The Polythermal Section of Ti-22Al-xNb (30-78at.%Ti) in Ti–Al–Nb System
Abstract
1. Introduction
2. Materials and Method
3. Results
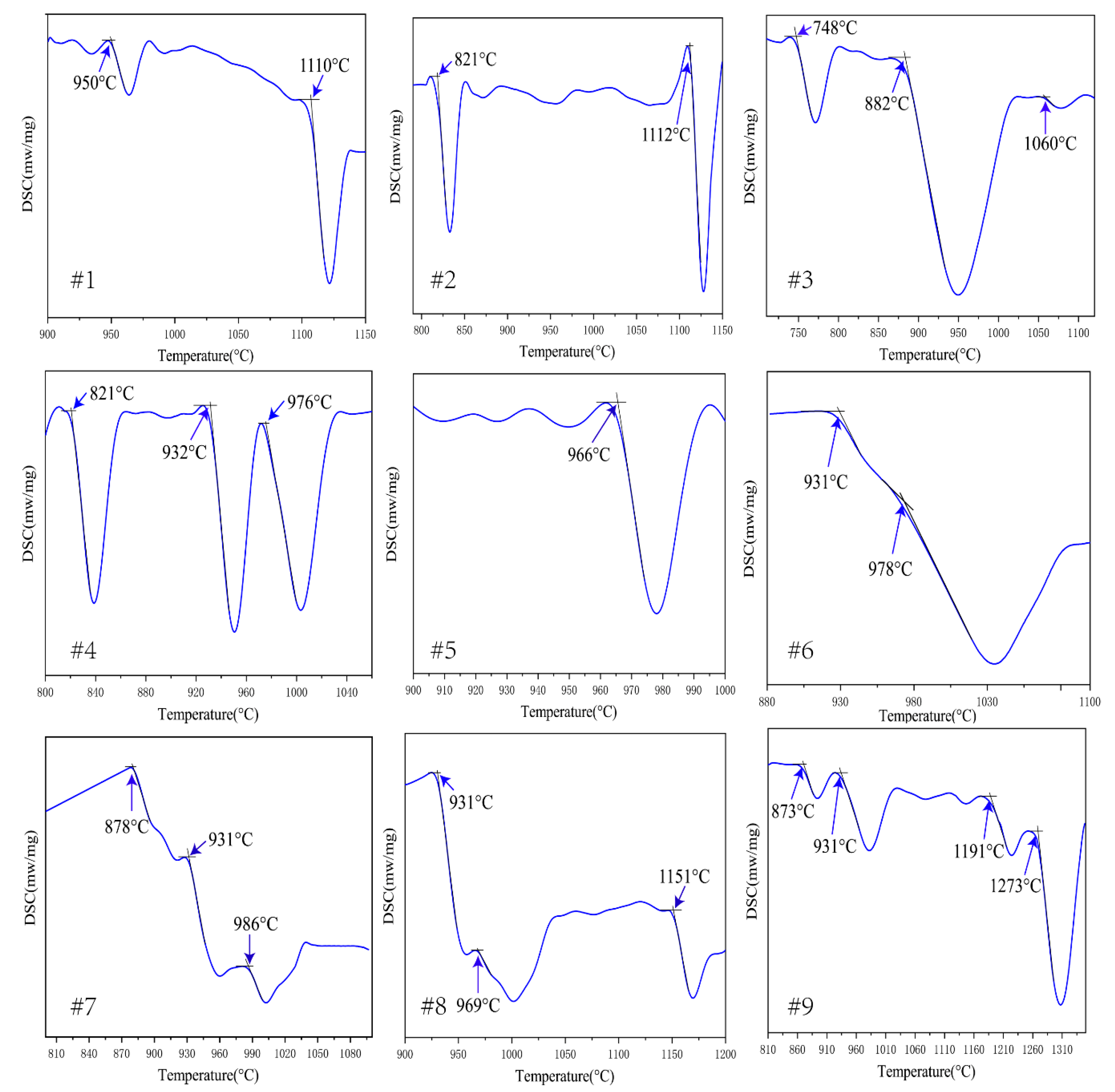
3.1. Analysis of DSC Results
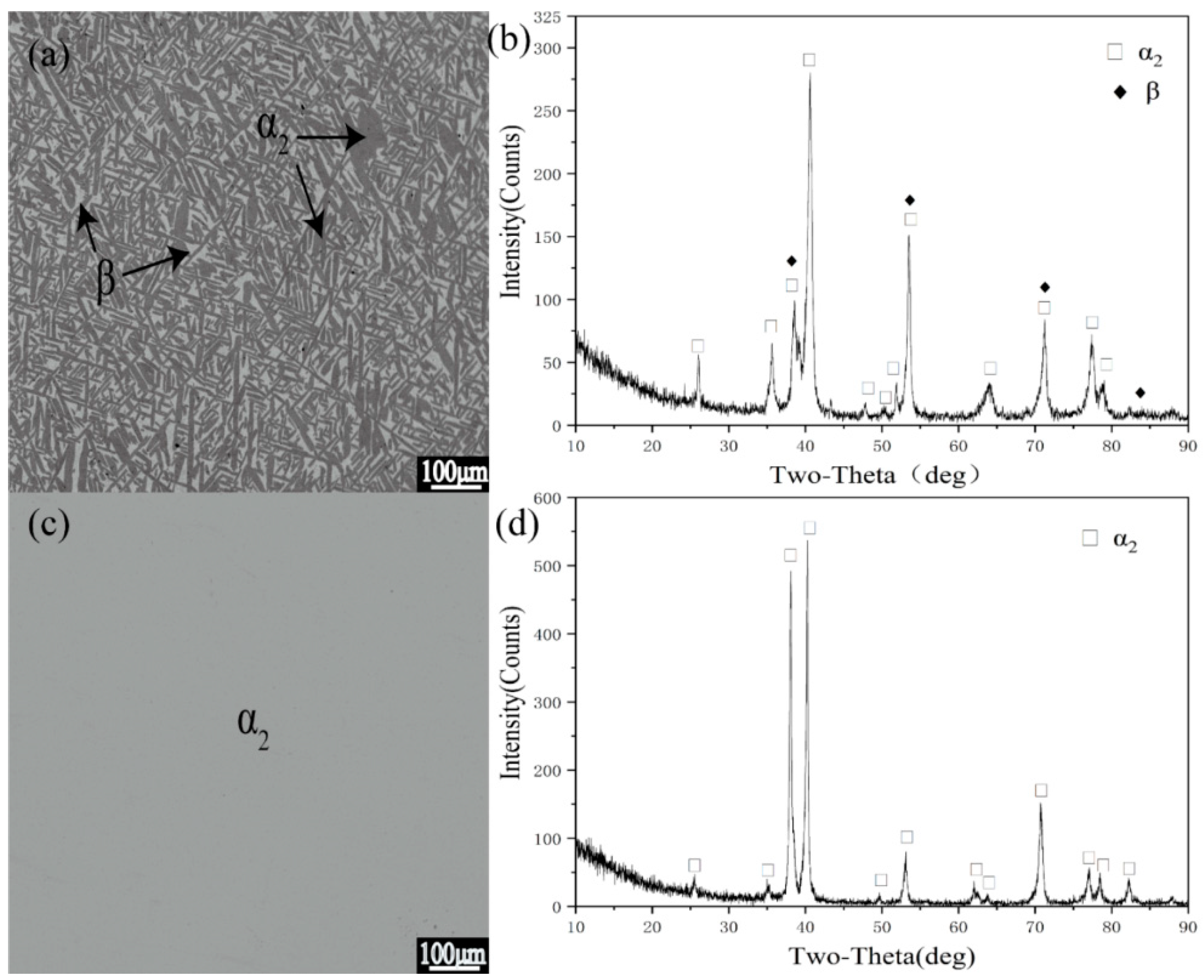
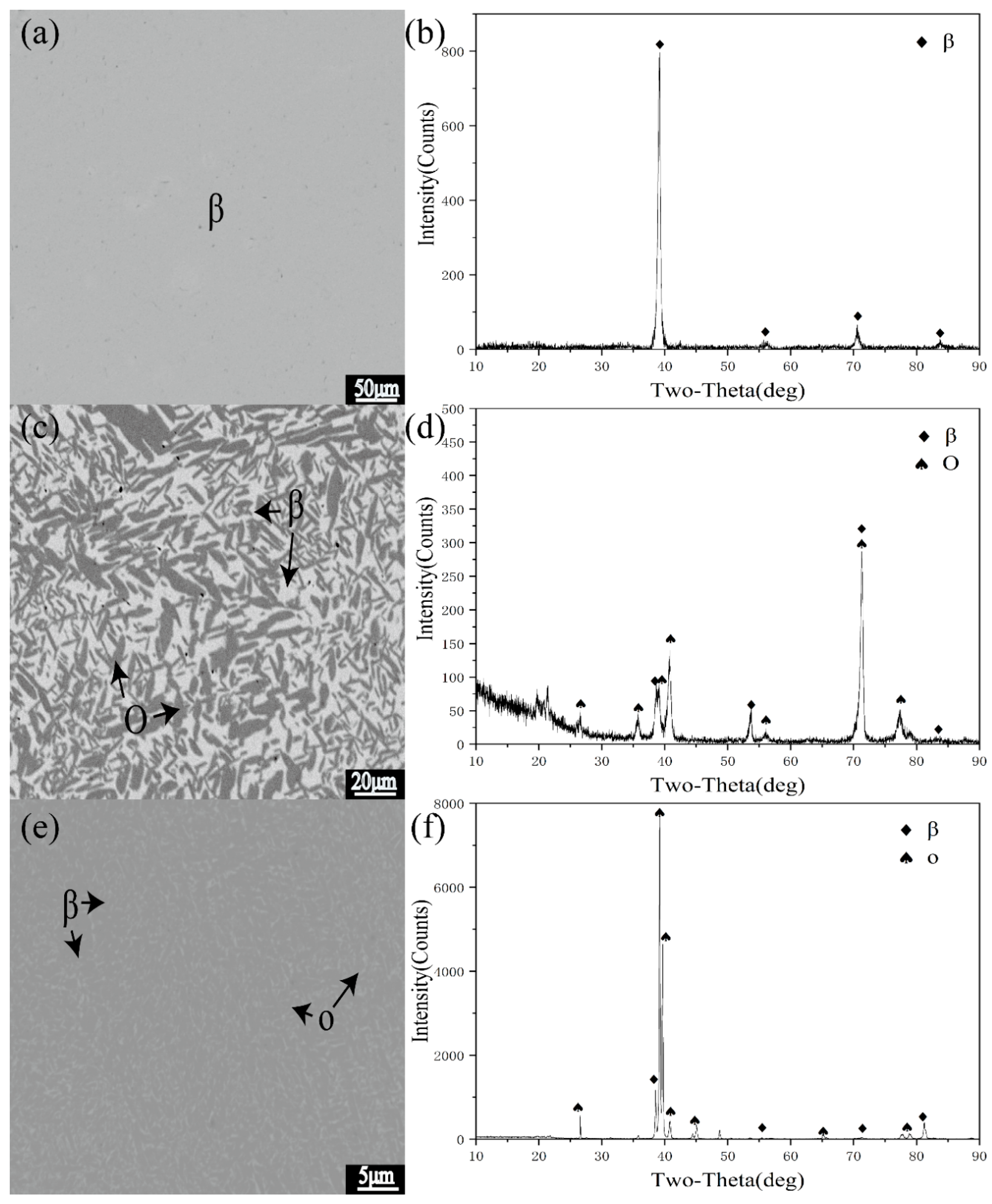
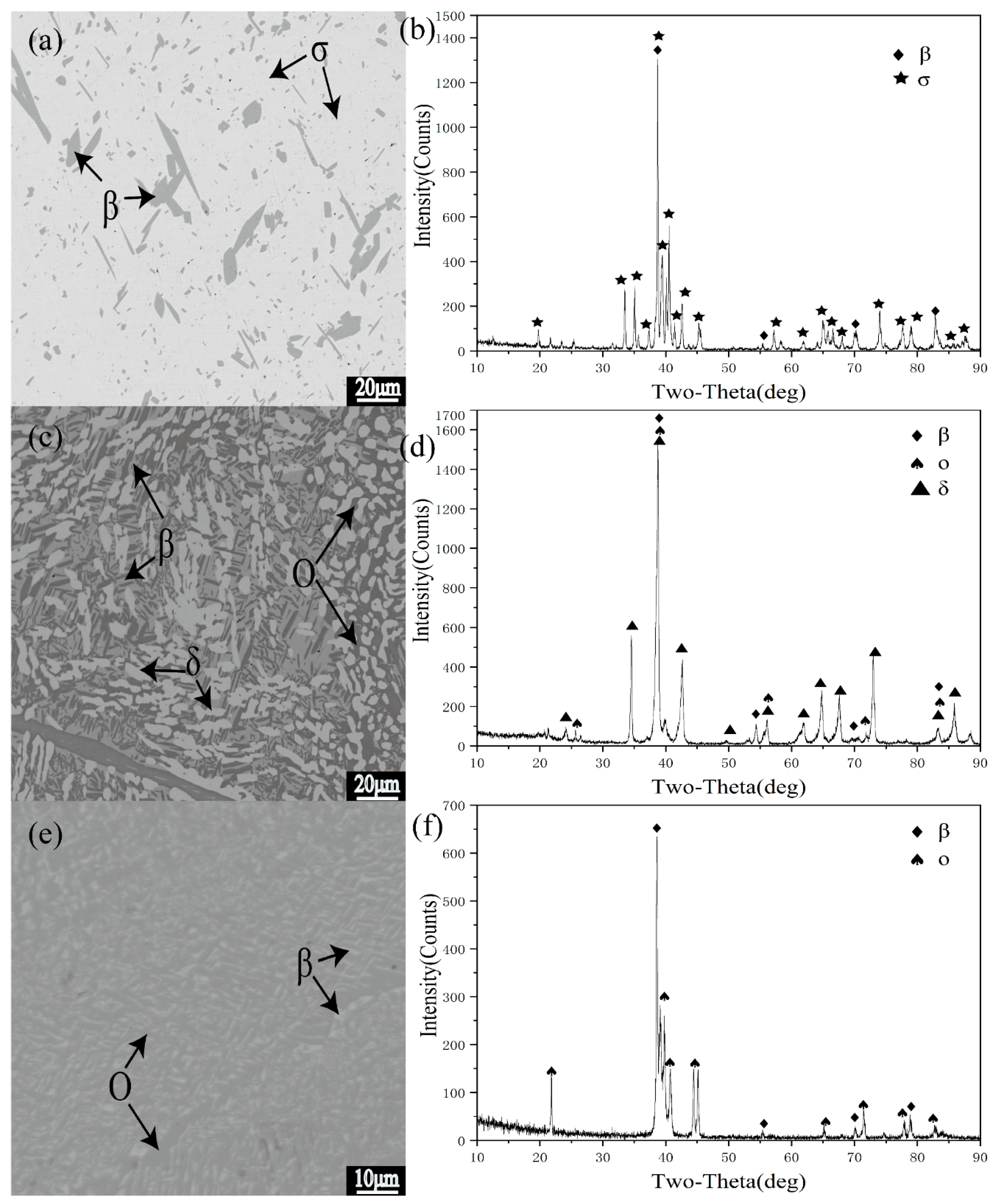
3.2. Analysis of Typical Alloys in Polythermal Section
3.3. Polythermal Section
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumpfert, J. Intermetallic Alloys Based on Orthorhombic Titanium Aluminide. Adv. Eng. Mater. 2010, 3, 851–864. [Google Scholar] [CrossRef]
- Pollock, T.M. Alloy design for aircraft engines. Nat. Mater. 2016, 15, 809–815. [Google Scholar] [CrossRef]
- Ding, X.F.; Lin, J.P.; Zhang, L.Q.; Su, Y.Q.; Chen, G.L. Microstructural control of TiAl–Nb alloys by directional solidification. Acta Mater. 2012, 60, 498–506. [Google Scholar] [CrossRef]
- Wang, S.; Xu, W.; Sun, W.; Zong, Y.; Chen, Y.; Shan, D. Study on Microstructure Evolution and Mechanical Properties of Ti2AlNb-Based Alloy under Canning Compression and Annealing. Metals 2019, 9, 980. [Google Scholar] [CrossRef]
- Bian, H.; Lei, Y.; Fu, W.; Hu, S.; Song, X.; Feng, J. Diffusion Bonding of Ti2AlNb Alloy and High-Nb-Containing TiAl Alloy: Interfacial Microstructure and Mechanical Properties. Metals 2018, 8, 1061. [Google Scholar] [CrossRef]
- Boehlert, C.J. The effects of forging and rolling on microstructure in O+BCC Ti-Al-Nb alloys. Mater. Sci. Eng. A 2000, 279, 118–129. [Google Scholar] [CrossRef]
