Optimization of Cu and Mn Dissolution from Black Coppers by Means of an Agglomerate and Curing Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Black Copper Sample
2.2. Ferrous Ions
2.3. Pretreatment and Subsequent Leaching in Reactors
2.4. Design of Experiments
3. Results and Discussions
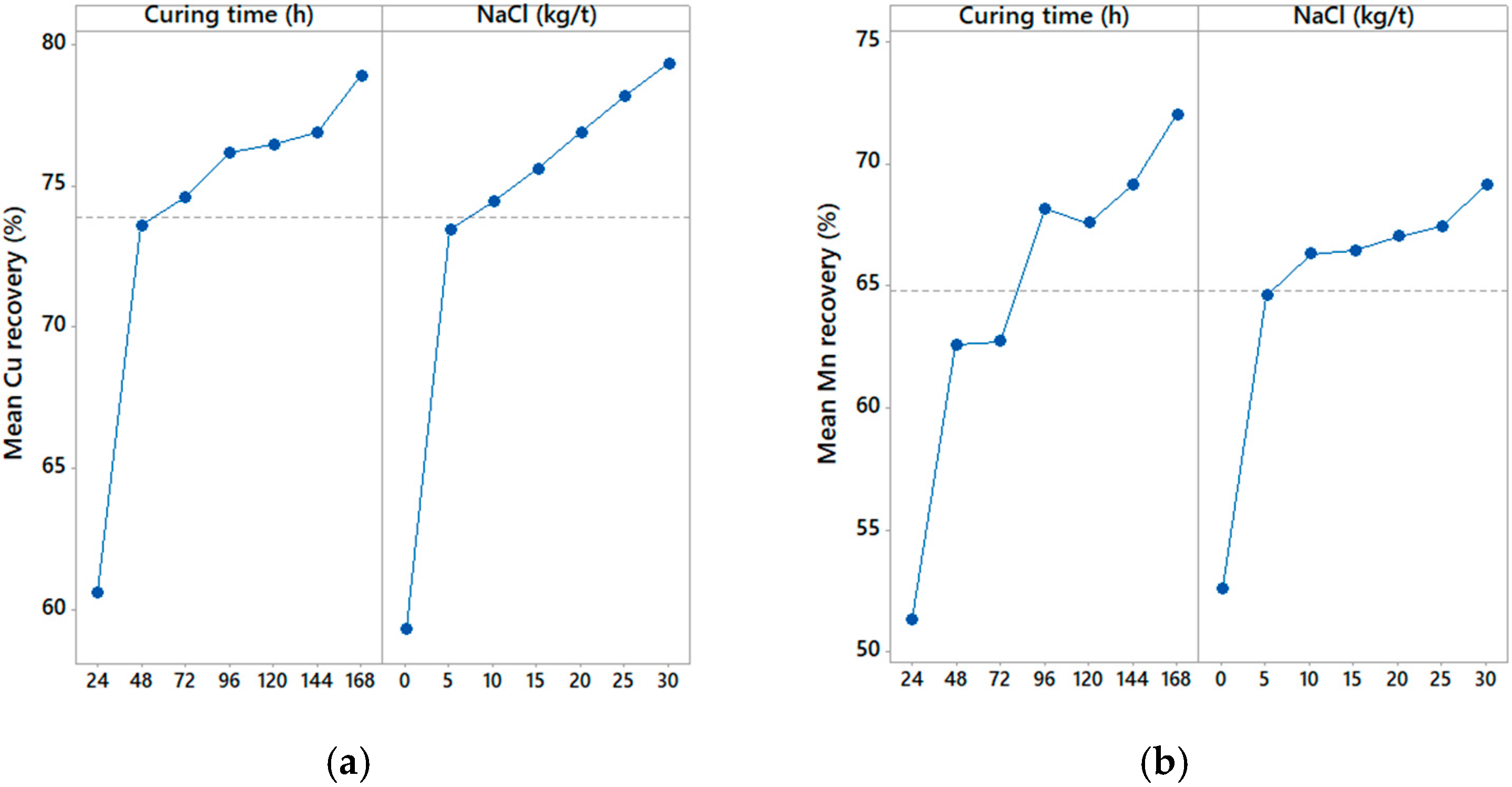
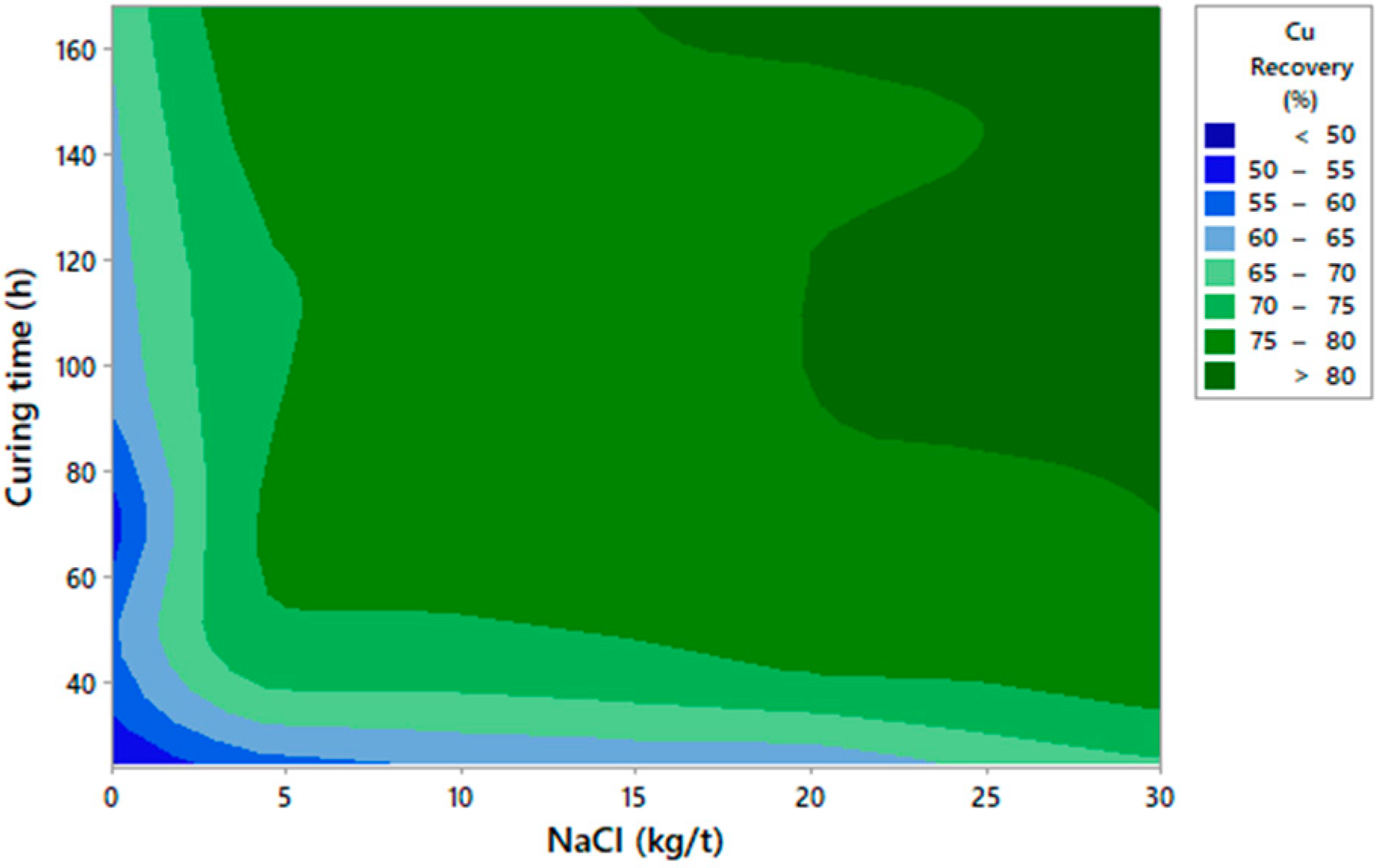
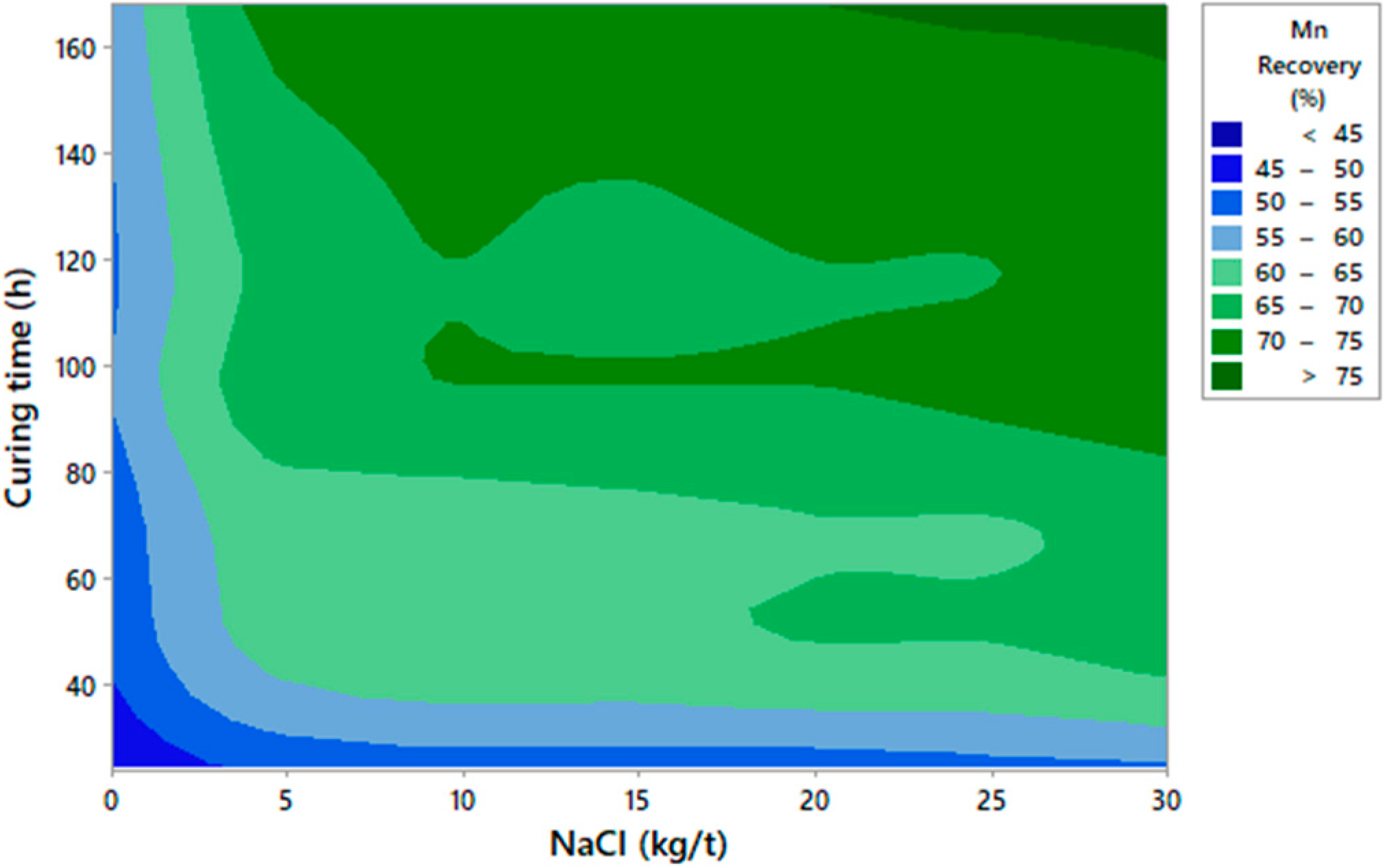
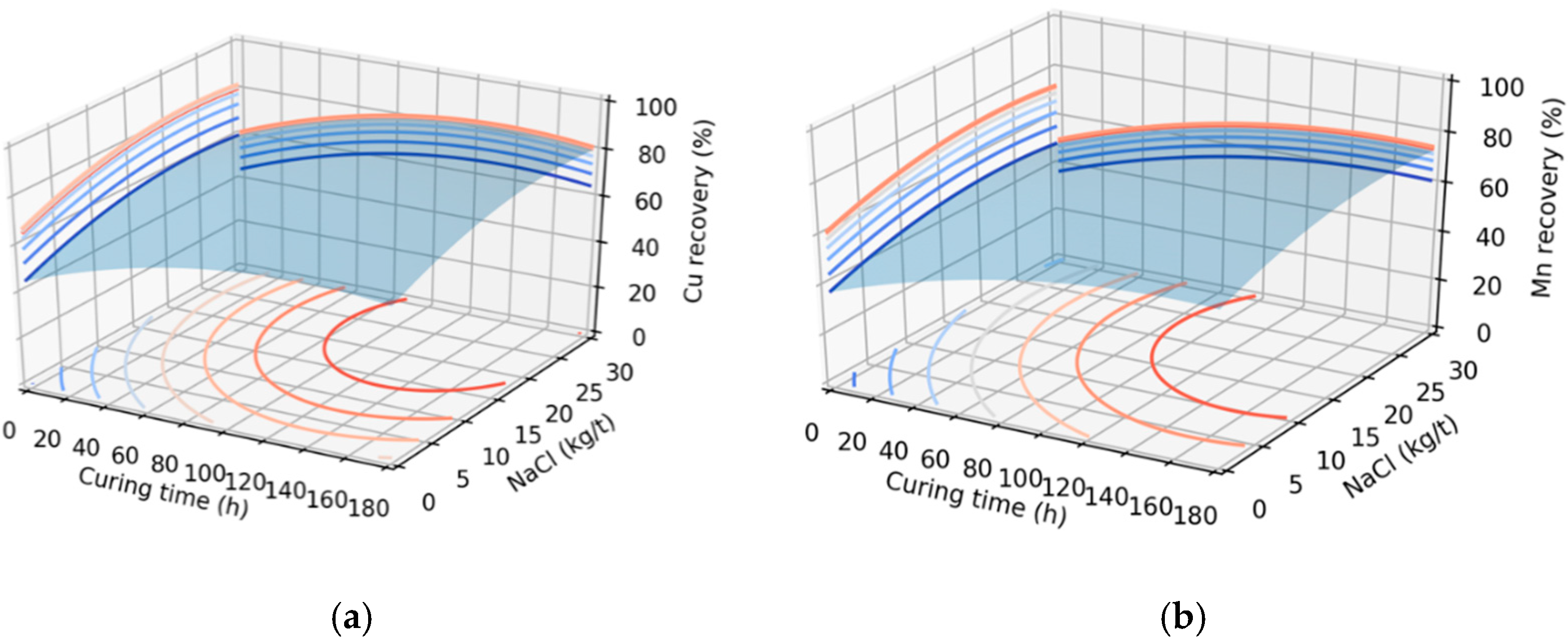
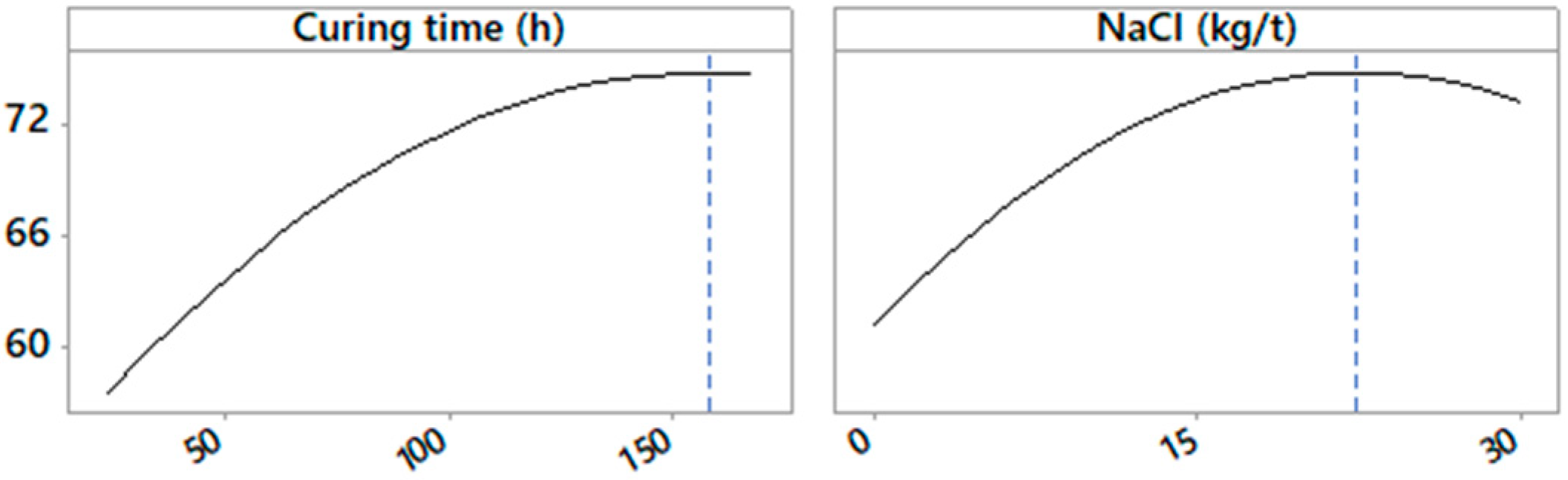
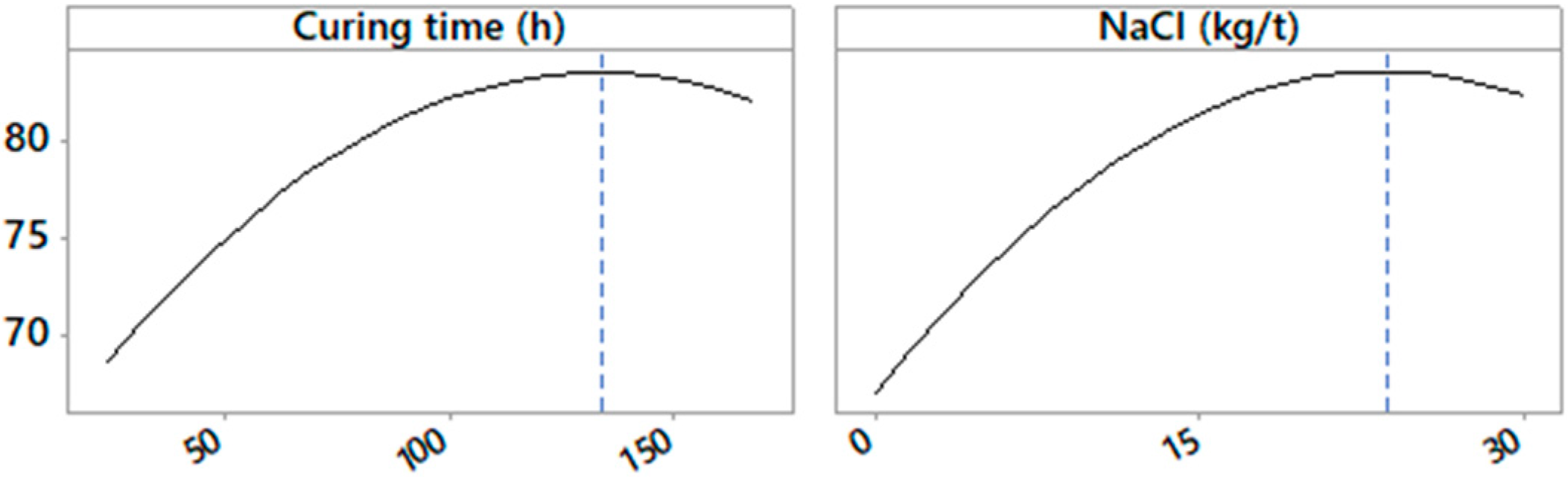
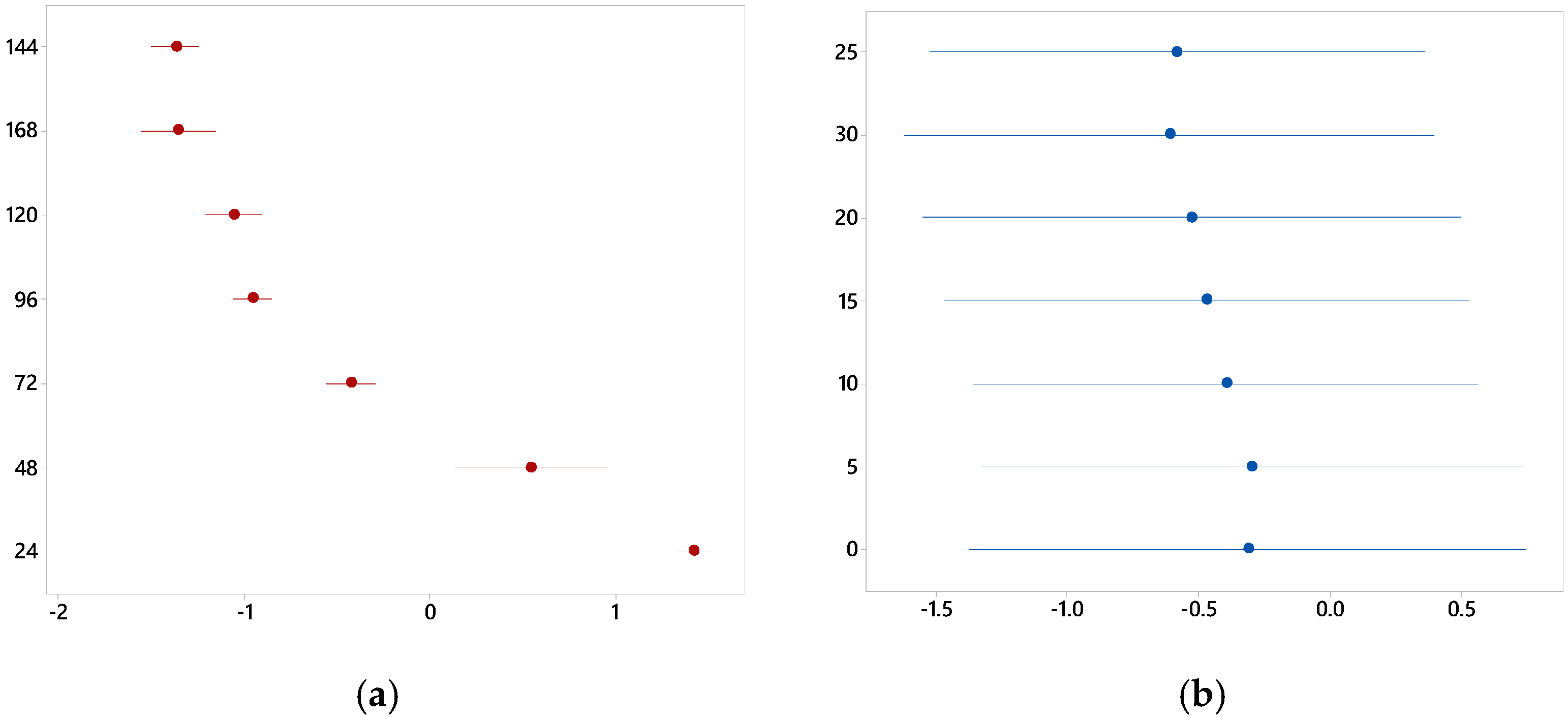
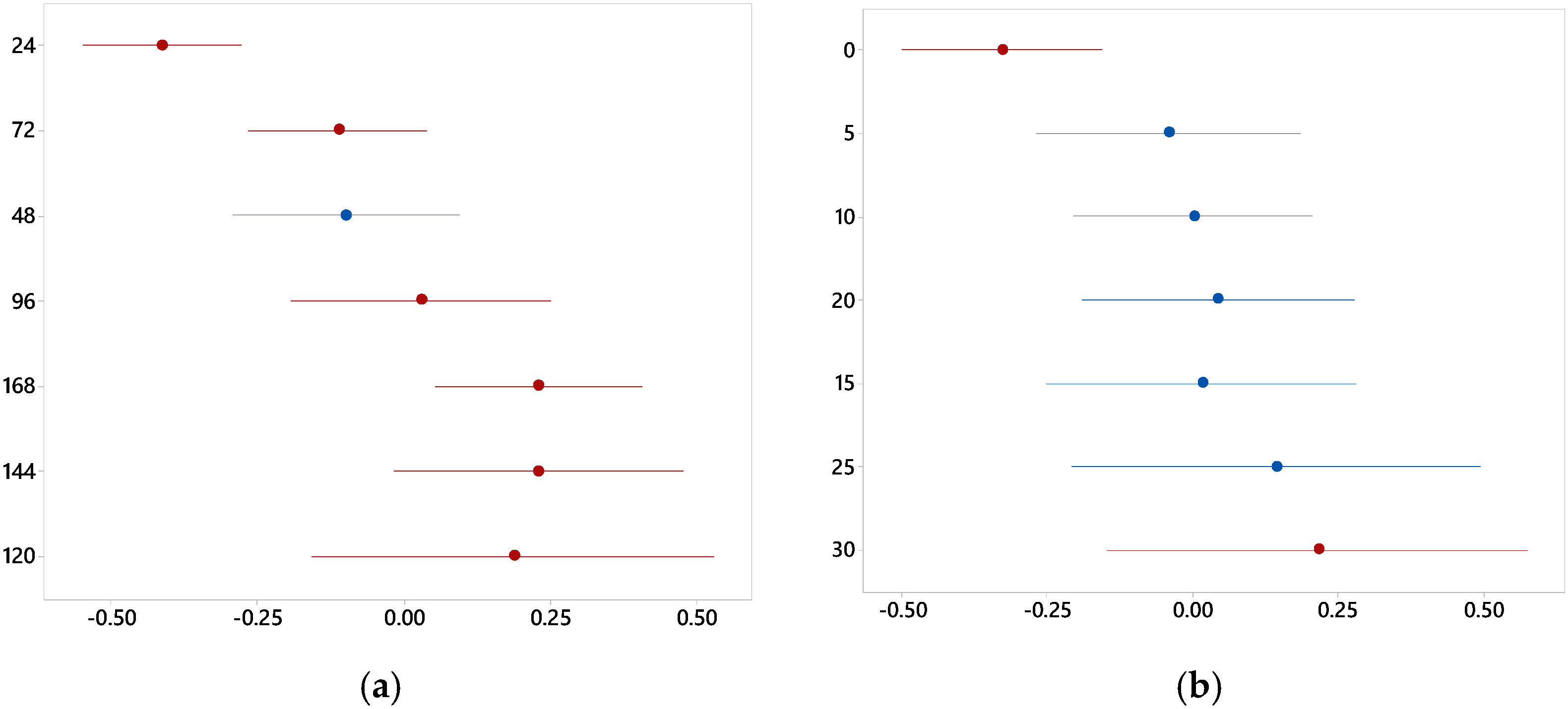
3.1. Graphic Analysis and Analytical Models
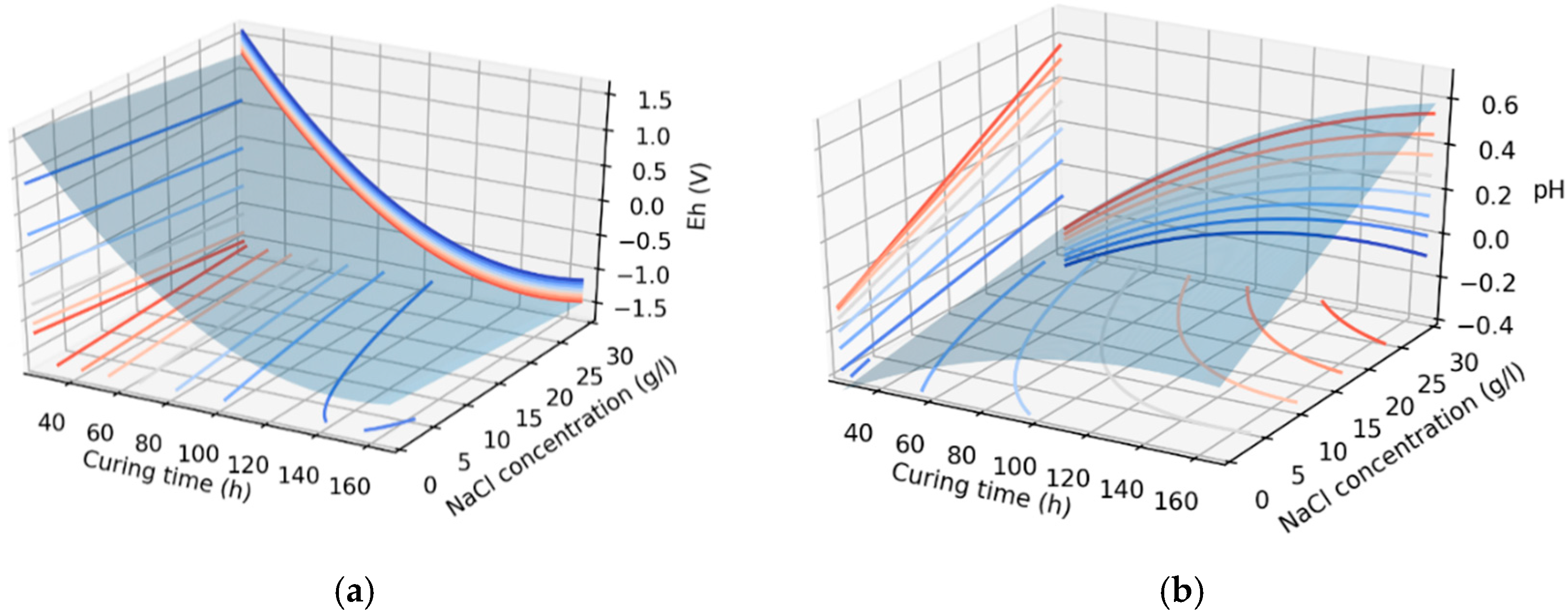
3.2. Effects of Redox Potential and pH on the System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Curing Time (h) | NaCl (kg/t) | Mn Recovery (%) | Cu Recovery (%) | Eh (V) | pH |
|---|---|---|---|---|---|---|
| 1 | 24 | 0 | 45 | 49 | 1.5 | −0.4 |
| 2 | 24 | 5 | 51 | 58 | 1.5 | −0.3 |
| 3 | 24 | 10 | 52 | 60 | 1.4 | −0.4 |
| 4 | 24 | 15 | 52 | 61 | 1.4 | −0.4 |
| 5 | 24 | 20 | 52 | 61 | 1.4 | −0.4 |
| 6 | 24 | 25 | 53 | 66 | 1.3 | −0.5 |
| 7 | 24 | 30 | 54 | 69 | 1.4 | −0.5 |
| 8 | 48 | 0 | 51 | 59 | 1 | −0.4 |
| 9 | 48 | 5 | 62 | 74 | 1 | −0.3 |
| 10 | 48 | 10 | 64 | 74 | 0.5 | −0.1 |
| 11 | 48 | 15 | 64 | 75 | 0.5 | −0.2 |
| 12 | 48 | 20 | 65 | 77 | 0.5 | 0.1 |
| 13 | 48 | 25 | 65 | 77 | 0.1 | 0.1 |
