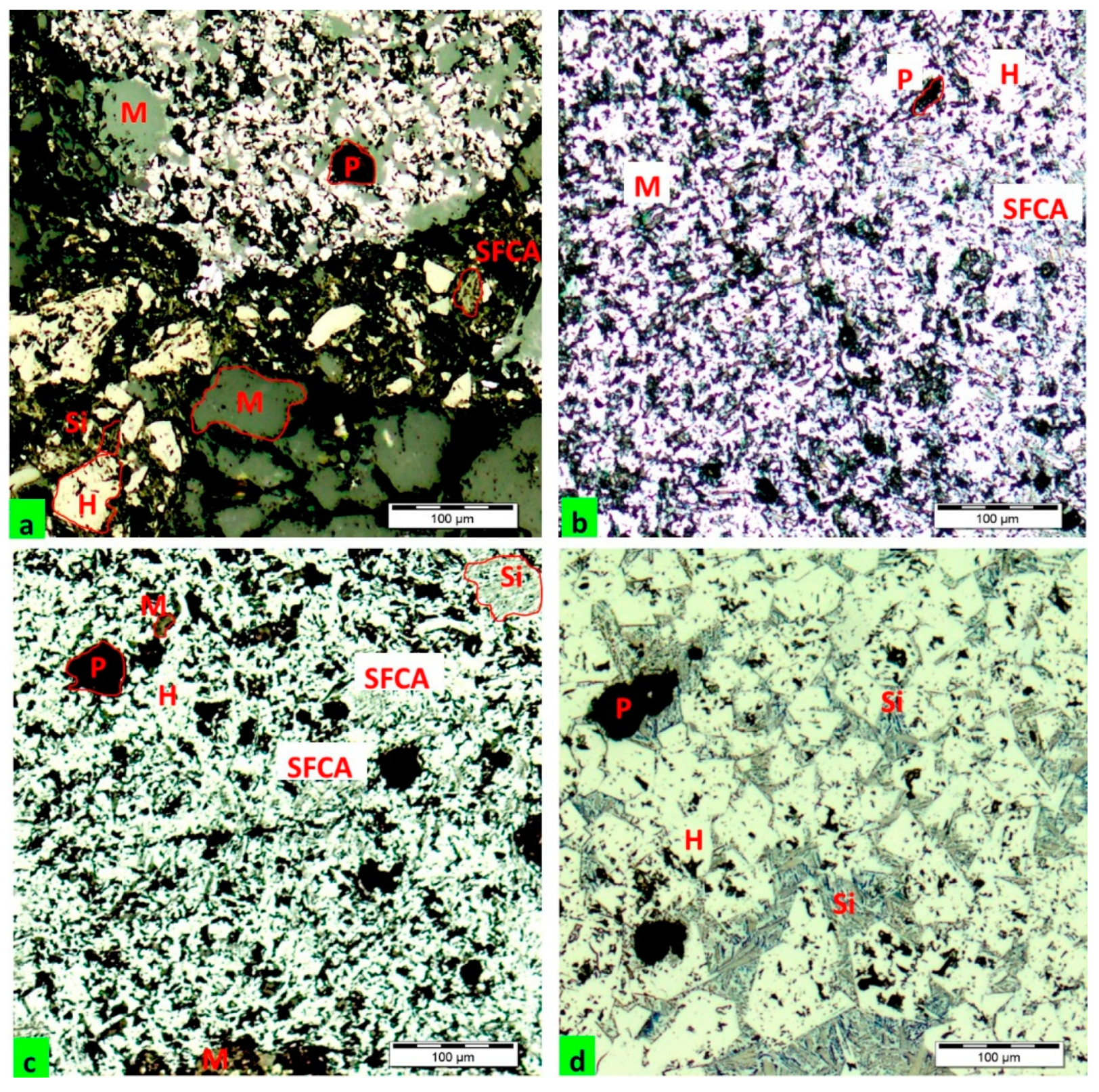
3.1.2. Substituted Agglomerates (b, c, d)
The visual assessment of agglomerate with 20% substitution of lignin (
Figure 1b) is different compared to the standard agglomerate. The agglomerate has a more fine-grained, heterogeneously porous structure. Larger pores and even cavities are visible, but small pores can be seen as well. Isolated areas of sintered ore grains can be found in the structure of the agglomerate. The cause of ore grain sintering is their melting accompanied by the formation of a smaller amount of liquid phase (melt) compared to the standard agglomerate. A small number of areas with grains of the original charge were observed. The predominant part of the agglomerate is characterized by the transition of sintered grains to the plastic state, i.e., to the area of softening. The mutual binding of grains is improved by the application of higher temperature; that is by the formation of a larger quantity of melt and extension of the contact surface of grains as a result of pressure deformation caused by sucked gas. The amount of liquid phase and its properties after the solidification and also after the recrystallization, as the case may be, will have a substantial effect on the change in the mineralogical composition of the agglomerate.