- Polozov, I.; Sufiiarov, V.; Popovich, A.; Masaylo, D.; Grigoriev, A. Synthesis of Ti-5Al, Ti-6Al-7Nb, and Ti-22Al-25Nb alloys from elemental powders using powder-bed fusion additive manufacturing. J. Alloys Compd. 2018, 763, 436–445. [Google Scholar] [CrossRef]
- Hagiwara, M.; Emura, S.; Araoka, A.; Yang, S.J.; Nam, S.W. The effect of lamellar morphology on tensile and high-cycle fatigue behavior of orthorhombic Ti-22Al-27Nb alloy. Metall. Mater. Trans. A 2004, 35, 2161–2170. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, W.; Xue, C.; Liang, X.; Zhang, J. Microstructure control and mechanical properties from isothermal forging and heat treatment of Ti–22Al–25Nb (at.%) orthorhombic alloy. Intermetallics 2015, 56, 79–86. [Google Scholar] [CrossRef]
- Kaltenbach, K.; Schulze, K.; Henig, E.T.; Gama, S.; Pinatti, D.G. A Contribution to the Ternary System Al-Nb-Ti. Z. Metallkd. 1989, 80, 535–539. [Google Scholar]
- Bendersky, L.A.; Boettinger, W.J.; Burton, B.P.; Biancaniello, F.S.; Shoemaker, C.B. The formation of ordered ω-related phases in alloys of composition Ti4Al3Nb. Acta Metall. Mater. 1990, 38, 931–943. [Google Scholar] [CrossRef]
- Chen, G.L.; Wang, X.T.; Ni, K.Q.; Hao, S.M.; Cao, J.X.; Ding, J.J.; Zhang, X. Investigation on the 1000, 1150 and 1400 °C isothermal section of the Ti-Al-Nb system. Intermetallics 1996, 4, 13–22. [Google Scholar] [CrossRef]
- Jewett, T.J. Comment on ‘Investigation on the 1000, 1150 and 1400 °C isothermal section of the Ti-Al-Nb system’. Intermetallics 1997, 5, 157–159. [Google Scholar] [CrossRef]