| 14 | 48 | 30 | 67 | 79 | 0.2 | 0.1 |
| 15 | 72 | 0 | 52 | 53 | −0.3 | −0.3 |
| 16 | 72 | 5 | 63 | 76 | −0.4 | −0.1 |
| 17 | 72 | 10 | 63 | 77 | −0.3 | −0.1 |
| 18 | 72 | 15 | 64 | 78 | −0.3 | −0.2 |
| 19 | 72 | 20 | 65 | 79 | −0.6 | −0.2 |
| 20 | 72 | 25 | 65 | 79 | −0.5 | −0.1 |
| 21 | 72 | 30 | 67 | 80 | −0.6 | 0.2 |
| 22 | 96 | 0 | 55 | 61 | −1.1 | −0.4 |
| 23 | 96 | 5 | 68 | 75 | −1 | −0.2 |
| 24 | 96 | 10 | 70 | 77 | −0.8 | 0.1 |
| 25 | 96 | 15 | 70 | 78 | −1 | 0.2 |
| 26 | 96 | 20 | 70 | 80 | −0.9 | 0.1 |
| 27 | 96 | 25 | 71 | 81 | −0.9 | 0.2 |
| 28 | 96 | 30 | 73 | 81 | −1 | 0.2 |
| 29 | 120 | 0 | 54 | 63 | −0.8 | −0.4 |
| 30 | 120 | 5 | 67 | 75 | −0.9 | 0.1 |
| 31 | 120 | 10 | 70 | 77 | −1 | 0.1 |
| 32 | 120 | 15 | 69 | 78 | −1.2 | 0.1 |
| 33 | 120 | 20 | 70 | 80 | −1.1 | 0.3 |
| 34 | 120 | 25 | 70 | 81 | −1.2 | 0.5 |
| 35 | 120 | 30 | 73 | 81 | −1.2 | 0.6 |
| 36 | 144 | 0 | 55 | 64 | −1.3 | −0.3 |
| 37 | 144 | 5 | 69 | 77 | −1.1 | 0.3 |
| 38 | 144 | 10 | 71 | 77 | −1.5 | 0.2 |
| 39 | 144 | 15 | 71 | 79 | −1.4 | 0.4 |
| 40 | 144 | 20 | 72 | 79 | −1.4 | 0.2 |
| 41 | 144 | 25 | 72 | 80 | −1.4 | 0.3 |
| 42 | 144 | 30 | 74 | 82 | −1.5 | 0.5 |
| 43 | 168 | 0 | 56 | 66 | −1.2 | −0.1 |
| 44 | 168 | 5 | 72 | 79 | −1.2 | 0.2 |
| 45 | 168 | 10 | 74 | 79 | −1.1 | 0.2 |
| 46 | 168 | 15 | 75 | 80 | −1.3 | 0.2 |
| 47 | 168 | 20 | 75 | 82 | −1.6 | 0.2 |
| 48 | 168 | 25 | 76 | 83 | −1.5 | 0.5 |
