Beta Titanium Alloys Produced from Titanium Hydride: Effect of Alloying Elements on Titanium Hydride Decomposition
Abstract
1. Introduction
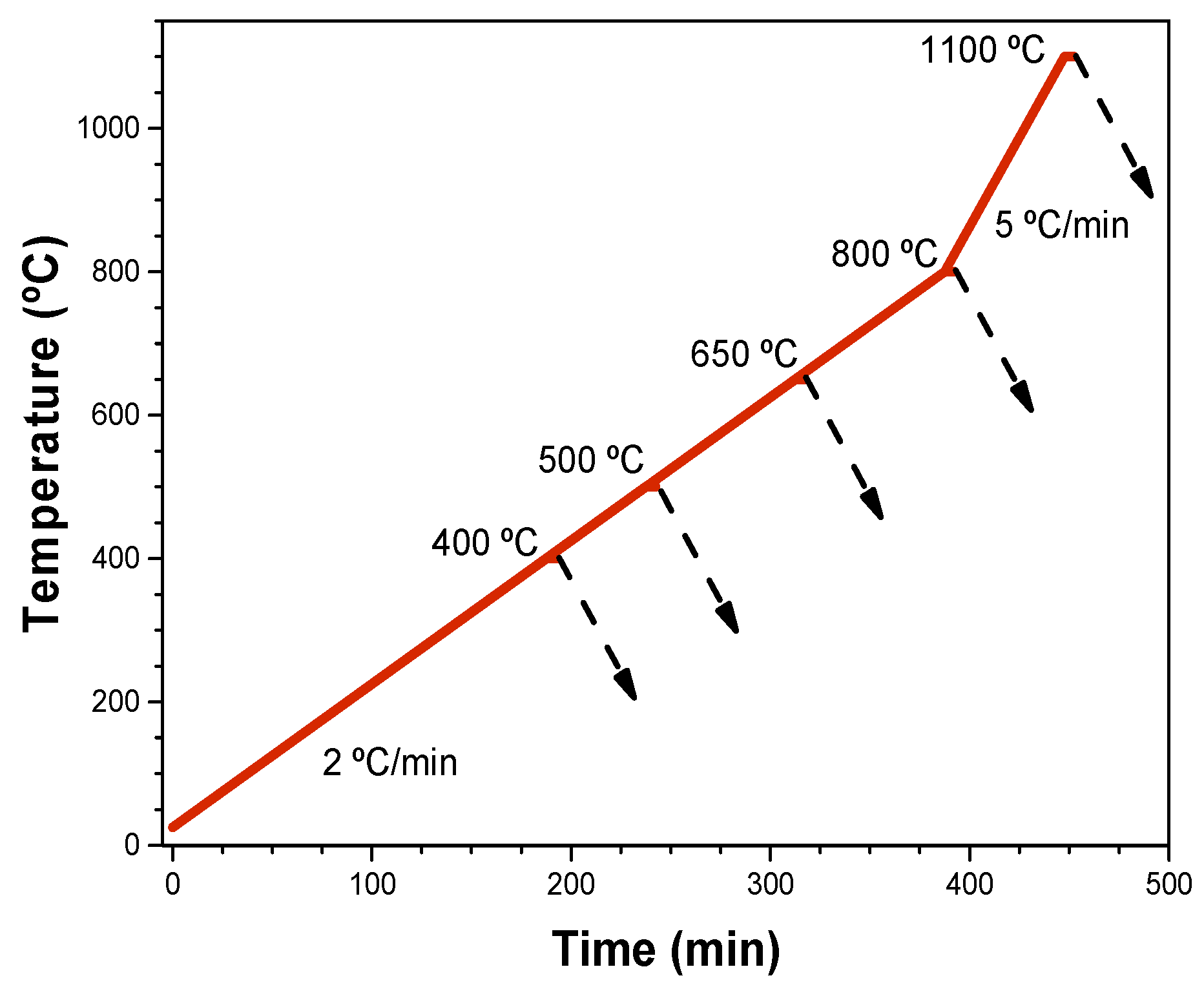
2. Experimental Procedure
3. Results and Discussion
3.1. Effect of Alloying Elements on Thermal Decomposition of Titanium Hydride
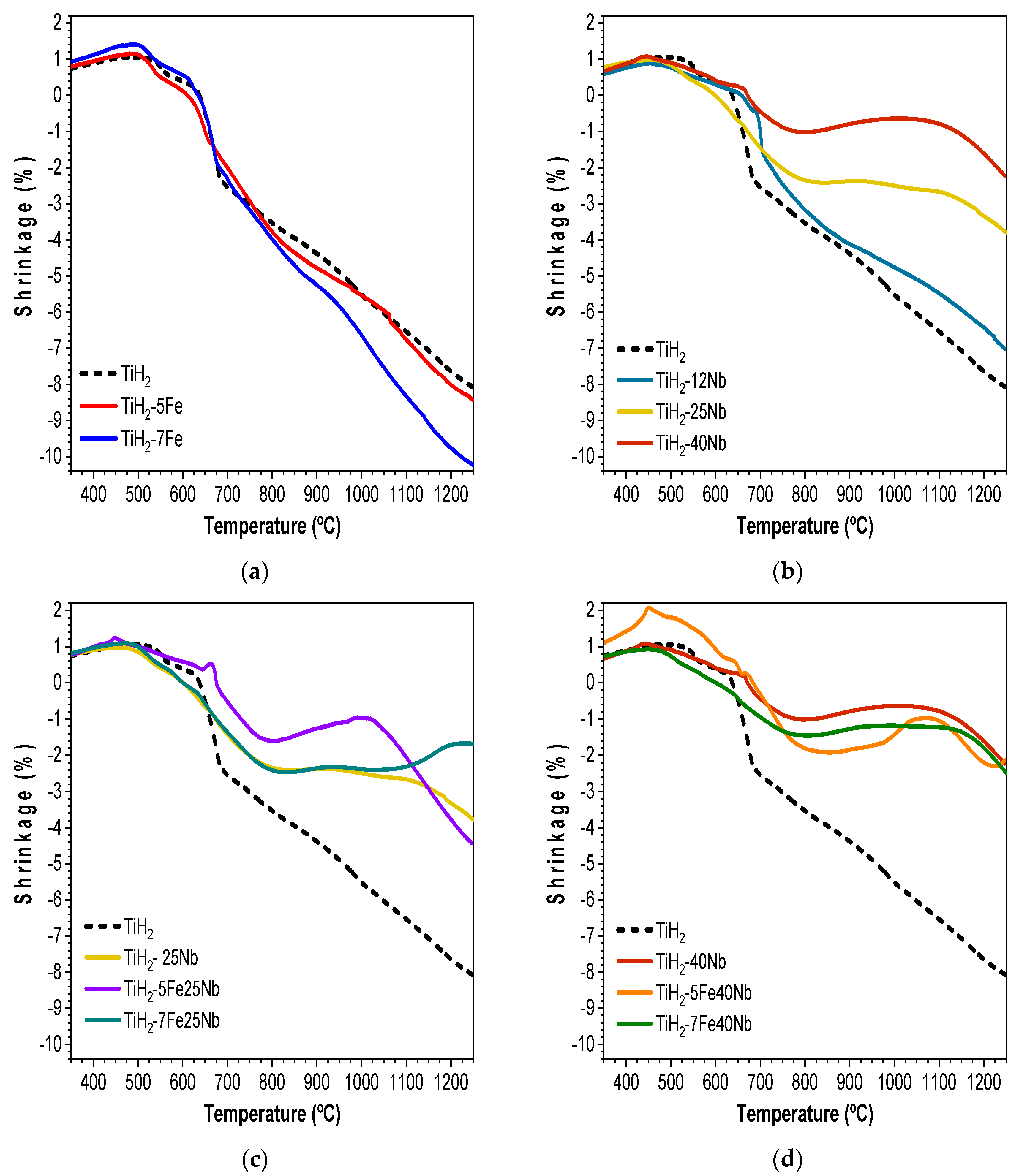
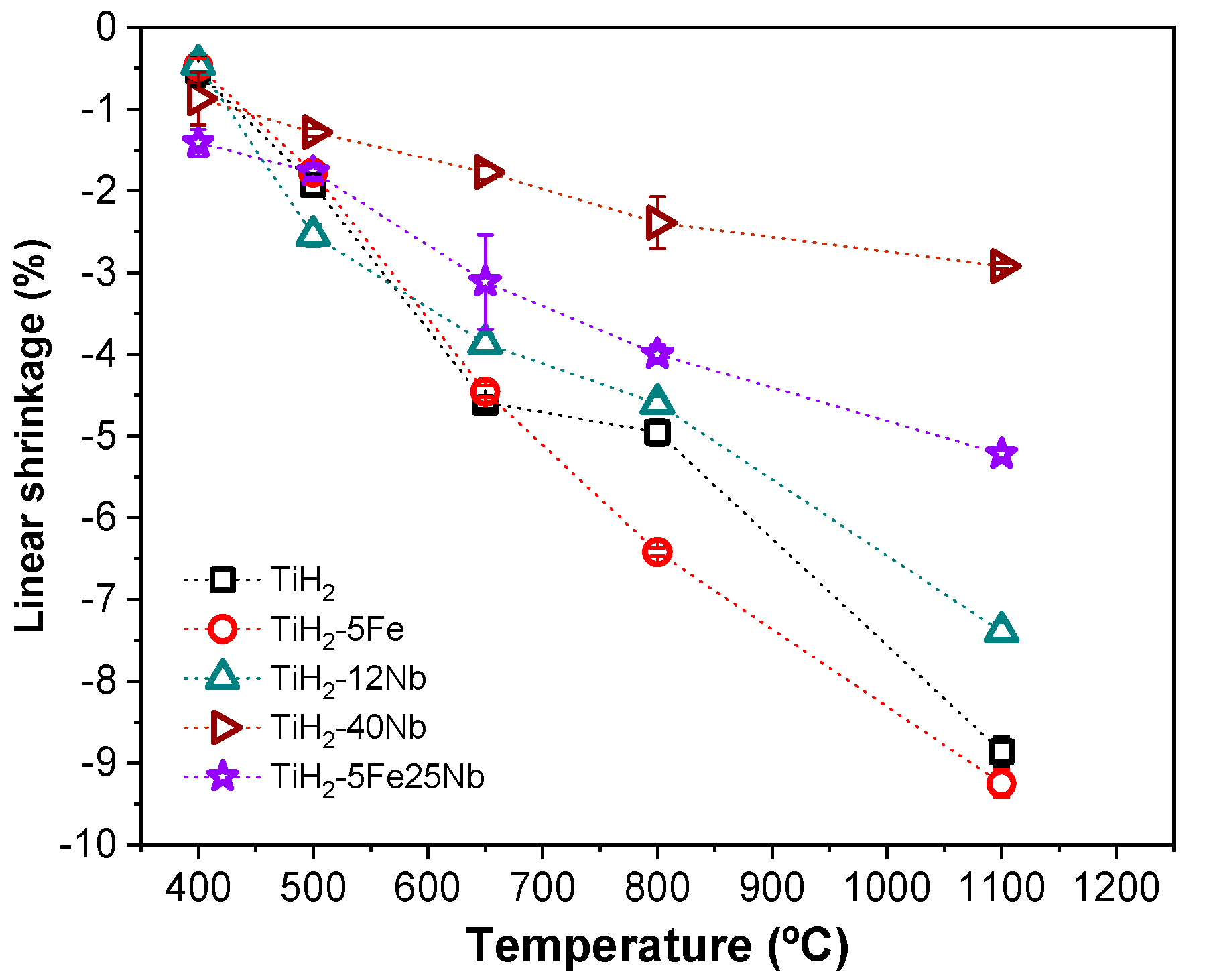
3.2. Shrinkage Evolution During the Dehydrogenation Process
3.3. Comparison of Mass Loss during the Dehydrogenation Process under Different Environments
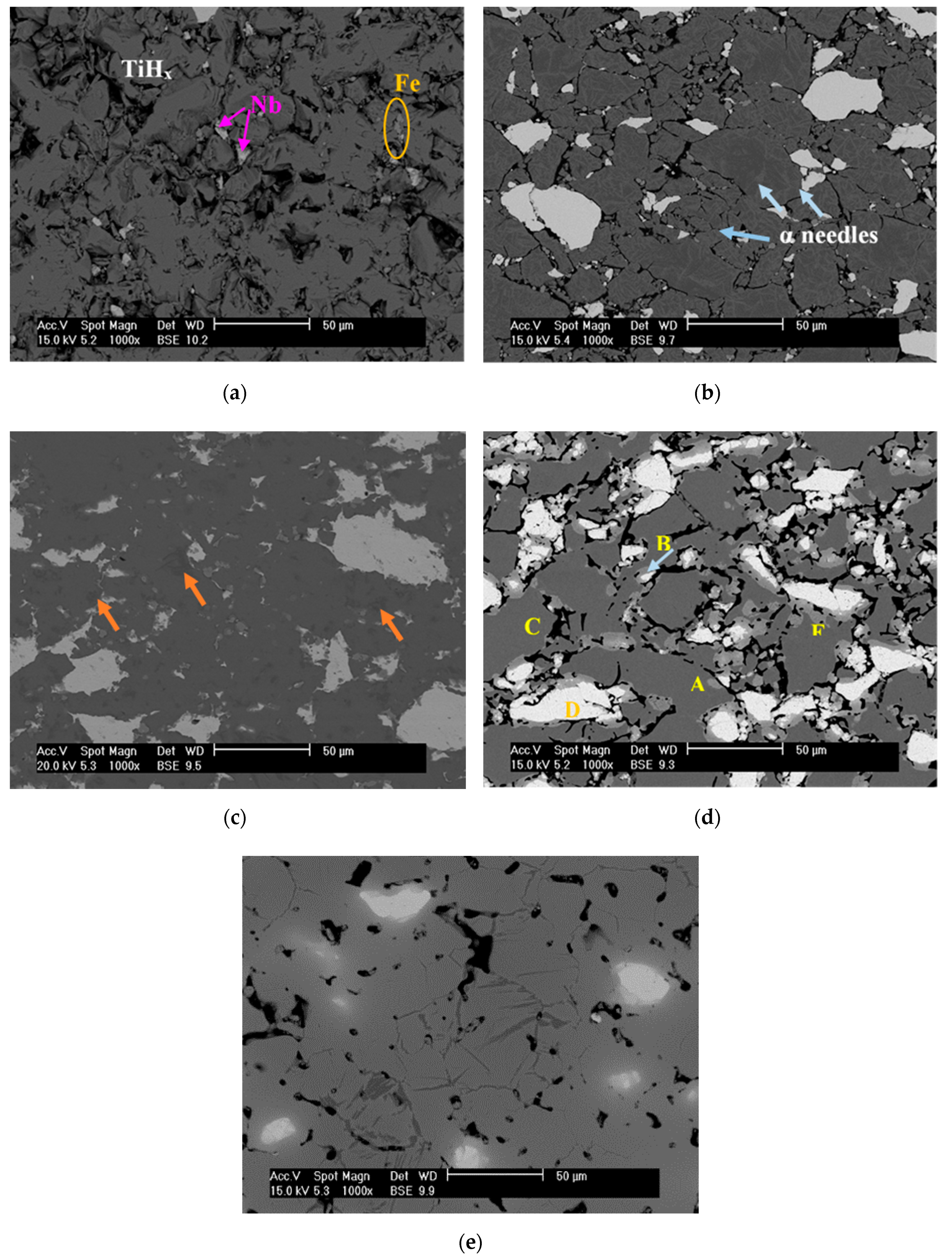