- Boehlert, C.J. The phase evolution and microstructural stability of an orthorhombic Ti-23Al-27Nb alloy. J. Phase Equilibria 1999, 20, 101–108. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Nb-Ti (Aluminum-Niobium-Titanium). J. Phase Equilibria Diffus. 2005, 26, 360–368. [Google Scholar] [CrossRef]
- Raghavan, V. ChemInform Abstract: Al—Nb—Ti (Aluminum-Niobium-Titanium). Cheminform 2012, 43. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Rex, S.; Velikanova, T.Y. The Al–B–Nb–Ti system: III. Thermodynamic re-evaluation of the constituent binary system Al–Ti. J. Alloys Compd. 2008, 465, 64–77. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Velikanova, T.Y. The Al–B–Nb–Ti system: IV. Experimental study and thermodynamic re-evaluation of the binary Al–Nb and ternary Al–Nb–Ti systems. J. Alloys Compd. 2009, 472, 133–161. [Google Scholar] [CrossRef]
- Kattner, U.R.; Boettinger, W.J. Thermodynamic calculation of the ternary Ti-Al-Nb system. Mater. Sci. Eng. A 1992, 152, 9–17. [Google Scholar] [CrossRef]
- Miracle, D.B.; Rhodes, C.G.; Foster, M.A. In Titanium ’95. M; The Cambridge University Press: Cambridge, UK, 1996; pp. 372–379. [Google Scholar]
- Li, L.; Liu, L.; Zhang, L.; Zeng, L.; Zhao, Y.; Bai, W.; Jiang, Y. Phase Equilibria of the Ti-Al-Nb System at 1000, 1100 and 1150 °C. J. Phase Equilibria Diffus. 2018, 39, 549–561. [Google Scholar] [CrossRef]
- Xu, S. Study on the Phase Equilibria and Typical Phase Transformations of Ti-Al-Nb Ternary System. Ph.D. Thesis, University of Science and Technology, Beijing, China, 2019. [Google Scholar]
