Electrochemical Investigation of the Effect of Process Parameters on the Corrosion Behavior of Aluminum-Cladded Pressure Vessel Steel Using a Friction Stir Diffusion Cladding Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Workpiece Preparations and the FSDC Process
2.2. Sample Preparation and Electrochemical Test
3. Results and Discussion
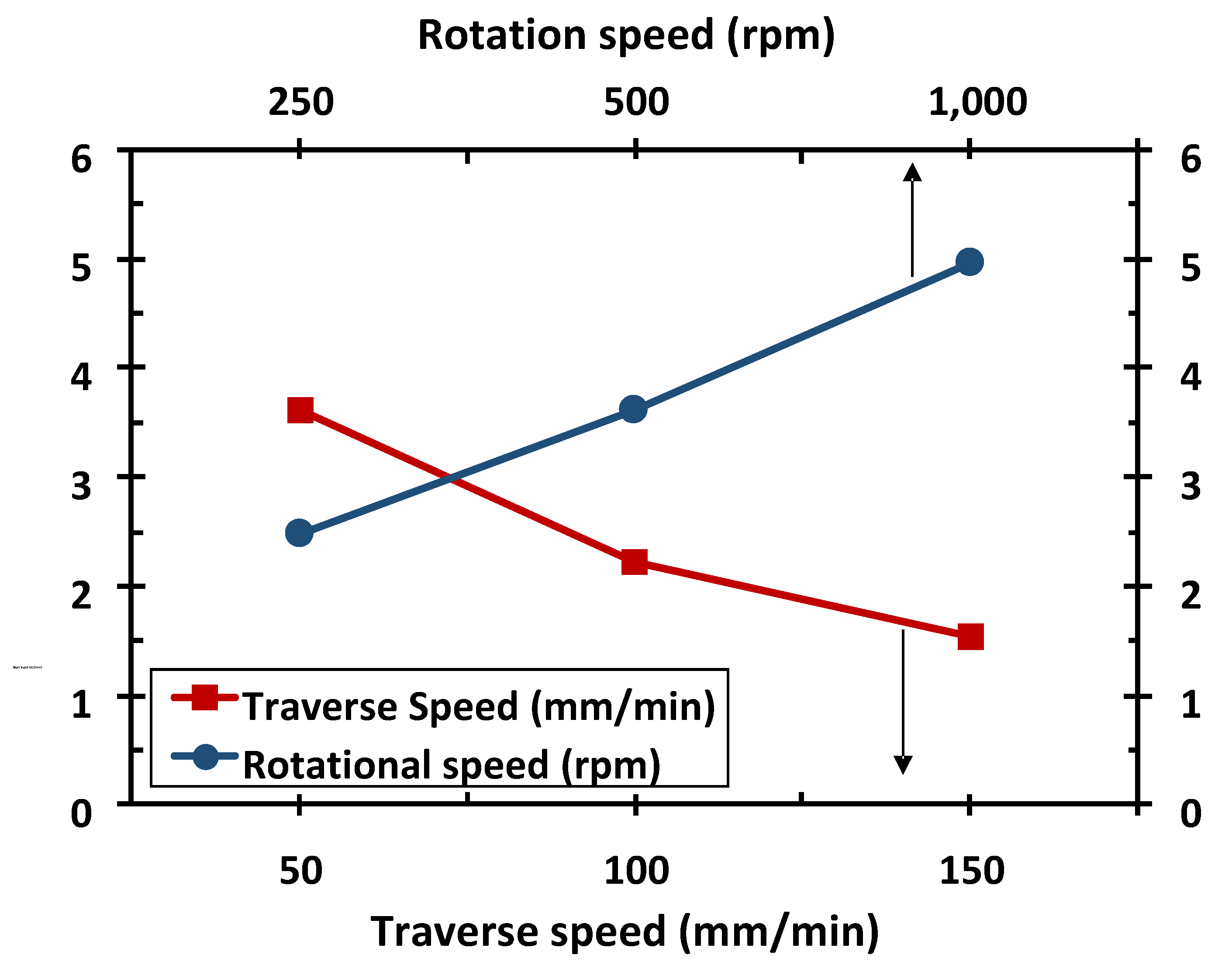
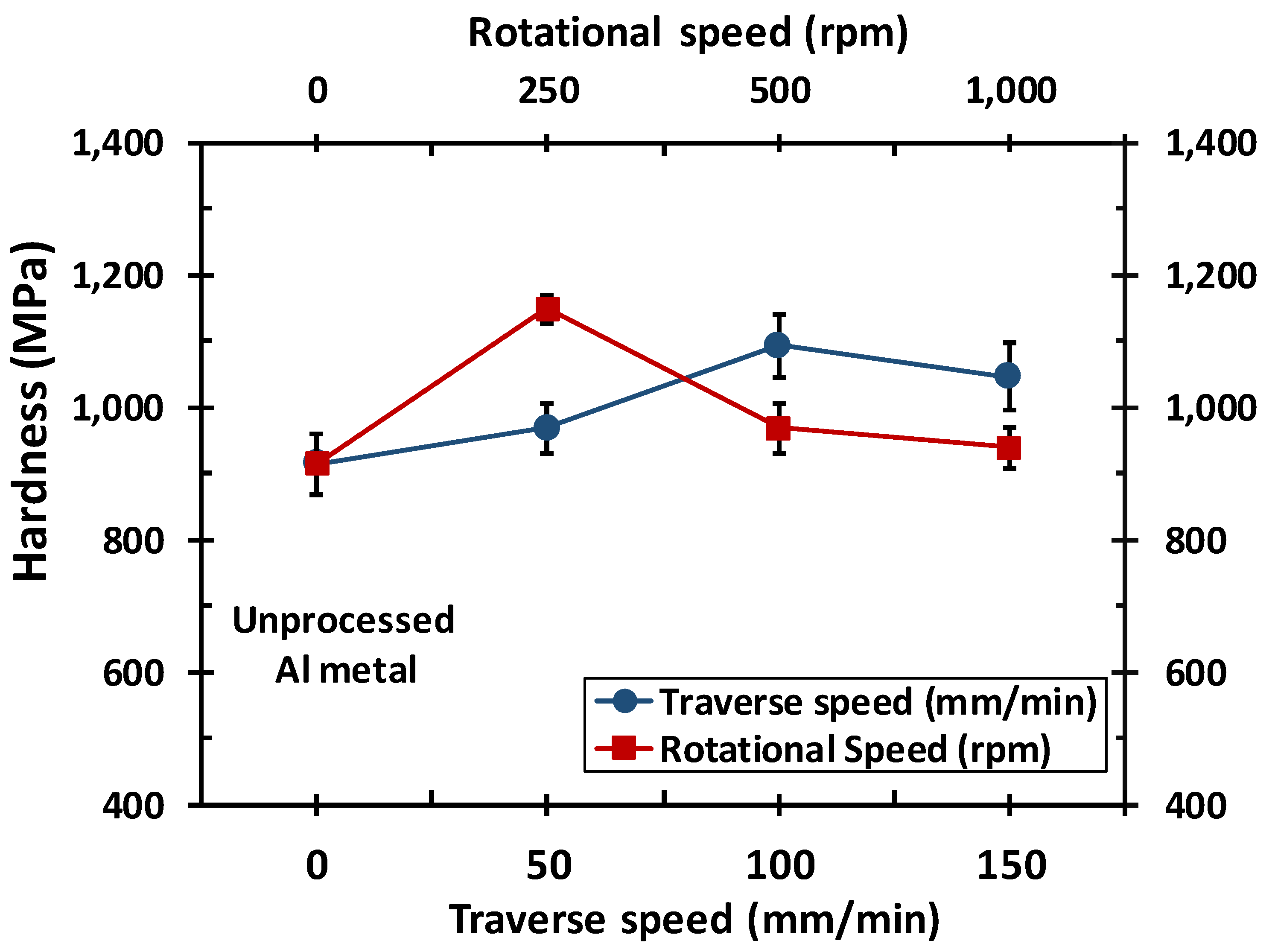
3.1. Dependent Process Variables and Heat Input