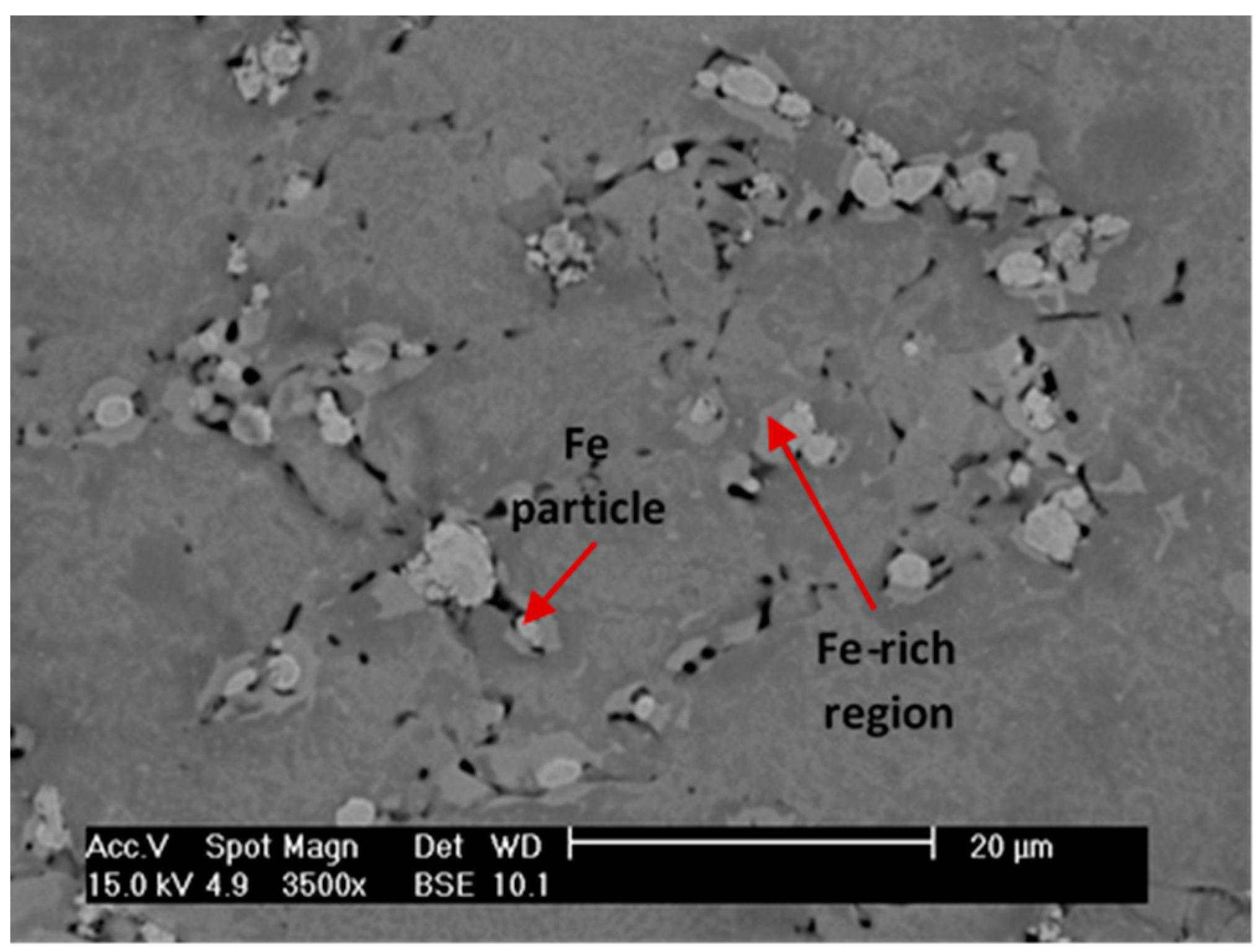
3.4. Microstructure Evolution during the TiH2 Decomposition
3.5. Effect of Fe and Nb Alloying Elements on Phase Evolution during Dehydrogenation Process
4. Conclusions
- Using thermal analysis (DTA, DIL) it is possible to follow the in-situ transformation during the whole dehydrogenation process and assess the effect of alloying elements. In contrast, for samples heated until a determined temperature and then cooled down (VAC), the dehydrogenation reactions were interrupted and some phase transformations could not be identified since they were reverted during cooling. The following reactions were identified for unalloyed TiH2 using conventional XRD:This transformation sequence was modified with the addition of Fe and Nb as alloying elements. The presence of these alloying elements reduced considerably the beta transus temperature, hence, it is suggested that beta→alpha reaction described in Equation (1) did not occur. Once δ-TiHx was transformed into β-Ti, this phase was maintained and became more stable as temperature increased and diffusion of Fe and Nb was produced, which allowed stabilization the β-Ti phase after cooling.The following transformation sequence was proposed for alloyed TiH2 with Fe and Nb during continuous heating:
- Fe was dissolved into Ti matrix at a lower temperature than when using Ti powders as starting material. Hence, dehydrogenation allowed chemical activation of the particle surface, promoting an earlier diffusion of the alloying elements. Small amount of hydrogen retained on the last dehydrogenation stage could have promoted the earlier diffusion of elements that exhibited higher diffusivity in BCC-Ti than HCP-Ti.