- Muraleedharan, K.; Nandy, T.K.; Banerjee, D.; Lele, S. Phase stability and ordering behaviour of the O phase in Ti-Al-Nb alloys. Intermetallics 1995, 3, 187–199. [Google Scholar] [CrossRef]
- Cupid, D.M.; Fabrichnaya, O.; Rios, O.; Ebrahimi, F.; Seifert, H.J. Thermodynamic re-assessment of the Ti-Al-Nb system. Int. J. Mater. Res. 2009, 100, 218–233. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Nb-Ti (Aluminum-Niobium-Titanium). J. Phase Equilibria Diffus. 2010, 31, 561. [Google Scholar] [CrossRef]
- Ding, J.J.; Hao, S.M. Reply to the “comment on ‘investigation on the 1000, 1150 and 1400 °C isothermal section of the Ti-Al-Nb system’ ”—Part II. Modification of 1000 and 1150 °C isothermal sections of the Ti-Al-Nb system. Intermetallics 1998, 6, 329–334. [Google Scholar] [CrossRef]
- Rollett, A.D.; Smith, P.R.; James, M.R. Texture and anisotropy of Ti-22Al-23Nb foil. Mater. Sci. Eng. A 1998, 257, 77–86. [Google Scholar] [CrossRef]
- Ohnuma, I.; Fujita, Y.; Mitsui, H.; Ishikawa, K.; Kainuma, R.; Ishida, K. Phase equilibria in the Ti–Al binary system. Acta Mater. 2000, 48, 3113–3123. [Google Scholar] [CrossRef]
- Kostov, A.; Friedrich, B. Selection of crucible oxides in molten titanium and titanium aluminum alloys by thermo-chemistry calculations. J. Min. Metall. 2005, 41, 113–125. [Google Scholar] [CrossRef]
- Masset, P.J.; Schütze, M. Thermodynamic Assessment of the Alloy Concentration Limits for the Halogen Effect of TiAl Alloys. Adv. Eng. Mater. 2008, 10, 666–674. [Google Scholar] [CrossRef]