| 49 | 168 | 30 | 76 | 83 | −1.6 | 0.4 |
References
- Cantallopts, J. Importancia y Oportunidades de la Minería en Chile en el Escenario Global; Comisión Chilena del Cobre: Santiago, Chile, 2016. [Google Scholar]
- COCHILCO. Proyección de la Producción de Cobre en Chile; Comisión Chilena del Cobre: Santiago, Chile, 2016. [Google Scholar]
- SERNAGEOMIN. Anuario de la Minería de Chile 2018; Servicio Nacional de Geología y Minería: Santiago, Chile, 2019. [Google Scholar]
- Toro, N.; Pérez, K.; Saldaña, M.; Jeldres, R.I.; Jeldres, M.; Cánovas, M. Dissolution of pure chalcopyrite with manganese nodules and waste water. J. Mater. Res. Technol. 2020, 9, 798–805. [Google Scholar] [CrossRef]
- Hernández, P.C. Estudio del Equilibrio Sólido-líquido de Sistemas Acuosos de Minerales de Cobre con agua de mar, Aplicado a Procesos de Lixiviación. Ph.D. Thesis, Facultad de Ingeniería, Universidad de Antofagasta, Antofagasta, Chile, 2013. [Google Scholar]
- Toro, N.; Saldaña, M.; Gálvez, E.; Cánovas, M.; Trigueros, E.; Castillo, J.; Hernández, P.C. Optimization of Parameters for the Dissolution of Mn from Manganese Nodules with the Use of Tailings in An Acid Medium. Minerals 2019, 9, 387. [Google Scholar] [CrossRef]
- Oyarzun, R.; Oyarzún, J.; Lillo, J.; Maturana, H.; Higueras, P. Mineral deposits and Cu-Zn-As dispersion-contamination in stream sediments from the semiarid Coquimbo Region, Chile. Environ. Geol. 2007, 53, 283–294. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Pincheira, M.; Dagnini, A.; Kelm, U.; Helle, S. Copper Pitch Y Copper Wad: Contraste Entre Las Fases Presentes En Las Cabezas Y En Los Ripios En Pruebas De Mina sur, Chuquicamata. In Proceedings of the X Congreso Geológico Chileno, Santiago, Chile, 6–10 October 2003; p. 10. [Google Scholar]
- Kojima, S.; Astudillo, J.; Rojo, J.; Tristá, D.; Hayashi, K.I. Ore mineralogy, fluid inclusion, and stable isotopic characteristics of stratiform copper deposits in the coastal Cordillera of northern Chile. Miner. Depos. 2003, 38, 208–216. [Google Scholar] [CrossRef]
- Cuadra, C.P.; Rojas, S.G. Oxide mineralization at the Radomiro Tomic porphyry copper deposit, Northern Chile. Econ. Geol. 2001, 96, 387–400. [Google Scholar]
- Pérez, K.; Toro, N.; Campos, E.; González, J.; Jeldres, R.I.; Nazer, A.; Rodriguez, M.H. Extraction of Mn from Black Copper Using Iron Oxides from Tailings and Fe2+ as Reducing Agents in Acid Medium. Metals 2019, 9, 1112. [Google Scholar]
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap leaching technology – current state, innovations and future directions: A review. Miner. Process. Extr. Metall. Rev. 2015. [Google Scholar] [CrossRef]
- Helle, S.; Pincheira, M.; Jerez, O.; Kelm, U. Sequential extraction to predict the leaching potential of refractory. In Proceedings of the Mineral Processing Congress, Sozopol, Bulgaria, 12–16 June 2013; pp. 109–111. [Google Scholar]