- Fe and Nb addition shifted the temperature of the initial and final stage of the dehydrogenation process with respect to unalloyed/pure TiH2.On one hand, Fe and Nb accelerated the beginning of the decomposition reaction, reducing the onset temperature of the first stage between 50–95 °C lower than TiH2. On the other hand, Fe and Nb could act as a barrier to the removal of the remaining hydrogen content retained in the Ti phase, delaying the offset temperature of the third stage between 15–50 °C more than TiH2.No significant differences were detected for the intermediate stage (second stage), which was considered the main decomposition reaction.
- Alloying elements’ addition, such as Fe and Nb, did not hinder the complete hydrogen elimination; however, they modified the decomposition process.
Author Contributions
Funding
Conflicts of Interest
References
- Amherd, A.H.; Frykholm, R.; Ebel, T.; Pyczak, F. Powder Metallurgy Strategies to Improve Properties and Processing of Titanium Alloys: A Review. Adv. Eng. Mater. 2017, 19, 1600743. [Google Scholar] [CrossRef]
- Froes, F.H.S.; Gungor, M.N.; Imam, M.A. Cost-Affordable Titanium: The Component Fabrication Perspective. JOM 2007, 59, 28–31. [Google Scholar] [CrossRef]
- Lutjering, G.; Williams, J. Titanium, 2nd ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Angelo, P.; Upadhyaya, G. Powder Metallurgy: Science, Technology and Applications; PHI Learning Pvt Ltd.: Delhi, India, 2009. [Google Scholar]
- Narasimhan, K.S.; Amuda, M.O.H. Powder Characterization. Ref. Modul. Mater. Sci. Mater. Eng. 2017. [Google Scholar] [CrossRef]
- Sibum, H. Titanium and Titanium Alloys—From Raw Material to Semi-finished Products. In Titanium and Titanium Alloys. Fundamentals and Applications; Leyens, C., Peters, M., Eds.; WILEY-VCH: Weinheim, Germany, 2003; pp. 231–245. [Google Scholar]
- Mccracken, C.G.; Barbis, D.P.; Deeter, R.C. Key characteristics of hydride—Dehydride titanium powder. Powder Metall. 2011, 54, 180–183. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Paramore, J.D.; Sun, P.; Chandran, K.S.R.; Zhang, Y.; Xia, Y.; Cao, F.; Koopman, M.; Free, M. Powder metallurgy of titanium–past, present, and future. Int. Mater. Rev. 2018, 63, 407–459. [Google Scholar] [CrossRef]
- Barbis, D.P.; Gasior, R.M.; Walker, G.P.; Capone, J.A.; Schaeffer, T.S. Titanium powders from the hydride-dehydride process. In Titanium Powder Metallurgy: Science, Technology and Applications; Qian, M., Froes, F., Eds.; Elsevier Inc Butterworth-Heinemann: Oxford, UK, 2015; pp. 101–116. [Google Scholar]
- Wang, C.; Zhang, Y.; Xiao, S.; Chen, Y. Sintering densification of titanium hydride powders. Mater. Manuf. Process. 2017, 32, 517–522. [Google Scholar] [CrossRef]
- Mei, L.; Wang, C.; Wei, Y.; Xiao, S.; Chen, Y. Effects of hydrogen content on powder metallurgy characteristic of titanium hydrides. Int. J. Hydrogen Energy 2018, 43, 7102–7107. [Google Scholar] [CrossRef]
- Robertson, I.M.; Schaffer, G.B. Comparison of sintering of titanium and titanium hydride powders. Powder Metall. 2010, 53, 12–19. [Google Scholar] [CrossRef]
- Ivasishin, O.; Moxson, V. Low-cost titanium hydride powder metallurgy. In Titanium Powder Metallurgy; Elsevier Inc.: Oxford, UK, 2015; pp. 117–148. [Google Scholar]
- Savvakin, D.H.; Humenyak, M.M.; Matviichuk, M.V.; Molyar, O.H. Role of hydrogen in the process of sintering of titanium powders. Mater. Sci. 2012, 47, 72–81. [Google Scholar] [CrossRef]
- Nyberg, E.; Miller, M.; Simmons, K.; Weil, S. Manufactures ‘need better quality titanium PM powders’. Met. Powder Rep. 2005, 60, 8–13. [Google Scholar] [CrossRef]