- Berghof-Hasselbächer, E.; Diliberto, S.; Gawenda, P.; Masset, P.J.; Schmidt, G.; Schütze, M. Metallographische Untersuchungen der Mikrostrukturen von technischen TiAl-Legierungen. Pract. Metallogr. 2010, 47, 65–83. [Google Scholar] [CrossRef]

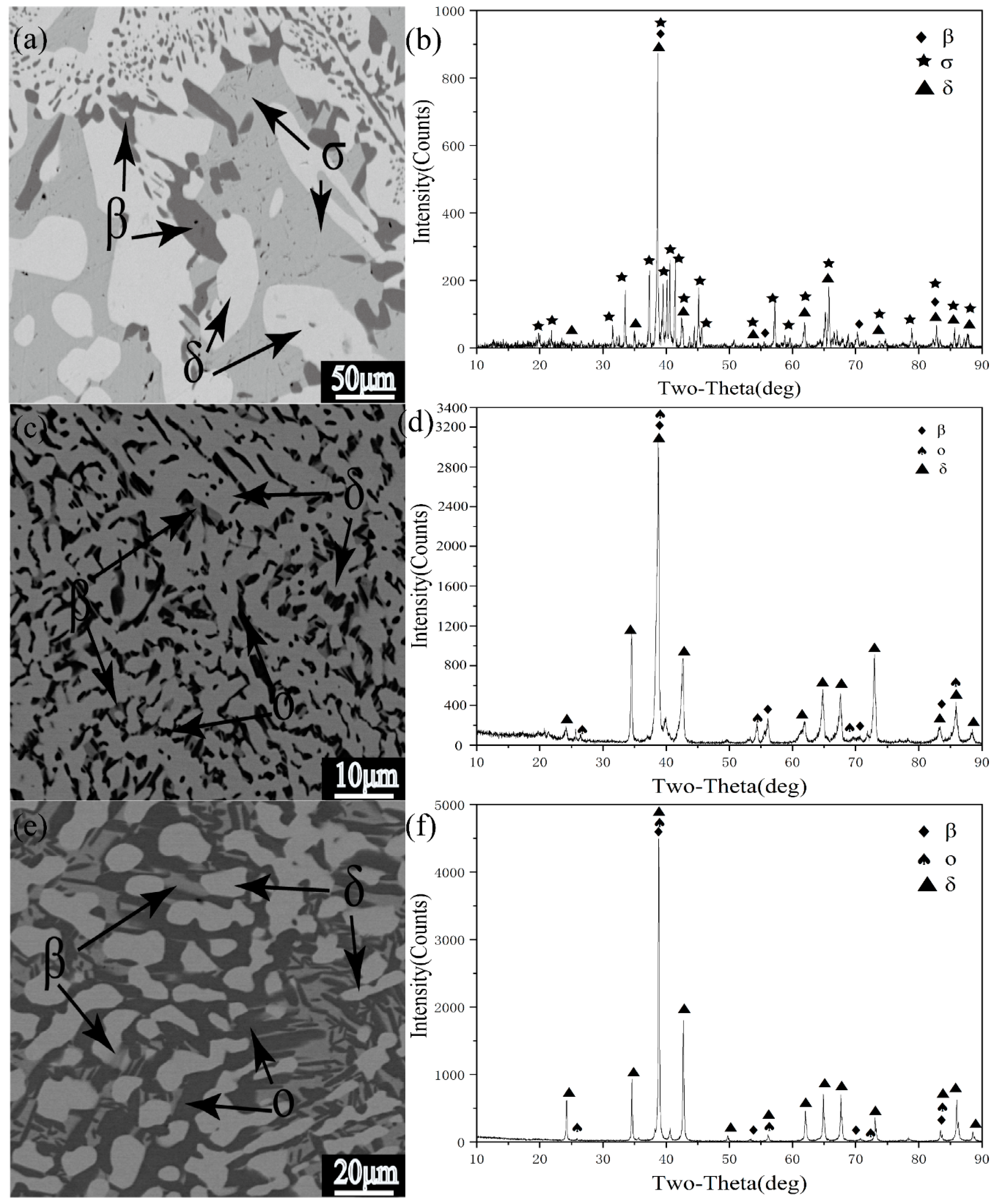
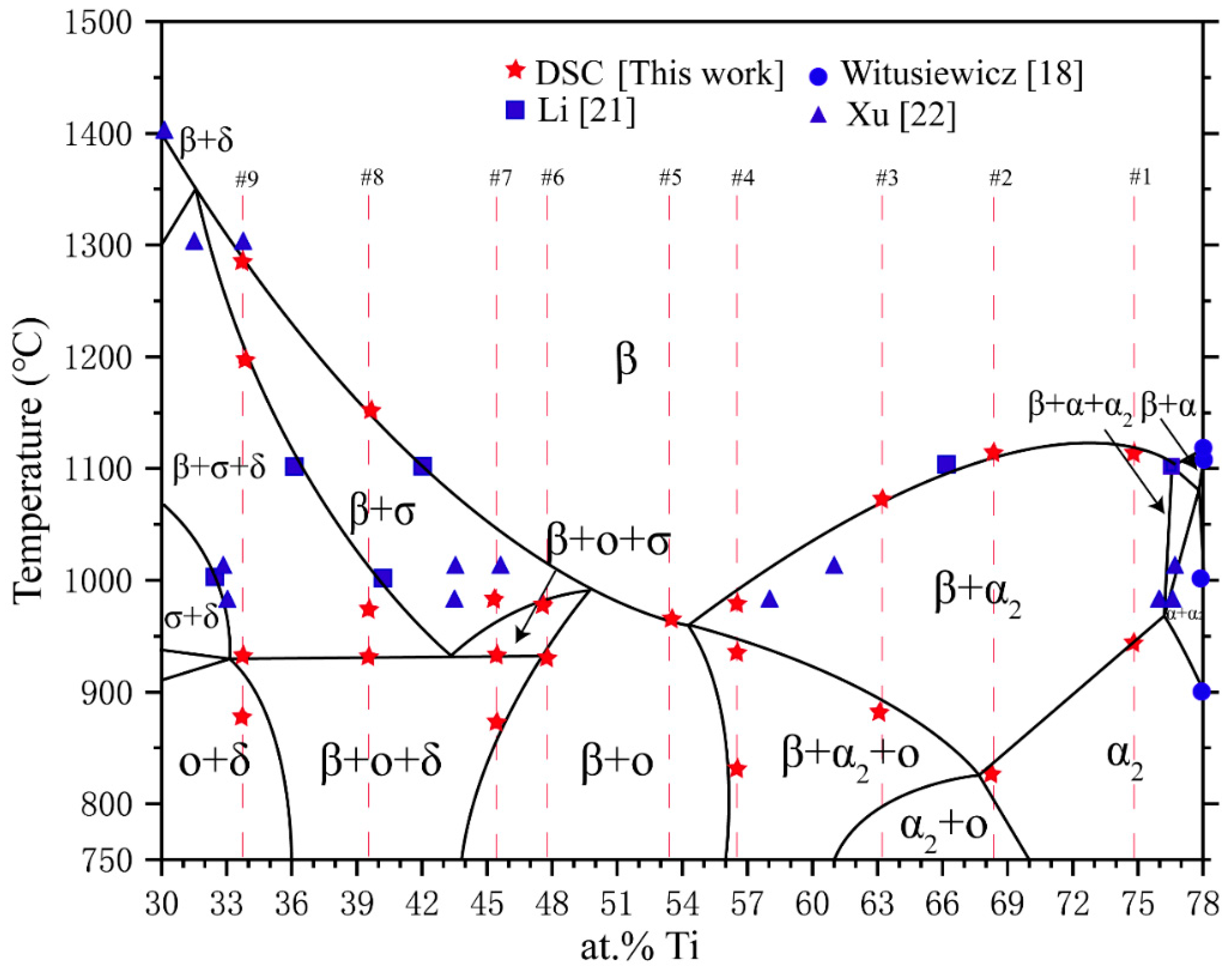
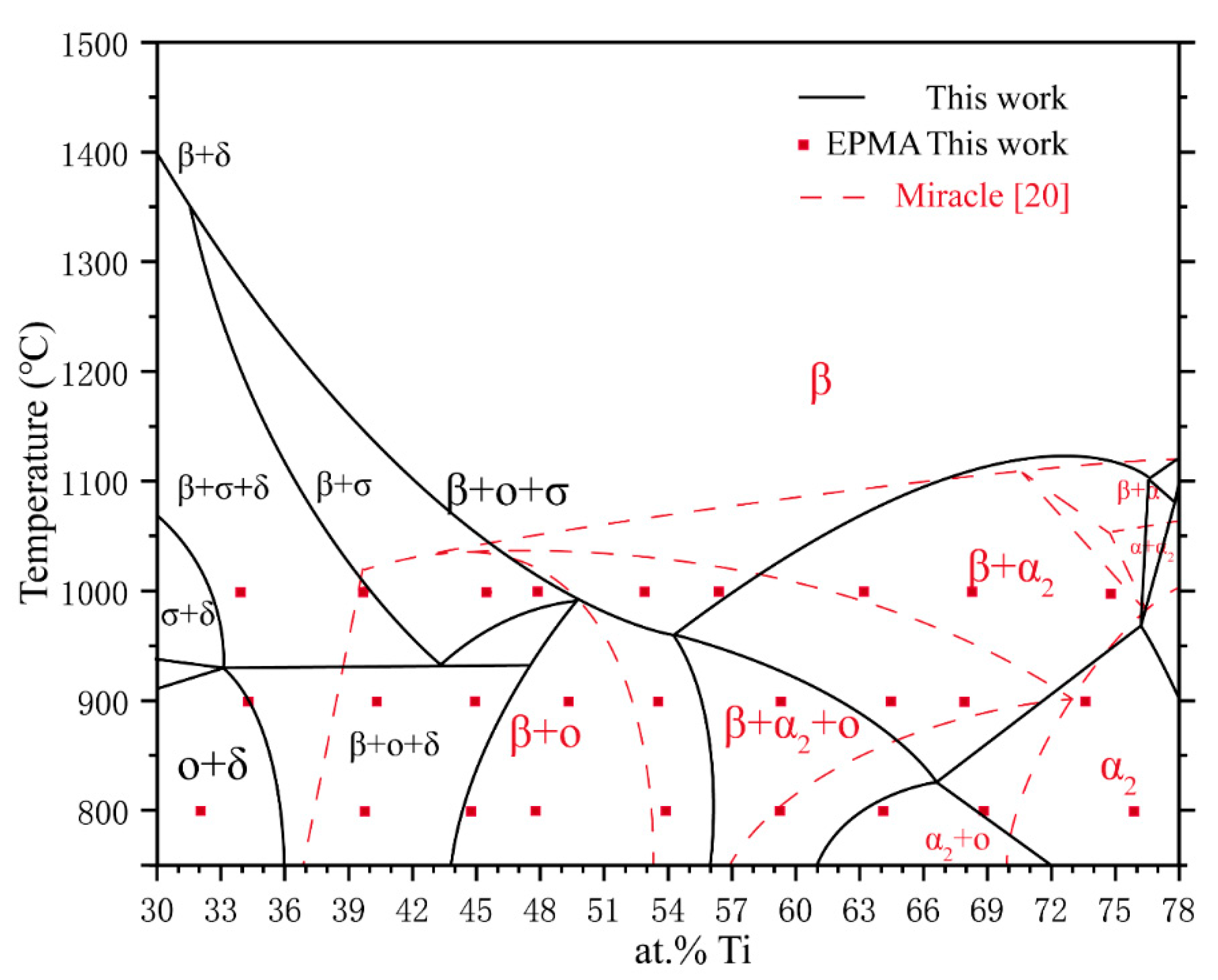
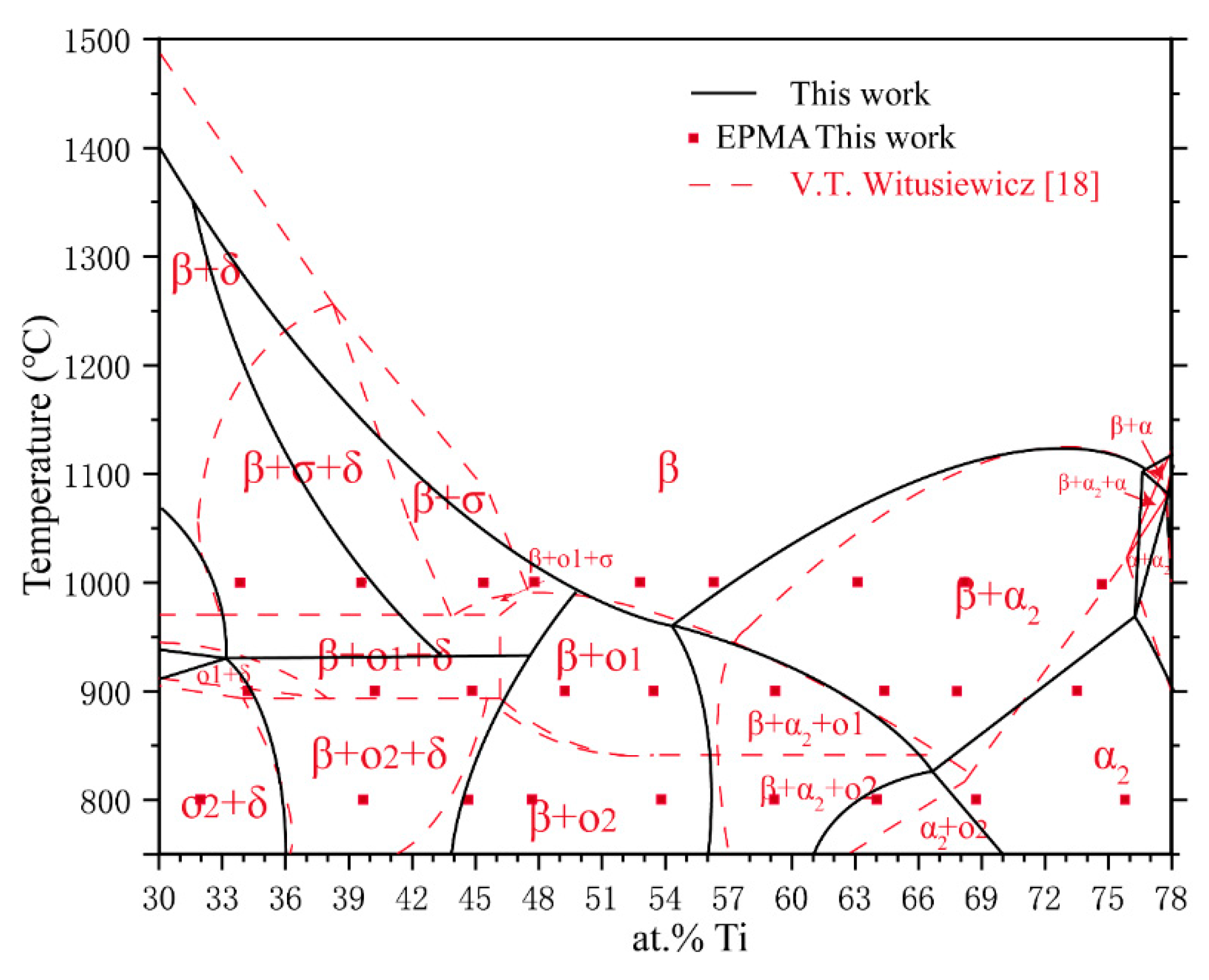
| Phase | Pearson Symbol | Space Group | Strukturbericht Designation | Prototype |
|---|---|---|---|---|
| (Al)(αAl), fcc_Al | cF4 | Fm-3m | A1 | Cu |
| α, (αTi), hcp_A3 | hp2 | P63/mmc | A3 | Mg |
| α2, Ti3Al | hP8 | P63/mmc | D019 | Ni3Sn |
| β, (βTi), bcc_A2 | cI2 | Im-3m | A2 | W |
| β0, bcc_B2 | cI2 | Pm-3m | B2 | CsCl |
| γ, γTiAl, TiAl | tP4 | P4/mmm | L10 | AuCu |
| δ, Nb3Al | cP8 | Pm-3n | A15 | Cr3Si |
| ℇ, (Ti1−xNbx)Al3, TiAl3(h), NbAl3 | tI8 | I4/mmm | D022 | TiAl3(h) |
| ε(l), TiAl3(l) | tI32 | I4/mmm | — | TiAl3(l) |
| ζ, Ti2+xAl5−x | tP28 | P4/mmm | — | Ti2Al5 |
| η, TiAl2 | tI24 | I41/amd | — | HfGa2 |
| σ, Nb2Al | tP30 | P42/mnm | D8b | σCrFe |
| Ti3Al5 | tP32 | P4/mbm | — | Ti3Al5 |
| O1, O, O1(h), Ti2NbAl | oC16 | Cmcm | — | NaHg |
| O2, O2(r), Ti2NbAl | oC16 | Cmcm | — | NaHg |
| τ, Ti4NbAl3 | hP6 | P63/mmc | B82 | Ni2In |
| γ1-Ti4Nb3Al9 | tP16 | P4/mmm | — | γ1-Ti4Nb3Al9 |
| No. | Alloy Compositions | ||
|---|---|---|---|
| Ti | Al | Nb | |
| 1 | 73 | 22 | 5 |
| 2 | 68 | 22 | 10 |
| 3 | 63 | 22 | 15 |
| 4 | 58 | 22 | 20 |
| 5 | 53 | 22 | 25 |
| 6 | 48 | 22 | 30 |