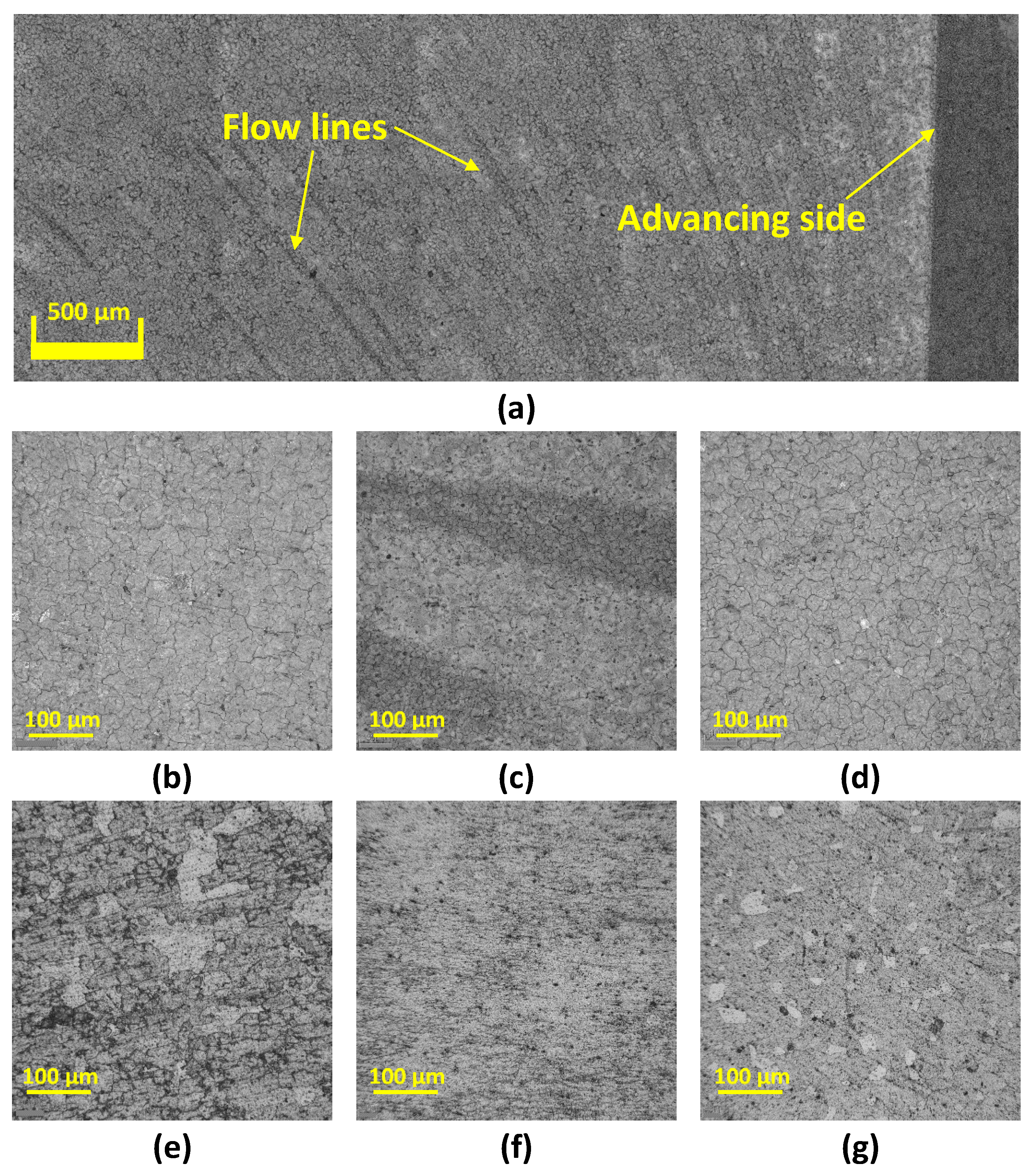
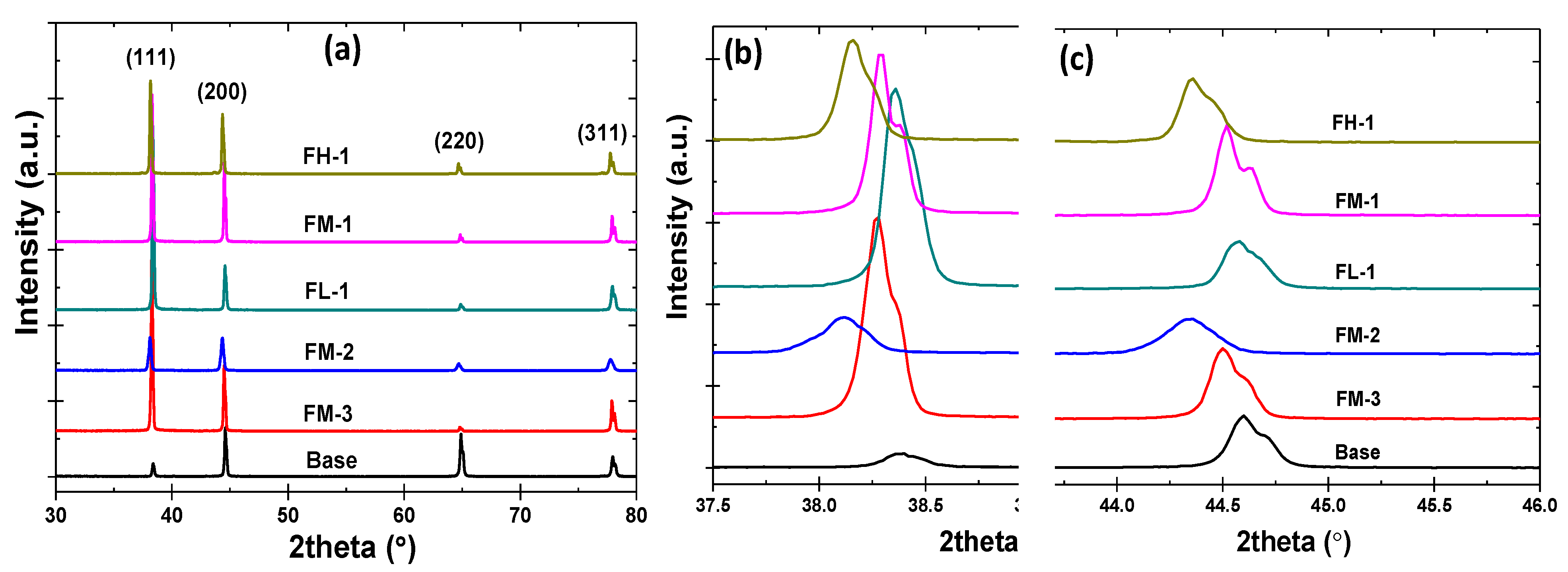
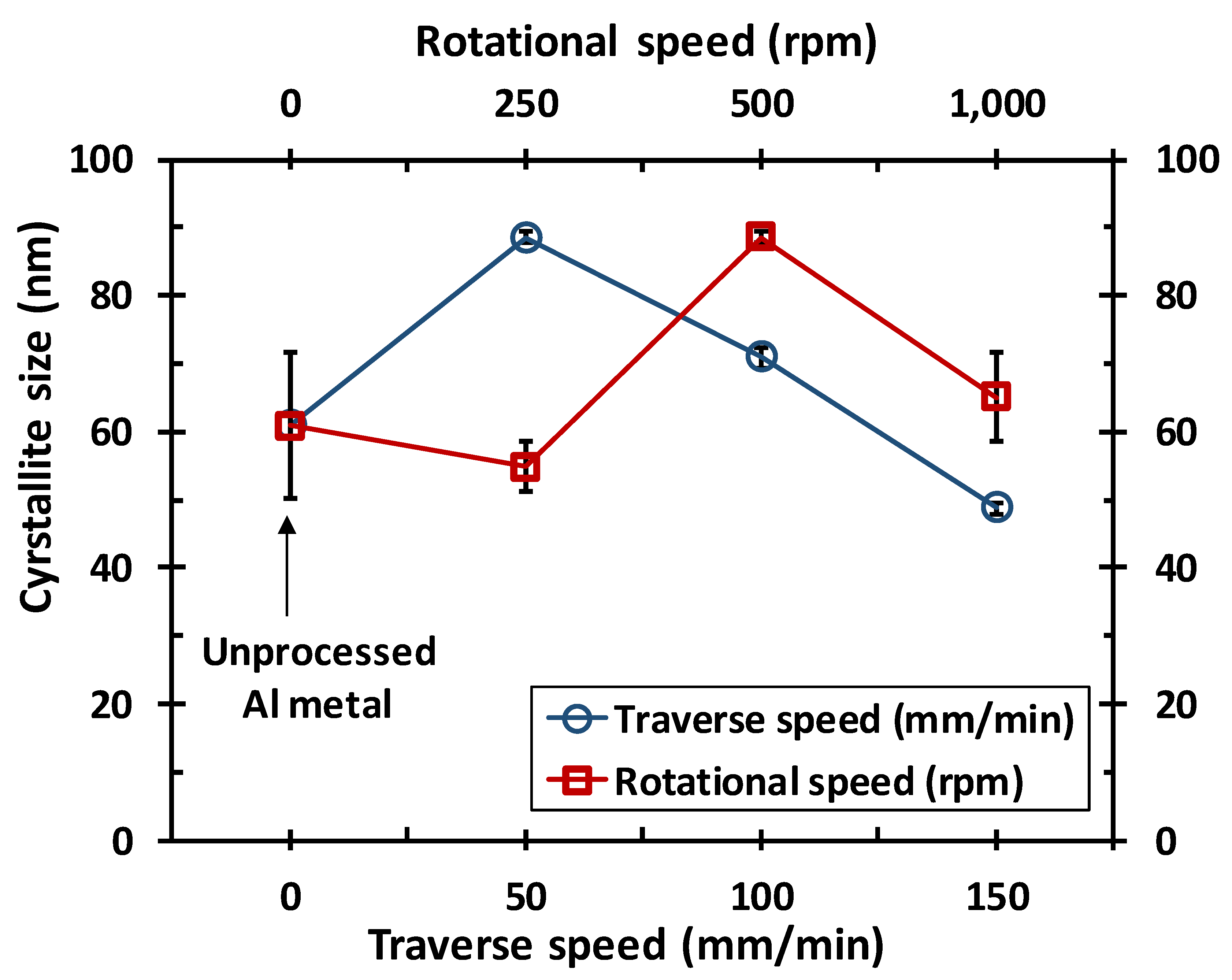
3.2. Microstructure Analysis
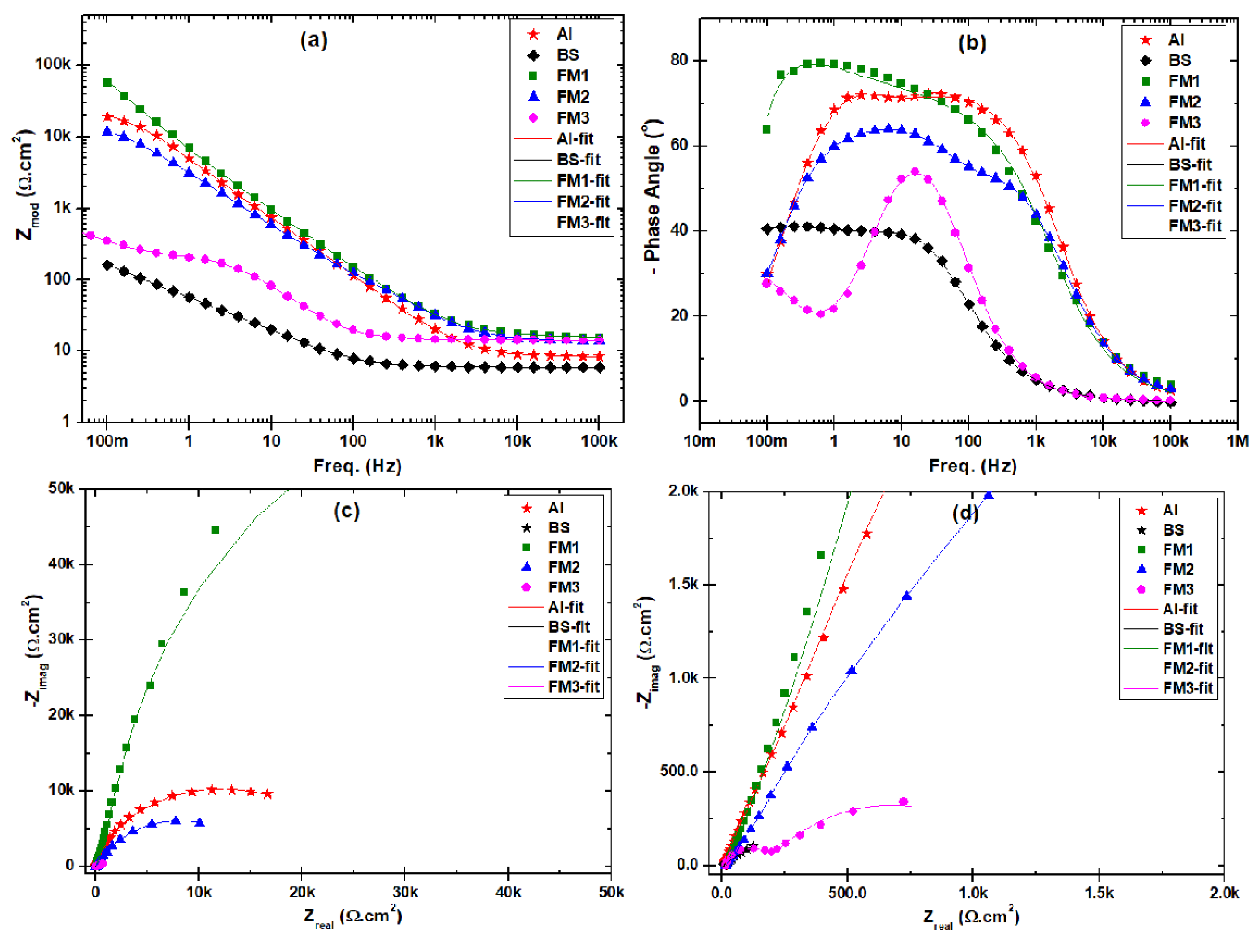
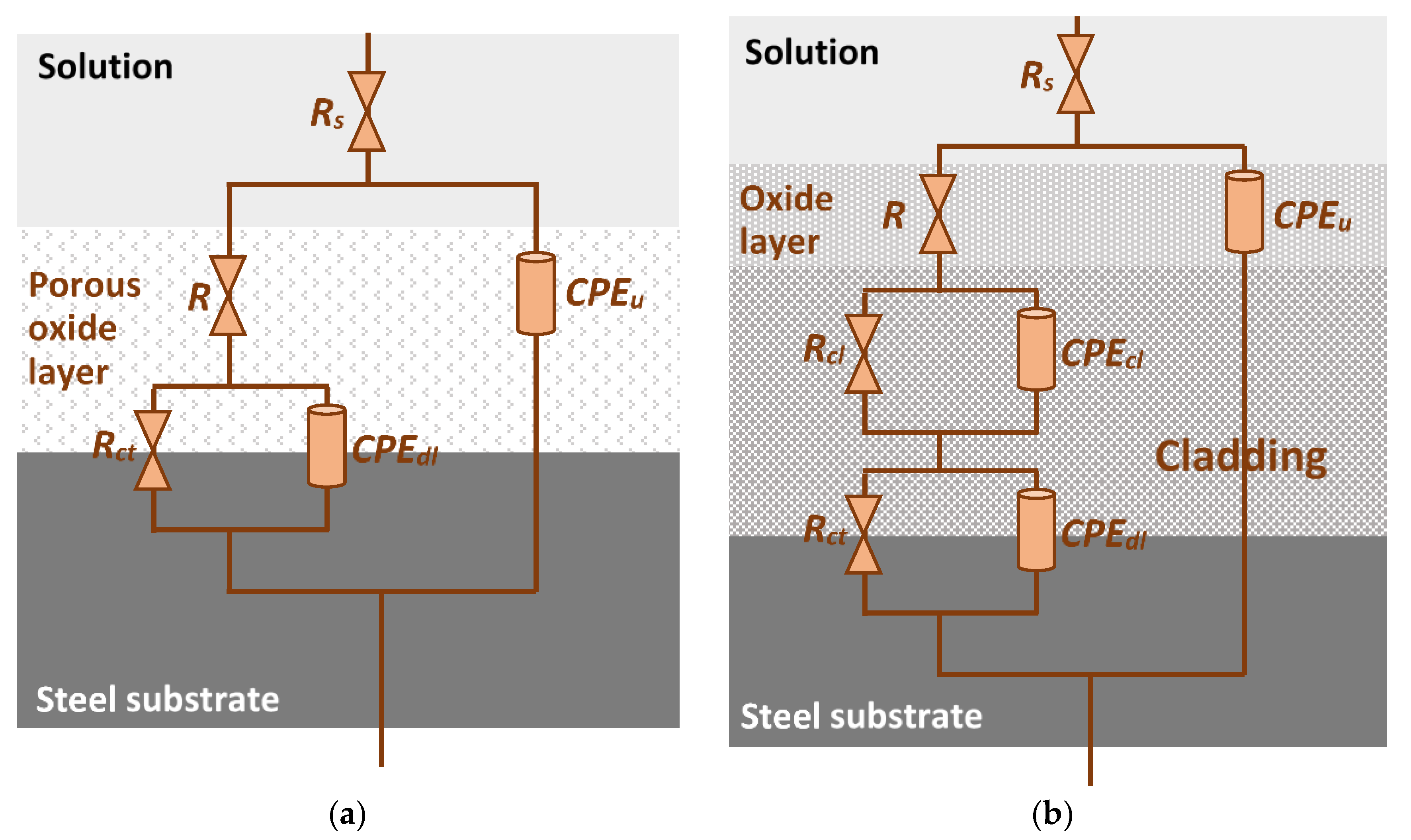