- Paramore, J.D.; Fang, Z.; Sun, P. Hydrogen sintering of Titanium and its alloys. In Titanium Powder Metallurgy; Elsevier Inc.: Oxford, UK, 2015; pp. 163–182. [Google Scholar]
- Kovalev, D.Y.; Prokudina, V.K.; Ratnikov, V.I.; Ponomarev, V.I. Thermal decomposition of TiH2: A TRXRD study. Int. J. Self-Propagating High-Temp. Synth. 2010, 19, 253–257. [Google Scholar] [CrossRef]
- Lehmhus, D.; Rausch, G. Tailoring titanium hydride decomposition kinetics by annealing in various atmospheres. Adv. Eng. Mater. 2004, 6, 313–330. [Google Scholar] [CrossRef]
- Peillon, N.; Fruhauf, J.B.; Gourdet, S.; Feraille, J.; Saunier, S.; Desrayaud, C. Effect of TiH2 in the preparation of MMC Ti based with TiC reinforcement. J. Alloys Compd. 2015, 619, 157–164. [Google Scholar] [CrossRef]
- Bhosle, V.; Baburaj, E.G.; Miranova, M.; Salama, K. Dehydrogenation of TiH2. Mater. Sci. Eng. A 2003, 356, 190–199. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Feng, J.C.; Cao, J. Kinetic study on nonisothermal dehydrogenation of TiH2 powders. Int. J. Hydrogen Energy 2009, 34, 3018–3025. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Zheng, L.R.; Chu, S.Q.; Wu, M.; An, P.F.; Zhang, J.; Hu, T.D. In-situ EXAFS study on the thermal decomposition of TiH2. Chin. Phys. C 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Jiménez, C.; Garcia-Moreno, F.; Pfretzschner, B.; Klaus, M.; Wollgarten, M.; Zizak, I.; Schumacher, G.; Tovar, M.; Banhart, J. Decomposition of TiH2 studied in situ by synchrotron X-ray and neutron diffraction. Acta Mater. 2011, 59, 6318–6330. [Google Scholar] [CrossRef]
- Ma, M.; Liang, L.; Wang, L.; Wang, Y.; Cheng, Y.; Tang, B.; Xiang, W.; Tan, X. Phase transformations of titanium hydride in thermal desorption process with different heating rates. Int. J. Hydrogen Energy 2015, 40, 8926–8934. [Google Scholar] [CrossRef]
- Prashanth, K.G. Influence of Mechanical Activation on Decomposition of Titanium Hydride. Mater. Manuf. Process. 2010, 25, 974–977. [Google Scholar] [CrossRef]
- Matijasevic-lux, B.; Banhart, J.; Fiechter, S.; Gorke, O.; Wanderka, N. Modification of titanium hydride for improved aluminium foam manufacture. Acta Mater. 2006, 54, 1887–1900. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung KW, K.; Hu, T.; Xu, Z.; Chung, J.C.; Chu, P.K. Hydrogen release from titanium hydride in foaming of orthopedic NiTi scaffolds. Acta Biomater. 2011, 7, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hur, B. The relationship between thermal decomposition properties of titanium hydride and the Al alloy melt foaming process. Mater. Lett. 2006, 60, 3635–3641. [Google Scholar] [CrossRef]
- Sharma, B.; Vajpai, S.K.; Ameyama, K. Microstructure and properties of beta Ti–Nb alloy prepared by powder metallurgy route using titanium hydride powder. J. Alloys Compd. 2016, 656, 978–986. [Google Scholar] [CrossRef]
- Chirico, C.; Tsipas, S.; Toptan, F.; Gordo, E. Development of Ti–Nb and Ti–Nb–Fe beta alloys from TiH2 powders. Powder Metall. 2019, 62, 44–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhang, Y.; Cheng, P.; Wei, Y.; Xiao, S.; Chen, Y. Fabrication of Low-cost Ti-1Al-8V-5Fe by Powder Metallurgy with TiH2 and FeV80 Alloy. Mater. Manuf. Process. 2017, 32, 1869–1873. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Lin, J.; Li, Y.; Ping, D.; Yamabe-Mitarai, Y.; Wen, C. Investigations into Ti–(Nb,Ta)–Fe alloys for biomedical applications. Acta Biomater. 2016, 32, 336–347. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, D.; Cheng, J.; Kit, J.; Tsoi, H.; Chen, J. Mechanical and biological properties of Ti–(0–25 wt%) Nb alloys for biomedical implants application. Regen. Biomater. 2020, 7, 119–127. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Y.; Zhou, K.; Huang, B. Effect of Fe addition on sintering behaviour of titanium powder. Powder Metall. 2003, 46, 246–250. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Lopez, V.H. The decomposition behavior of as-received and oxidized TiH2 foaming-agent powder. Mater. Sci. Eng. A 2003, 357, 258–263. [Google Scholar] [CrossRef]