| 7 | 43 | 22 | 35 |
| 8 | 38 | 22 | 40 |
| 9 | 33 | 22 | 45 |
| No. | Heat Treatment | Phase Constitution | Phase Composition, at.% | Phase Fraction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Phase 1/2/3 | Phase 1 | Phase 2 | Phase 3 | Phase1:2:3 | |||||
| Al | Ti | Al | Ti | Al | Ti | ||||
| 1 | 1000 °C 720 h | β/α2 | 16.7 | 75.9 | 23.7 | 74.3 | - | - | 0.53:0.47 |
| 900 °C 1440 h | α2 | 21.6 | 73.7 | - | - | - | - | 1 | |
| 800 °C 2160 h | α2 | 21.6 | 75.8 | - | - | - | - | 1 | |
| 2 | 1000 °C 720 h | β/α2 | 18.4 | 68.3 | 23.3 | 68.3 | - | - | 0.58:0.42 |
| 900 °C 1440 h | β/α2 | 14.3 | 67.9 | 23.4 | 67.9 | - | - | 0.59:0.41 | |
| 800 °C 2160 h | α2/O | 22.5 | 68.9 | 18.0 | 68.9 | - | - | 0.42:0.58 | |
| 3 | 1000 °C 720 h | β/α2 | 20.4 | 61.9 | 24.6 | 65.3 | - | - | 0.60:0.40 |
| 900 °C 1440 h | β/α2 | 15.7 | 61.3 | 23.4 | 64.7 | - | - | 0.62:0.38 | |
| 800 °C 2160 h | α2/O | 22.7 | 64.9 | 15.3 | 61.2 | - | - | 0.43:0.57 | |
| 4 | 1000 °C 720 h | β/α2 | 20.5 | 53.5 | 24.5 | 61.2 | - | - | 0.63:0.37 |
| 900 °C 1440 h | β/α2/O | 15.6 | 56.8 | 23.5 | 62.9 | 23.5 | 60.0 | 0.6:0.03:0.37 | |
| 800 °C 2160 h | β/α2/O | 18.3 | 52.1 | 23.0 | 59.4 | 13.0 | 56.0 | 0.58:0.02:0.4 | |
| 5 | 1000 °C 720 h | β | 22.5 | 53.2 | - | - | - | - | 1 |
| 900 °C 1440 h | β/O | 23.9 | 53.9 | 15.7 | 52.3 | - | - | 0.57:0.43 | |
| 800 °C 2160 h | β/O | 23.9 | 54.5 | 19.4 | 52.8 | - | - | 0.6:0.4 | |
| 6 | 1000 °C 720 h | β/σ | 22.3 | 47.0 | 26.8 | 57.8 | - | - | 0.64:0.36 |
| 900 °C 1440 h | β/O | 23.1 | 50.0 | 15.2 | 47.0 | - | - | 0.55:0.45 | |