- Kanungo, S.B.; Jena, P.K. Reduction leaching of manganese nodules of Indian Ocean origin in dilute hydrochloric acid. Hydrometallurgy 1988, 21, 41–58. [Google Scholar] [CrossRef]
- Kanungo, S.B. Rate process of the reduction leaching of manganese nodules in dilute HCl in presence of pyrite. Part II: Leaching behavior of manganese. Hydrometallurgy 1999, 52, 331–347. [Google Scholar] [CrossRef]
- Kanungo, S.B. Rate process of the reduction leaching of manganese nodules in dilute HCl in presence of pyrite. Part I. Dissolution behaviour of iron and sulphur species during leaching. Hydrometallurgy 1999, 52, 313–330. [Google Scholar] [CrossRef]
- Zakeri, A.; Bafghi, M.S.; Shahriari, S. Dissolution kinetics of manganese dioxide ore in sulfuric acid in the presence of ferrous ion. Iran. J. Mater. Sci. Eng. 2007, 4, 22–27. [Google Scholar]
- Bafghi, M.S.; Zakeri, A.; Ghasemi, Z.; Adeli, M. Reductive dissolution of manganese ore in sulfuric acid in the presence of iron metal. Hydrometallurgy 2008, 90, 207–212. [Google Scholar] [CrossRef]
- Pérez, K.; Villegas, Á.; Saldaña, M.; Jeldres, R.I.; González, J.; Toro, N. Initial investigation into the leaching of manganese from nodules at room temperature with the use of sulfuric acid and the addition of foundry slag—Part II. Sep. Sci. Technol. 2020, 1–6. [Google Scholar] [CrossRef]