- Esteban, P.G.; Bolzoni, L.; Ruiz-Navas, E.M.; Gordo, E. PM processing and characterisation of Ti-7Fe low cost titanium alloys. Powder Metall. 2011, 54, 242–252. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.F.; Tang, H.P.; Liu, C.T.; Liu, B.; Huang, B.Y. Design of powder metallurgy titanium alloys and composites. Mater. Sci. Eng. A 2006, 418, 25–35. [Google Scholar] [CrossRef]
- Gibbs, G.B.; Graham, D.; Tomlin, D.H. Diffusion in titanium and titanium—Niobium alloys. Philos. Mag. 1963, 8, 1269–1282. [Google Scholar] [CrossRef]
- Soma, P.; Paul, A. Interdiffusion in Nb-Mo, Nb-Ti and Nb-Zr systems. Defect Diffus. Forum 2012, 323–325, 491–496. [Google Scholar]
- Zhu, L.; Zhang, Q.; Chen, Z.; Wei, C.; Cai, G.M.; Jiang, L.; Jin, Z.; Zhao, J.C. Measurement of interdiffusion and impurity diffusion coefficients in the bcc phase of the Ti–X (X = Cr, Hf, Mo, Nb, V, Zr) binary systems using diffusion multiples. J. Mater. Sci. 2017, 52, 3255–3268. [Google Scholar] [CrossRef]
- Nakajima, H.; Koiwa, M. Diffusion in Titanium. ISIJ Int. 1991, 31, 757–766. [Google Scholar] [CrossRef]
- Froes, F.H.; Senkov, O.N.; Qazi, J.I. Hydrogen as a temporary alloying element in titanium alloys: Thermohydrogen processing. Int. Mater. Rev. 2004, 49, 227–245. [Google Scholar] [CrossRef]
- O’Flynn, J.; Corbin, S.F. The influence of iron powder size on pore formation, densification and homogenization during blended elemental sintering of Ti–2.5Fe. J. Alloys Compd. 2015, 618, 437–448. [Google Scholar] [CrossRef]
- Ivasishin, O.M.; Demidik, A.N.; Savvakin, D.G. Use of titanium hydride for the synthesis of titanium aluminides from powder materials. Powder Metall. Met. Ceram. 1999, 38, 482–487. [Google Scholar] [CrossRef]
- Pieraggi, B. Diffusion and solid-state reactions. In Developments in High Temperature Corrosion and Protection of Materials; Woodhead, P., Gao, W., L, Z.W., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 9–35. [Google Scholar]
- Peartt, R.F.; Tomlin, D.H. Diffusion of elements in beta-titanium. Acta Metall. 1962, 10, 123–134. [Google Scholar] [CrossRef]
- Lee, C.M.; Ju, C.P.; Lin, J.H.C. Structure property relationship of cast Ti-Nb alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef]
- Cremasco, A.; Andrade, P.N.; Contieri, R.J.; Lopes, E.S.N.; Afonso, C.R.M.; Caram, R. Correlations between aging heat treatment, ω phase precipitation and mechanical properties of a cast Ti-Nb alloy. Mater. Des. 2011, 32, 2387–2390. [Google Scholar] [CrossRef]
- Bönisch, M.; Calin, M.; Waitz, T.; Panigrahi, A.; Zehetbauer, M.; Gebert, A.; Skrotzki, W.; Eckert, J. Thermal stability and phase transformations of martensitic Ti-Nb alloys. Sci. Technol. Adv. Mater. 2013, 14, 055004. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Xiao, S.L.; Tian, J.; Chen, Y.Y.; Huang, Y.D. Microstructure and dry wear properties of Ti-Nb alloys for dental prostheses. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2009, 19, s639–s644. [Google Scholar] [CrossRef]
- Ebel, T.; Beißig, T.; Ebner, S.; Luo, X.; Nagaram, A.B. Reduction of the embrittlement effect of binder contamination in MIM processing of Ti alloys. Powder Metall. 2017, 60, 157–166. [Google Scholar] [CrossRef]