| 800 °C 2160 h | β/O | 24.8 | 49.5 | 18.6 | 46.7 | - | - | 0.61:0.39 | |
| 7 | 1000 °C 720 h | β/σ | 21.5 | 44.8 | 27.2 | 56.8 | - | - | 0.67:0.33 |
| 900 °C 1440h | β/O/δ | 23.3 | 52.1 | 14.6 | 47.7 | 19.7 | 33.4 | 0.04:0.4:0.56 | |
| 800 °C 2160 h | β/O | 22.6 | 45.1 | 17.7 | 42.1 | - | - | 0.58:0.42 | |
| 8 | 1000 °C 720 h | β/σ/δ | 19.5 | 46.7 | 25.0 | 59.1 | 20.4 | 31.0 | 0.11:0.37:0.52 |
| 900 °C 1440 h | β/O/δ | 14.7 | 46.9 | 23.6 | 52.8 | 20.1 | 34.5 | 0.02:0.41:0.57 | |
| 800 °C 2160 h | β/O/δ | 23.7 | 35.3 | 24.8 | 42.5 | 21.8 | 29.3 | 0.01:0.41:0.58 | |
| 9 | 1000 °C 720 h | β/σ/δ | 19.3 | 42.4 | 22.6 | 54.3 | 19.6 | 30.3 | 0.52:0.09:0.39 |
| 900 °C 1440 h | O/δ | 22.8 | 50.1 | 20.3 | 32.5 | - | - | 0.41:0.59 | |
| 800 °C 2160 h | O/δ | 25.8 | 43.3 | 19.3 | 33.2 | - | - | 0.40:0.60 | |
| No. | Composition | Crucible Type | Mass (mg) | Rate (°C/min) | Phase Transformation | Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 | Ti74.8Al21.6Nb3.6 | Al2O3 | 13.56 | 20 | α2 ↔ α2 + β | 950(±10) |
| α2 + β ↔ β | 1110(±10) | |||||
| 2 | Ti68.3Al22.2Nb9.5 | Al2O3 | 17.75 | 20 | α2 ↔α2 + β | 821(±10) |
| α2 + β ↔ β | 1112(±10) | |||||
| 3 | Ti63.2Al22Nb14.8 | Al2O3 | 14.45 | 20 | O + α2 ↔α2 + β+ O | 748(±10) |
| α2 + β + O ↔ α2 + β | 882(±10) | |||||
| α2 + β ↔ β | 1060(±10) | |||||
| 4 | Ti56.4Al22Nb21.6 | Al2O3 | 18.73 | 20 | β + O ↔ α2 + β + O | 821(±10) |
| α2 + β + O ↔ α2 + β | 932(±10) | |||||
| α2 + β ↔ β | 976(±10) | |||||
| 5 | Ti53.2Al22.5Nb24.3 | Al2O3 | 26.67 | 20 | β + O ↔ β | 966(±10) |