It can be stated, based on the results of optical microscopy (
Figure 2b), that the structure of agglomerate is homogeneous, made up of fine-grained aggregates connected by the melt phase with relatively evenly distributed pores. The structure of agglomerate consists of several mineral phases, while the major ones are hematite and fine-grained magnetite, silicoferrites of calcium and aluminum (SFCA), dicalcium silicate and the glassy phase as in the case of the standard agglomerate. These phases are complexly distributed together with pores.
Compared to the previous agglomerate, the agglomerate with 50% substitution of coke (
Figure 1c) also has a fine-grained structure, but it is more porous, characterized by both larger and smaller pores, which is related to the level of substitution with biomass and the kinetics of combustion. Pores are formed in places where the fuel has burned out and their diameters vary during melting of adjacent grains when edges become rounded and small cavities are enlarged due to the increase of surface tension. The dimension of the resulting pores depends on the size of fuel grains.
The amount of the formed binding melt phase, assessed on the basis of macroanalysis, is smaller compared to the previous agglomerate. Grains of the original ore, grains coated with a film of the melt phase or grains partially modified in the sintering process are visible in the photo documentation.
The microstructure of the agglomerate (
Figure 2c) is more heterogeneous compared to the previous agglomerate. Areas with larger pores were observed in the image. Grains of hematite and magnetite are located in the matrix of glassy silicates and silicoferrites of calcium and aluminum (SFCA). Magnetite is the prevailing oxide mineral. The sintered ore grains are connected without a significant change in their shape, which is an accompanying sign of the formation of a smaller quantity of melt phase owing to the decrease in temperature in the sintered layer. The needle-like formations of dicalcium silicate are well visible in the image.
The agglomerate with 86% substitution of coke has a different morphologic nature of macrostructure (
Figure 1d) compared to the previous agglomerates. The original ore grains coated with a fine film of the melt phase prevail in the structure of agglomerate. This nature of the agglomerate suggests the formation of a very low quantity of melt phase as a result of the insufficient temperature between the grains of the charge. The amount of heat released by combustion of fuel and the amount of accumulated heat in the sintered layer could not provide the sufficient thermal conditions for the formation of an optimal quantity of the melt phase. Reactions between solid phases immediately affecting the formation of the melt phase play an important role in the formation of the agglomeration melt. At a low temperature, only a part of the charge melts and a large portion of products of reactions in the solid state pass unchanged to the structure of the final agglomerate.
The structure of agglomerate comprises the primary and secondary grains of hematite, magnetite and probably also the silicate represented mainly by larnite. The predominant part of the secondary magnetite was formed by the reduction of the primary hematite, which is the basic ferriferous component of the initial metal-bearing charge. The grains of magnetite are characterized by irregular shapes corresponding to polyhedrons with sharp edges.
The mineralogical composition of agglomerates with different substitution levels of the applied fuel was identified using the X-ray analysis. The quantification of mineralogical composition using the Rietveld method was carried out by the powder diffraction technique. The mineralogical compositions of various produced agglomerates are given in
Table 7. These results show how the use of a substitute fuel affected the amount of different minerals in the agglomerate.
The share of substitute fuel and the formation of mineralogical compounds of the agglomerate in the course of the sintering process are shown in
Figure 3. These minerals are represented by complex calcium ferrites in association with iron oxides and a limited amount of silicates. Quartz and wüstite are present in small quantity.