- Amigó, A.; Vicente, A.; Afonso, C.R.M.; Amigó, V. Mechanical properties and the microstructure of β Ti-35Nb-10Ta-xFe alloys obtained by powder metallurgy for biomedical applications. Metals 2019, 9, 76. [Google Scholar] [CrossRef]
- Zhao, D.; Chang, K.; Ebel, T.; Qian, M.; Willumeit, R.; Yan, M.; Pyczak, F. Microstructure and mechanical behavior of metal injection molded Ti-Nb binary alloys as biomedical material. J. Mech. Behav. Biomed. Mater. 2013, 28, 171–182. [Google Scholar] [CrossRef]
- Hosnie, S.M.; Yahaya, M.; Haris, N.A.; Todd, I. Fabrication of porous β-Type Ti-40Nb alloys incorporated with TiH2 via powder metallurgy processing route under reducing environment. J. Mech. Eng. 2017, 2, 99–112. [Google Scholar]
- Schur, D.V.; Zaginaichenko, S.Y.; Adejev, V.M.; Voitovich, V.B.; Lyashenko, A.A.; Trefilov, V.I. Phase transformations in titanium hydrides. Int. J. Hydrogen Energy 1996, 21, 1121–1124. [Google Scholar] [CrossRef]



| Composition 1 | DTA/TGA/DIL * | VAC |
|---|---|---|
| TiH2 | X | X |
| TiH2-5Fe | X | X |
| TiH2-7Fe | X | - |
| TiH2-12Nb 1 | X | X |
| TiH2-25Nb | X | - |
| TiH2-5Fe-25Nb | X | X |
| TiH2-7Fe-25Nb | X | - |
| TiH2-40Nb | X | X |
| TiH2-5Fe-40Nb | X | - |
| TiH2-7Fe-40Nb | X | - |
| Sample | Temperature (°C) | |||||
|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | ||||
| Onset | Peak | Onset | Peak | Onset | Offset | |
| TiH2 | 475 | 510 | 555 | 592 | 650 | 830 |
| TiH2-5Fe | 395 | 500 | 555 | 623 | 700 | 880 |
| TiH2-Fe | 385 | 500 | 555 | 620 | 700 | 875 |
| TiH2-12Nb | 380 | 470 | 535 | 600 | 645 | 850 |
| TiH2-25Nb | 385 | 490 | 545 | 613 | 645 | 870 |
| TiH2-40Nb | 390 | 480 | 540 | 596 | 630 | 830 |
| TiH2-5Fe25Nb | 410 | 485 | 535 | 585 | 645 | 815 |
| TiH2-7Fe25Nb | 405 | 495 | 542 | 695 | 630 | 840 |
| TiH2-5Fe40Nb | 420 | 487 | 535 | 608 | 635 | 855 |
| TiH2-Fe40Nb | 425 | 490 | 545 | 600 | 630 | 845 |
| Samples | 400 °C | 500 °C | 650 °C | 800 °C | 1100 °C | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TGA | VAC | TGA | VAC | TGA | VAC | TGA | VAC | TGA | VAC | |
| TiH2 | 2.3 | 16.7 | 1.7 | 76.8 | 66.3 | 98.9 | 89.1 | 99.8 | 94.8 | 99.1 |
| TiH2-5Fe | <1 | 14.9 | 9.9 | 74.7 | 57.1 | 90.6 | 87.8 | 97.4 | 97.7 | 98.1 |
| TiH2-12Nb | 5.9 | 18.4 | 9.5 | 84.0 | 58.7 | 97.3 | 83.8 | 97.7 | 89.9 | 98.1 |
| TiH2-40Nb | <1 | 24.7 | 15.0 | 92.8 | 70.9 | 96.0 | 93.9 | 99.7 | 98.4 | 99.7 |
| TiH2-5Fe25Nb | 8.5 | 14.0 | 7.0 | 84.6 | 62.9 | 97.9 | 86.7 | 98.3 | 90.6 | 98.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirico, C.; Tsipas, S.A.; Wilczynski, P.; Gordo, E. Beta Titanium Alloys Produced from Titanium Hydride: Effect of Alloying Elements on Titanium Hydride Decomposition. Metals 2020, 10, 682. https://doi.org/10.3390/met10050682
Chirico C, Tsipas SA, Wilczynski P, Gordo E. Beta Titanium Alloys Produced from Titanium Hydride: Effect of Alloying Elements on Titanium Hydride Decomposition. Metals. 2020; 10(5):682. https://doi.org/10.3390/met10050682
Chicago/Turabian StyleChirico, Caterina, Sophia Alexandra Tsipas, Pablo Wilczynski, and Elena Gordo. 2020. "Beta Titanium Alloys Produced from Titanium Hydride: Effect of Alloying Elements on Titanium Hydride Decomposition" Metals 10, no. 5: 682. https://doi.org/10.3390/met10050682
APA StyleChirico, C., Tsipas, S. A., Wilczynski, P., & Gordo, E. (2020). Beta Titanium Alloys Produced from Titanium Hydride: Effect of Alloying Elements on Titanium Hydride Decomposition. Metals, 10(5), 682. https://doi.org/10.3390/met10050682