| 6 | Ti47.8Al22.4Nb29.8 | Al2O3 | 22.23 | 20 | β + O + δ ↔ β + O + σ | 931(±10) |
| β + O + σ ↔ β + σ | 978(±10) | |||||
| β + σ ↔ β | - | |||||
| 7 | Ti45.5Al21.4Nb33.1 | Pt | 14.78 | 20 | β + O ↔ β + O + δ | 878(±3) |
| β + O + δ ↔ β + O + σ | 931(±3) | |||||
| β + O + σ ↔ β + σ | 986(±3) | |||||
| β + σ ↔ β | - | |||||
| 8 | Ti39.7Al21.2Nb39.1 | Pt | 15.67 | 20 | Β +O + δ ↔ δ + β + σ | 931(±3) |
| δ + β + σ ↔ β + σ | 969(±3) | |||||
| β + σ ↔ β | 1151(±3) | |||||
| 9 | Ti33.9Al21.1Nb45 | Al2O3 | 11.96 | 20 | O + δ ↔ β + O + δ | 873(±10) |
| β + O + δ ↔ δ + β + σ | 931(±10) | |||||
| δ + β + σ ↔ β + σ | 1191(±10) | |||||
| β + σ ↔ β | 1273(±10) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, L.-B.; Zhang, L.-G.; Yang, J.-J.; Masset, P.J. The Polythermal Section of Ti-22Al-xNb (30-78at.%Ti) in Ti–Al–Nb System. Metals 2020, 10, 871. https://doi.org/10.3390/met10070871
Zhao Y, Liu L-B, Zhang L-G, Yang J-J, Masset PJ. The Polythermal Section of Ti-22Al-xNb (30-78at.%Ti) in Ti–Al–Nb System. Metals. 2020; 10(7):871. https://doi.org/10.3390/met10070871
Chicago/Turabian StyleZhao, Yun, Li-Bin Liu, Li-Gang Zhang, Jia-Jun Yang, and Patrick J. Masset. 2020. "The Polythermal Section of Ti-22Al-xNb (30-78at.%Ti) in Ti–Al–Nb System" Metals 10, no. 7: 871. https://doi.org/10.3390/met10070871
APA StyleZhao, Y., Liu, L.-B., Zhang, L.-G., Yang, J.-J., & Masset, P. J. (2020). The Polythermal Section of Ti-22Al-xNb (30-78at.%Ti) in Ti–Al–Nb System. Metals, 10(7), 871. https://doi.org/10.3390/met10070871