It is clear from the X-ray analysis that there was no change in the mineralogical composition, i.e., no formation of new minerals, at 20% and 50% substitution of coke breeze with biomass. There was a substantial change in the share of individual minerals, as it is shown in
Figure 3. At 20% and 50% substitution, there was a decrease in the majority oxide (magnetite, hematite) and increase in the bonding phases represented by silicoferrites (SFCA-I and SFCA) and silicates (larnite, hedenbergite). The share of non-assimilated SiO
2 (quartz) drops slightly compared to the standard agglomerate. At 86% substitution, the share of SiO
2 rose by 2.3%. It may indicate a lower rate of agglomeration ore assimilation, which is the main carrier of SiO
2 in the sintering charge.
Magnetite is the majority oxide in the standard agglomerate and its share at 20% substitution with biomass is almost identical (−0.3%) with the standard agglomerate. A more marked decrease by 12.3% occurred at 50% substitution. By increasing the substitution to 86%, the share of magnetite rose to the value of 41.2%, which corresponds to the increase by 5.9 against the standard.
The share of hematite against the standard agglomerate at 20% substitution was reduced by 6.4% and it dropped by 7.3% at 50% substitution due to the formation of bonding ferritic phase. Different nature of change was observed at 86% substitution. Its share against the standard agglomerate rose by 4.3%, which is presumably linked to the absence of the formation of silicoferrites of calcium and aluminum and the preferential formation of calcium silicate–larnite.
On the basis of the above, it can be stated that as the partial substitution of coke with biomass of 20% and 50% increased, the amount of both oxide decreased. On the contrary, a considerably opposite trend is observed at 86% substitution, i.e., increase in magnetite and thus in hematite as well. The increased content of hematite and magnetite in the agglomerate at 86% substitution was related to the presence of the original grains of sintering ore and concentrate, in which no mineralogical change occurred at low temperature.
Silicoferrites of calcium and aluminum are the key bonding phase in agglomerates on which the overall quality of agglomerate depends. In the standard agglomerate, there are both forms of complex silicoferrites SFCA-I and SFCA, while the higher values are achieved by the high iron modification of SFCA-I. As the substitution increases up to 50%, both forms of SFCA are growing, while the high iron form SFCA-I dominates just like in the case of the standard agglomerate. Neither of the forms of silicoferrites of calcium and aluminum was identified in the agglomerate by increasing the substitution to the value of 86%. By contrast, the share of the silicate Ca2SiO4 rose sharply. While its value was growing slightly and rose by 1.1% against the standard agglomerate at the substitution of up to 50%, a step change and an increase by 14.9% is observed at 86% substitution.
A new phase—wüstite (FeO)—was identified in the agglomerate with 86% substitution. The presence of wüstite was not confirmed in other agglomerates. The effect of FeO on the formation of ferrites is negative because it stabilizes magnetite and Fe
2O
3, which is bound in it [
4]. Due to the higher reactivity of lignin, the vertical sintering rate increased, which resulted in shortening of the total sintering time and thus shortening of the time during which potential oxidation of FeO could occur. This could be the cause of the higher amount of FeO in the agglomerate with the highest (86%) substitution of coke with lignin, when the lowest temperatures were reached in the sintered layer.
Silicoferrites of calcium and aluminum, which function as the bonding phase in the structure of agglomerate, are characterized by good strength and reducibility. Their absence at 86% substitution and the presence of larnite result in reduced strength and possibly also decreased reducibility of the agglomerate. Crumbly, disintegrating agglomerate is the outcome of the above.
In most cases, the identified silicate bonding phase constitutes an undesired phase. However, it is an accompanying phase in the production of all basic agglomerates. The silicate, which is in most cases represented as larnite (2CaO.SiO2) or hedenbergite, does not ensure sufficient strength and formation of quality agglomerate.