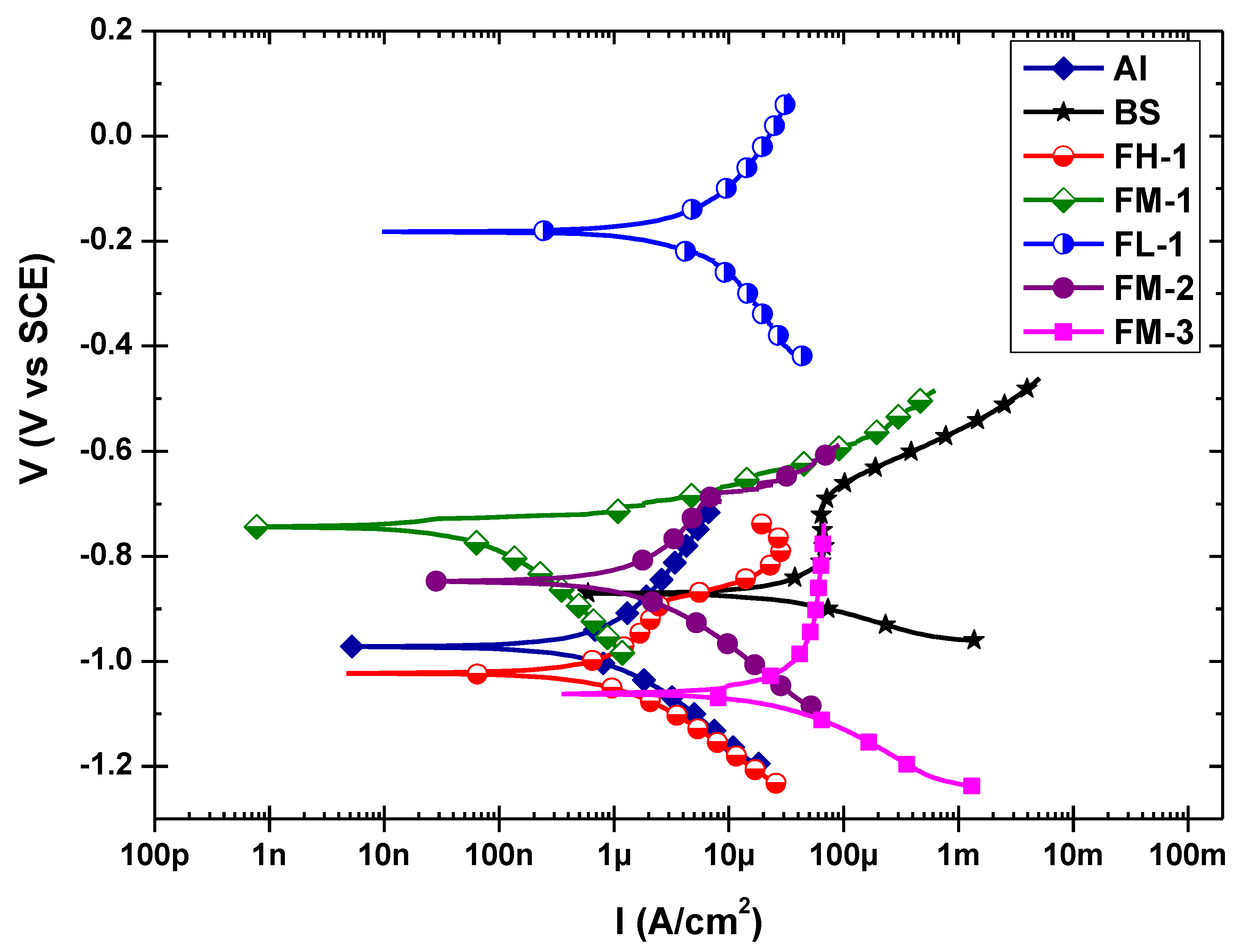
3.3. Corrosion Behavior
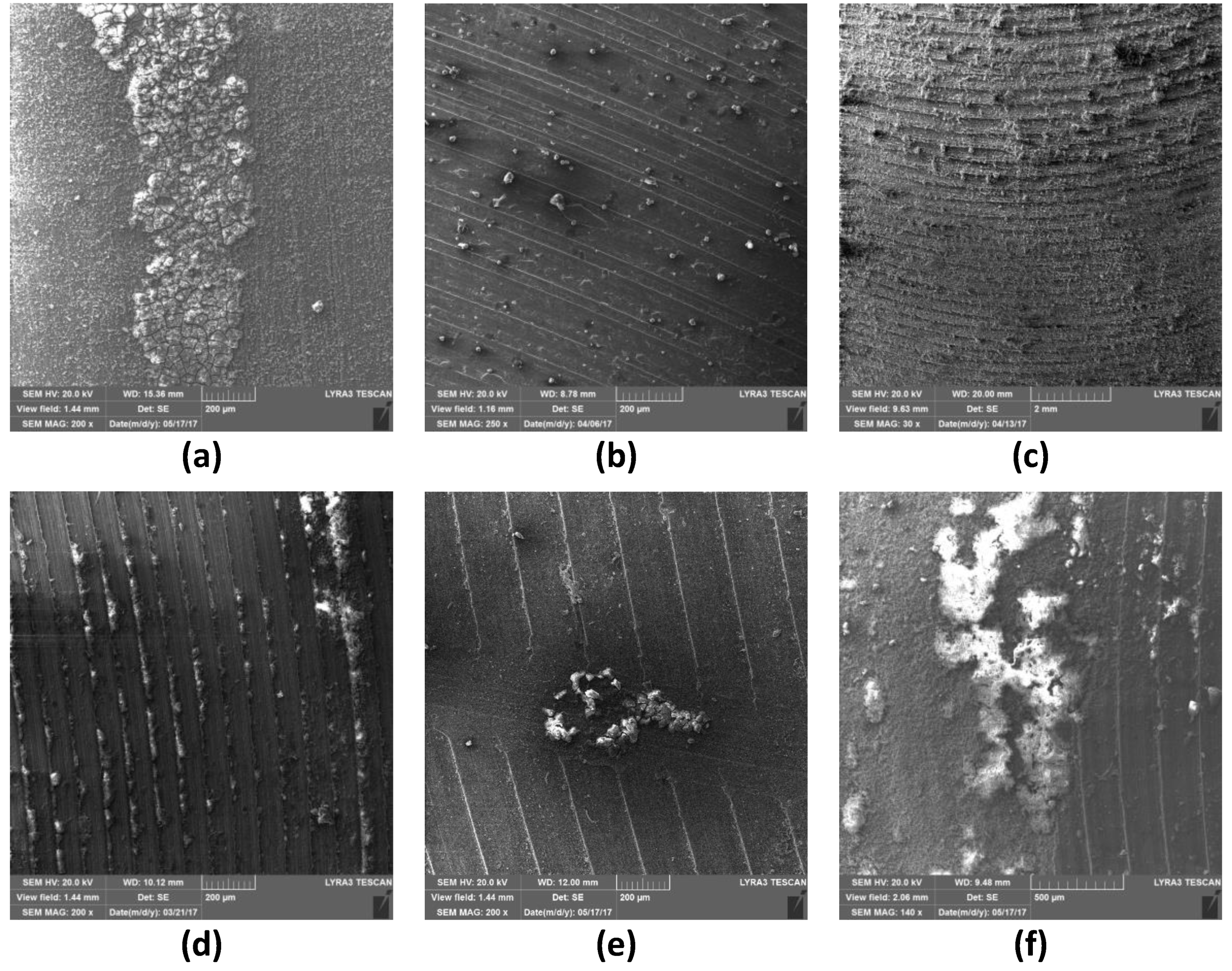
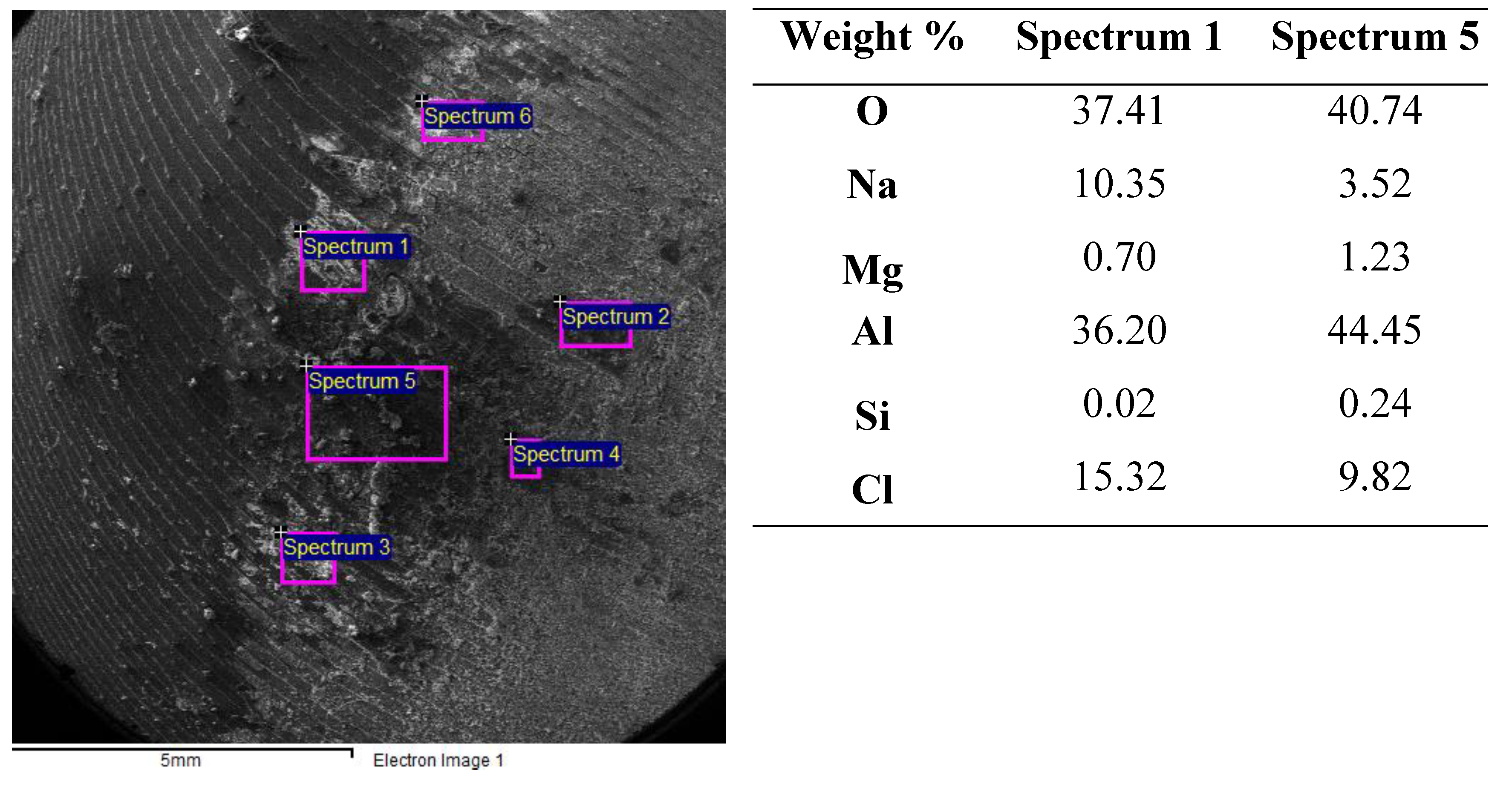
3.4. Surface Examination after Corrosion
4. Conclusions
- The FSDC processing of steel substrate minimized its corrosion rate, which was found to be dependent on processing parameters.
- Optimum process conditions for general corrosion resistance were found to be for medium tool rotational speed and low traverse speed. While improvement of passivity of the cladded system occurred at medium traverse speed and high traverse speed.
- EIS and PDP results revealed that the general corrosion resistance decreases upon increasing the traverse speed.