- Torres, D.; Pérez, K.; Trigueros, E.; Jeldres, R.I.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Reducing-Effect of Chloride for the Dissolution of Black Copper. Metals 2020, 10, 123. [Google Scholar] [CrossRef]
- Hernández, P.C.; Taboada, M.E.; Herreros, O.O.; Graber, T.A.; Ghorbani, Y. Leaching of chalcopyrite in acidified nitrate using seawater-based media. Minerals 2018, 8, 238. [Google Scholar] [CrossRef]
- Cerda, C.P.; Taboada, M.E.; Jamett, N.E.; Ghorbani, Y.; Hernández, P.C. Effect of pretreatment on leaching primary copper sulfide in acid-chloride media. Minerals 2018, 8, 1. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C. Hydrometallurgical process for recovery of cobalt from zinc plant residue. Hydrometallurgy 2002, 63, 225–234. [Google Scholar] [CrossRef]
- Toro, N.; Saldaña, M.; Castillo, J.; Higuera, F.; Acosta, R. Leaching of Manganese from Marine Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Tailings. Minerals 2019, 9, 289. [Google Scholar] [CrossRef]
- Montgomery, D.C. Montgomery: Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Kenett, R.; Zacks, S. Modern Industrial Statistics: With Applications in R, MINITAB and JPM; John Wiley & Sons: Hoboken, NJ, USA, 2017; p. 91. [Google Scholar]
- Cintas, P.G.; Almagro, L.M.; Llabres, X.T.-M. Industrial Statistics with Minitab; John Wiley & Sons: West Sussex, UK, 2012. [Google Scholar]