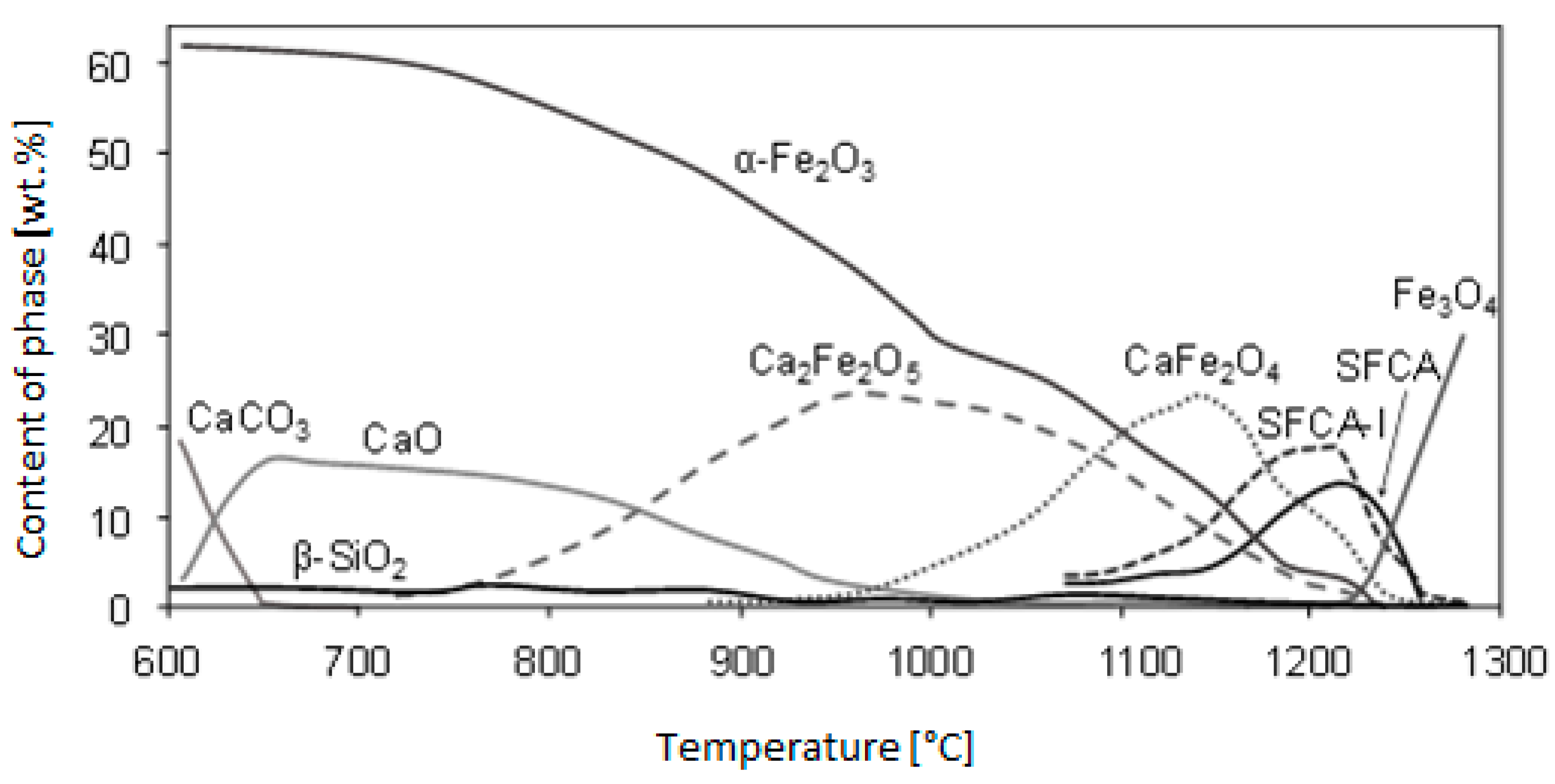
Webster et al. [
5] examined the formation of complex silicoferrites and observed the formation of SFCA and SFCA-I from chemically pure reagents Fe
2O
3, SiO
2, CaCO
3 and Al(OH)
3 in a slightly reducing atmosphere of pO
2 = 10
−3 atm. The course of calcium ferrite formation from the reactions in the solid state to the formation of the melt is the outcome of this study, which is illustrated in
Figure 4.
It is apparent from the said dependence that thermal conditions play an important role in the sintered layer. The issue of creation and formation of complex silicoferrites has been addressed by a number of research papers [
6,
7,
8,
9,
10,
11,
12,
13,
14].
Fuel plays a crucial role in the assurance of thermal conditions in the sintered layer, while each fuel has its specific properties and is a carrier of a varying number of components for the sintering mixture. The above applies particularly when the substitution of coke breeze is performed according to the energy value. The preservation of the energy value of a fuel mixture is directly related to the amount of fixed carbon in fuels. As it follows from
Table 2, the substitute fuel has four times lower content of fixed carbon and incomparably higher content of volatile combustible. The volatile combustible reduces the calorific value of fuel and shifts its ignition temperature to lower values. The mixture of fuels with two ignition temperatures expands the combustion zone and decreases the maximum reached temperature.