- An intermediate tool rotational speed of 500 rpm resulted in optimum value for anticorrosion properties, while the passivation property was improved at the rotational speed of 1000 rpm and traverse speeds of 50 mm/min.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buchanan, V.; Shipway, P.; McCartney, D. Microstructure and abrasive wear behaviour of shielded metal arc welding hardfacings used in the sugarcane industry. Wear 2007, 263, 99–110. [Google Scholar] [CrossRef]
- Rao, K.P.; Ram, G.J.; Stucker, B. Effect of friction stir processing on corrosion resistance of aluminum–copper alloy gas tungsten arc welds. Mater. Des. 2010, 31, 1576–1580. [Google Scholar] [CrossRef]
- Mabuwa, S.; Msomi, V. Review on Friction Stir Processed TIG and Friction Stir Welded Dissimilar Alloy Joints. Metals 2020, 10, 142. [Google Scholar] [CrossRef]
- Ibrahim, A.B.; Al-Badour, F.A.; Adesina, A.Y.; Merah, N. Effect of process parameters on microstructural and mechanical properties of friction stir diffusion cladded ASTM A516-70 steel using 5052 Al alloy. J. Manuf. Processes 2018, 34, 451–462. [Google Scholar] [CrossRef]
- Al-Badour, F.; Merah, N.; Mohamed, O.; Bazoune, A.; Shuaib, A. Optimizing Process Conditions in Friction Stir-Diffusion Lap Welding of Aluminum Alloy With Cold Rolled Steel. In Proceedings of the ASME 2018 13th International Manufacturing Science and Engineering Conference, College Station, TX, USA, 18–22 June 2018. [Google Scholar] [CrossRef]
- Ibrahim, A.B.; Al-Badour, F.A.; Suleiman, R.; Adesina, A.Y.; Sorour, A.A. Friction Stir Diffusion Cladding of 5052 Aluminum Alloy on Cold-Rolled Carbon Steel. In Proceedings of the 17th Middle East Corrosion Conference and Exhibition, Manama, Bahrain, 3 October–30 November 2018; pp. 1–13. [Google Scholar]
- Argade, G.; Shukla, S.; Liu, K.; Mishra, R. Friction stir lap welding of stainless steel and plain carbon steel to enhance corrosion properties. J. Mater. Process. Technol. 2018, 259, 259–269. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, Y.; Haghshenas, M.; Nguyen, T.; Galloway, J.; Gerlich, A. Interfacial microstructure and properties of copper clad steel produced using friction stir welding versus gas metal arc welding. Mater. Charact. 2015, 104, 1–9. [Google Scholar] [CrossRef]
- Li, B.; Shen, Y.; Luo, L.; Hu, W. Effects of processing variables and heat treatments on Al/Ti-6Al-4V interface microstructure of bimetal clad-plate fabricated via a novel route employing friction stir lap welding. J. Alloys Compd. 2016, 658, 904–913. [Google Scholar] [CrossRef]
- Li, H.; Qin, W.; Galloway, A.; Toumpis, A. Friction surfacing of aluminium alloy 5083 on DH36 steel plate. Metals 2019, 9, 479. [Google Scholar] [CrossRef]
- Davis, J.R. Stainless Steels; ASM international: Novelty, OH, USA, 1994. [Google Scholar]
- Bajakke, A.; Jambagi, C.; Malik, R.; Deshpande, S. Friction Stir Processing: An Emerging Surface Engineering Technique. In Surface Engineering of Modern Materials. Engineering Materials; Gupta, K., Ed.; Springer: Cham, UK, 2020. [Google Scholar] [CrossRef]
- Xu, W.; Liu, J. Microstructure and pitting corrosion of friction stir welded joints in 2219-O aluminum alloy thick plate. Corros. Sci. 2009, 51, 2743–2751. [Google Scholar] [CrossRef]
- Surekha, K.; Murty, B.; Rao, K.P. Microstructural characterization and corrosion behavior of multipass friction stir processed AA2219 aluminium alloy. Surf. Coat. Technol. 2008, 202, 4057–4068. [Google Scholar] [CrossRef]
- Surekha, K.; Murty, B.; Rao, K.P. Effect of processing parameters on the corrosion behaviour of friction stir processed AA 2219 aluminum alloy. Solid State Sci. 2009, 11, 907–917. [Google Scholar] [CrossRef]
- Surekha, K.; Murty, B.; Rao, K.P. Comparison of corrosion behaviour of friction stir processed and laser melted AA 2219 aluminium alloy. Mater. Des. 2011, 32, 4502–4508. [Google Scholar] [CrossRef]
- Shamsudeen, S.; John, E.R.D. Effect of Welding on Pitting and Intergranular Corrosion Behavior of Marine Grade Aluminum Alloy. Mater. Perform. Charact. 2019, 8, 555–570. [Google Scholar] [CrossRef]
- Shanavas, S.; Dhas, J.E.R.; Murugan, N. Weldability of marine grade AA 5052 aluminum alloy by underwater friction stir welding. Int. J. Adv. Manuf. Technol. 2018, 95, 4535–4546. [Google Scholar] [CrossRef]
- Mohammadtaheri, M. A new metallographic technique for revealing grain boundaries in aluminum alloys. Metall. Microstruct. Anal. 2012, 1, 224–226. [Google Scholar] [CrossRef]
- ASME. BPVC Section IX-Welding, Brazing and Fusing Qualifications; ASME: New York, NY, USA, 2017. [Google Scholar]
- Fu, R.; Xu, H.; Luan, G.; Dong, C.; Zhang, F.; Li, G. Top surface microstructure of friction-stir welded AA2524-T3 aluminum alloy joints. Mater. Charact. 2012, 65, 48–54. [Google Scholar] [CrossRef]
- Kaufman, J.G. Introduction to Aluminum Alloys and Tempers; ASM International: Novelty, OH, USA, 2000. [Google Scholar]
- Atlas Steel Documents. Available online: http://www.atlassteels.com.au/documents/Atlas_Aluminium_datasheet_5052_rev_Oct_2013.pdf (accessed on 3 February 2020).