- Dean, A.; Voss, D.; Draguljić, D. Design and Analysis of Experiments; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Lu, J.; Dreisinger, D.; West-Sells, P. Acid curing and agglomeration for heap leaching. Hydrometallurgy 2017, 167, 30–35. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Torres, D.; Toro, N. Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods. Hydrometallurgy 2018, 181, 215–220. [Google Scholar] [CrossRef]
- Hernández, P.C.; Dupont, J.; Herreros, O.O.; Jimenez, Y.P.; Torres, C.M. Accelerating copper leaching from sulfide ores in acid-nitrate-chloride media using agglomeration and curing as pretreatment. Minerals 2019, 9, 250. [Google Scholar]
- Toro, N.; Herrera, N.; Castillo, J.; Torres, C.; Sepúlveda, R. Initial Investigation into the Leaching of Manganese from Nodules at Room Temperature with the Use of Sulfuric Acid and the Addition of Foundry Slag—Part I. Minerals 2018, 8, 565. [Google Scholar] [CrossRef]
| Mineral (% Mass) | Black Copper |
|---|---|
| Native Cu/Cuprite/Tenorite | 0.12 |
| Cu-Mn Wad | 78.90 |
| Chrysocolla | 16.72 |
| Other Cu Minerals | 2.69 |
| Goethite | 0.01 |
| Quartz | 1.41 |
| Feldspars | 0.02 |
| Kaolinite Group | 0.01 |
| Muscovite/Sericite | 0.01 |
| Chlorite/Biotite | 0.01 |
| Others | 0.09 |
| Total | 100 |
| Curing Time (h) | NaCl (kg/t) |
|---|---|
| 24 | 0 |
| 48 | 5 |
| 72 | 10 |
| 96 | 15 |
| 120 | 20 |
| 144 | 25 |
| 168 | 30 |
| Model/Variable | Curing Time (h) | NaCl (kg/t) | Optimal (%) |
|---|---|---|---|