The maximum temperatures and sintering time with the substitution of coke with biomass documented in
Table 8 confirm this fact.
The obtained results of temperature curves along the height of the sintered layer match the previous research on the creation and formation of the ferritic phases in the structure of agglomerate.
The formation of agglomerate with satisfactory strength properties is conditioned by the accomplishment of such thermal conditions in the sintered layer that would provide the complete melting of charge in one part of microvolumes, the partial melting of charge in another part of microvolumes and the preservation of the solid state in other microvolumes. The rate of melt formation, the amount of melt, the chemical and mineralogical composition and the method of agglomerate cooling determine the final properties of the agglomerate, including its strength. With a small amount of sintering melt in the sintered layer, a low-strength diffusion bond is formed, and the agglomerate has a low strength. This was reflected in the agglomerate with 86% substitution of coke with lignin. The resulting strength is also influenced by the mineralogical composition of the agglomerate, in which hematite and magnetite have the highest strength in the final structure of the agglomerate, followed by calcium ferrites and SFCA complex compounds. On the contrary, dicalcium silicate (Ca
2SiO
4) has the lowest strength. The SFCA complex compounds generally form a bonding phase in the agglomerate. The effect of coke powder substitution with lignin on the strength properties of the agglomerate shows certain differences. In a series of sinterings with lignin, the agglomerate showed a lower abrasion compared to the standard at up to 50% substitution of coke, which was also reflected in an increase in the strength of the agglomerate. These agglomerates had an ISO
+6.3 strength in the range of 63–65% and an ISO
−0.5 abrasion index in the range of 6–7%. A further increase in the substitution coefficient has already had a negative impact on the strength properties. At 86% substitution, the ISO
+6.3 strength of 55% and the ISO
−0.5 abrasion index of 10% were achieved. Industrially produced agglomerates have the ISO
+6.3 strengths in the range of 65–78% and the ISO
−0.5 abrasion index of 4–9% [
15], which also corresponds to laboratory-produced agglomerates with the substitution of coke with lignin up to 50%. The proportion of silicoferrites of calcium and aluminum (SFCA) increased in the resulting structure of agglomerates produced with up to 50% substitution of coke with lignin, which could have a positive effect on the final strength of these agglomerates, as these compounds formed an appropriate bonding phase between iron oxides, calcium ferrites and calcium silicates.
The properties of produced agglomerate are determined by the requirements of blast furnace technology and consequently its production technology. Chemical, physical, mechanical and metallurgical properties constitute the essential requirements for the blast furnace agglomerate. The reducibility is very important for estimate agglomerate suitability to blast furnace process. The reducibility of agglomerates produced using lignin was realized in high-temperature reducibility test. The test sample is reduced isothermally at 950 °C using a reducing gas (CO and N2) and in certain time intervals, its weight is determined until the degree of reduction reaches 65%. The reducibility index is expressed as a rate of reduction, i.e., the time needed to achieve the 60% degree of reduction. The reducibility index (dR/dt) of standard agglomerate was 1.08%/min. The agglomerates with substitution of coke powder by lignin reached the higher values of reducibility (dR/dt = 1.12–1.25%/min). From the point of view of the metallurgical properties of the agglomerate, we can evaluate the replacement of coke with lignin positively.
Increasing volume of coke substitution with biomass results in the decrease of maximum temperatures in the sintered layer and shortening of the sintering time. This is indicated by the faster progress of the combustion zone in the sintered layer due to the higher reactivity of biomass.
It follows from the above that the heat generated by the mixed fuel at 86% substitution does not suffice for the formation of the silicoferrite phase, which has probably caused the deficit of SFCA-I and SFCA phases in the structure of agglomerate.
The agglomerate always contains desired and suitable phases and, conversely, undesired and unsuitable phases. Redistribution of quantity between the appropriate phases (e.g., decreasing the proportion of iron oxides and increasing the proportion of SFCA and calcium ferrites) does not significantly decrease the quality of the agglomerate—which was also achieved at 20–50% substitution of coke with lignin. In addition, these agglomerates had standard chemical, physical and mechanical properties and lower emissions of nitrogen and sulfur oxides were achieved in the production.