- Ralston, K.; Fabijanic, D.; Birbilis, N. Effect of grain size on corrosion of high purity aluminium. Electrochim. Acta 2011, 56, 1729–1736. [Google Scholar] [CrossRef]
- Ralston, K.; Birbilis, N.; Davies, C. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Murugan, B.; Thirunavukarasu, G.; Kundu, S.; Kailas, S.V. Influence of tool traverse speed on structure, mechanical properties, fracture behavior, and weld corrosion of friction stir welded joints of aluminum and stainless steel. Adv. Eng. Mater. 2019, 21, 1800869. [Google Scholar] [CrossRef]
- Rambabu, G.; Naik, D.B.; Rao, C.V.; Rao, K.S.; Reddy, G.M. Optimization of friction stir welding parameters for improved corrosion resistance of AA2219 aluminum alloy joints. Defence Technol. 2015, 11, 330–337. [Google Scholar] [CrossRef]
- Gollapudi, S. Grain size distribution effects on the corrosion behaviour of materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar] [CrossRef]
- Ralston, K.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005–075013. [Google Scholar] [CrossRef]
- Anas, N.; Abioye, T.; Anasyida, A.; Dhindaw, B.; Zuhailawati, H.; Ismail, A. Microstructure, mechanical and corrosion properties of cryorolled-AA5052 at various solution treatment temperatures. MRE 2020, 7, 016535. [Google Scholar] [CrossRef]
- Moeini, G.; Sajadifar, S.V.; Engler, T.; Heider, B.; Niendorf, T.; Oechsner, M.; Böhm, S. Effect of Friction Stir Processing on Microstructural, Mechanical, and Corrosion Properties of Al-Si12 Additive Manufactured Components. Metals 2020, 10, 85. [Google Scholar] [CrossRef]
- D’Antuono, D.S.; Gaies, J.; Golumbfskie, W.; Taheri, M. Grain boundary misorientation dependence of β phase precipitation in an Al–Mg alloy. Scr. Mater. 2014, 76, 81–84. [Google Scholar] [CrossRef]


| Materials | Composition (at.%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Fe | Mg | Cr | Others | Al | |
| A516-70 Steel | 0.31 | 0.45 | 1.3 | 0.035 | 0.035 | Bal. | - | - | - | - |
| 5052 Al alloy | - | 0.29 | 0.086 | - | - | 0.325 | 2.26 | 0.247 | 0.029 | Bal. |
| Sample ID | Rotation Speed (rpm) | Travel Speed (mm/min) | Interface Strength 1 (N/mm)/Bonding | Standard Deviation (N/mm) | Remark |
|---|---|---|---|---|---|
| FL-1 | 250 | 50 | 291 | 23 | Passed |
| FL-2 | 250 | 100 | Defective bonding | Dropped | |
| FL-3 | 250 | 150 | Defective bonding | Dropped | |
| FM-1 | 500 | 50 | 457 | 30 | Passed |
| FM-2 | 500 | 100 | 399 | 29 | Passed |
| FM-3 | 500 | 150 | 315 | 32 | Passed |
| FH-1 | 1000 | 50 | 293 | 3 | Passed |
| FH-2 | 1000 | 100 | 428 | 55 | Dropped |
| FH-3 | 1000 | 150 | 51/Weak bonding | 6 | Dropped |
| Sample ID | APR | Forging Force [N] | Torque [N.m] |
|---|---|---|---|
| FM-1 | 0.1 | 7099.8 | 57.6 |
| FM-2 | 0.2 | 9845.2 | 70.5 |
| FM-3 | 0.3 | 13225 | 73.9 |
| FH-1 | 0.05 | 5351.4 | 39.5 |
| FL-1 | 0.2 | 8237.1 | 78.7 |
| Samples | Rs (Ωcm2) | CPEu (µF/cm2) | nu | R (Ωcm2) | Rct (kΩcm2) | CPEdl (µF/cm2) | ndl | Rcl (Ωcm2) | CPEcl (µF/cm2) | ncl | Eeis (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A516-70 | 5.91 | 6890 | 0.51 | 26.18 | 0.164 | 1670 | 0.79 | - | - | - | |
| FH-1 | 14.20 | 8.29 | 0.94 | 9.16 | 29.9 | 7.36 | 0.94 | 23.6 | 46.9 | 0.76 | 99.45 |
| FM-1 | 15.61 | 32.7 | 0.42 | 3.98 | 61.9 | 48.1 | 0.78 | 175 | 0.0183 | 0.97 | 99.74 |
| FL-1 | 14.50 | 169 | 1.00 | 5.62 | 2.05 | 543 | 0.78 | 2.39 | 506 | 0.54 | 92.02 |
| FM-2 | 13.70 | 45.7 | 0.90 | 214 | 12.2 | 34.3 | 0.81 | 415 | 65.7 | 0.71 | 98.66 |
| FM-3 | 14.25 | 150 | 0.52 | 5.69 | 0.348 | 346 | 0.87 | 941 | 6550 | 0.75 | 53.03 |
| Samples | Icorr (μA/cm2) | Ecorr (mV vs SCE) | βa (V/Decade) | βc (V/Decade) | Epdp (%) |
|---|---|---|---|---|---|
| A516-70 | 41.90 | −870 | 248 | 752 | - |
| 5052-H32 | 1.25 | −972 | 352 | 197 | - |
| FH-1 | 1.18 | −1020 | 285 | 133 | 97.2 |
| FM-1 | 0.98 | −744 | 256 | 204 | 97.7 |
| FL-1 | 3.43 | −182 | 151 | 138 | 91.8 |
| FM-2 | 2.84 | −848 | 414 | 199 | 93.2 |
| FM-3 | 20.70 | −1060 | 152 | 150 | 50.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Badour, F.A.; Adesina, A.Y.; Ibrahim, A.B.; Suleiman, R.K.; Merah, N.; Sorour, A.A. Electrochemical Investigation of the Effect of Process Parameters on the Corrosion Behavior of Aluminum-Cladded Pressure Vessel Steel Using a Friction Stir Diffusion Cladding Process. Metals 2020, 10, 623. https://doi.org/10.3390/met10050623
Al-Badour FA, Adesina AY, Ibrahim AB, Suleiman RK, Merah N, Sorour AA. Electrochemical Investigation of the Effect of Process Parameters on the Corrosion Behavior of Aluminum-Cladded Pressure Vessel Steel Using a Friction Stir Diffusion Cladding Process. Metals. 2020; 10(5):623. https://doi.org/10.3390/met10050623
Chicago/Turabian StyleAl-Badour, Fadi A., Akeem Y. Adesina, Almigdad B. Ibrahim, Rami K. Suleiman, Neçar Merah, and Ahmad A. Sorour. 2020. "Electrochemical Investigation of the Effect of Process Parameters on the Corrosion Behavior of Aluminum-Cladded Pressure Vessel Steel Using a Friction Stir Diffusion Cladding Process" Metals 10, no. 5: 623. https://doi.org/10.3390/met10050623
APA StyleAl-Badour, F. A., Adesina, A. Y., Ibrahim, A. B., Suleiman, R. K., Merah, N., & Sorour, A. A. (2020). Electrochemical Investigation of the Effect of Process Parameters on the Corrosion Behavior of Aluminum-Cladded Pressure Vessel Steel Using a Friction Stir Diffusion Cladding Process. Metals, 10(5), 623. https://doi.org/10.3390/met10050623