| Mn recovery | 157.8 | 22.4 | 74.9 |
| Cu recovery | 133.0 | 23.6 | 83.7 |
| Response | Variable | F-Value | p-Value |
|---|---|---|---|
| Eh (V) | Curing time | 225.96 | 0.000 |
| NaCl concentration | 0.1 | 0.996 | |
| pH | Curing time | 8.07 | 0.000 |
| NaCl concentration | 2.79 | 0.022 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldaña, M.; Gálvez, E.; Jeldres, R.I.; Díaz, C.; Robles, P.; Sinha, M.K.; Toro, N. Optimization of Cu and Mn Dissolution from Black Coppers by Means of an Agglomerate and Curing Pretreatment. Metals 2020, 10, 657. https://doi.org/10.3390/met10050657
Saldaña M, Gálvez E, Jeldres RI, Díaz C, Robles P, Sinha MK, Toro N. Optimization of Cu and Mn Dissolution from Black Coppers by Means of an Agglomerate and Curing Pretreatment. Metals. 2020; 10(5):657. https://doi.org/10.3390/met10050657
Chicago/Turabian StyleSaldaña, Manuel, Edelmira Gálvez, Ricardo I. Jeldres, Catalina Díaz, Pedro Robles, Manish Kumar Sinha, and Norman Toro. 2020. "Optimization of Cu and Mn Dissolution from Black Coppers by Means of an Agglomerate and Curing Pretreatment" Metals 10, no. 5: 657. https://doi.org/10.3390/met10050657
APA StyleSaldaña, M., Gálvez, E., Jeldres, R. I., Díaz, C., Robles, P., Sinha, M. K., & Toro, N. (2020). Optimization of Cu and Mn Dissolution from Black Coppers by Means of an Agglomerate and Curing Pretreatment. Metals, 10(5), 657. https://doi.org/10.3390/met10050657








