Formation and Properties of Amorphous Multi-Component (CrFeMoNbZr)Ox Thin Films
Abstract
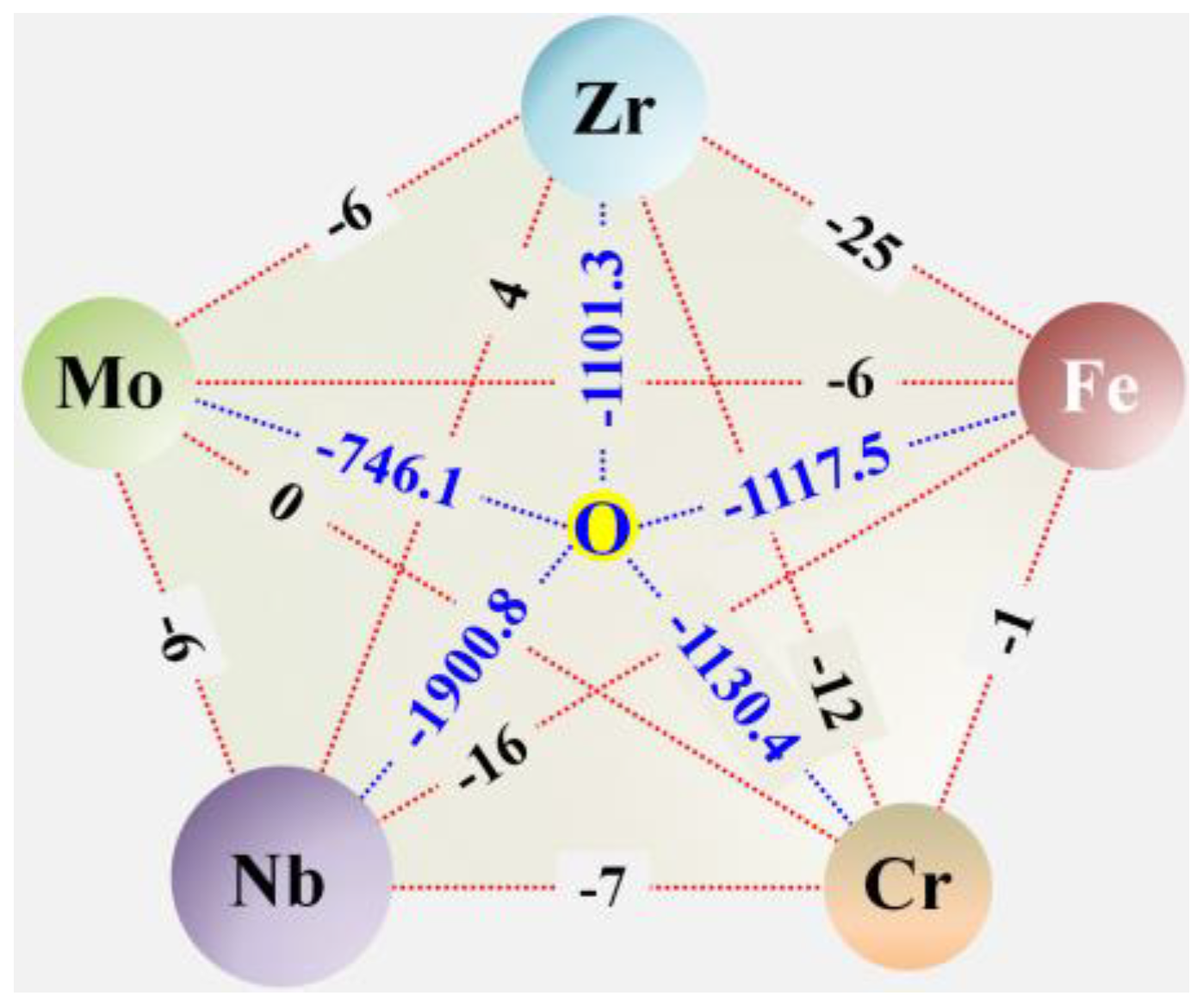
1. Introduction
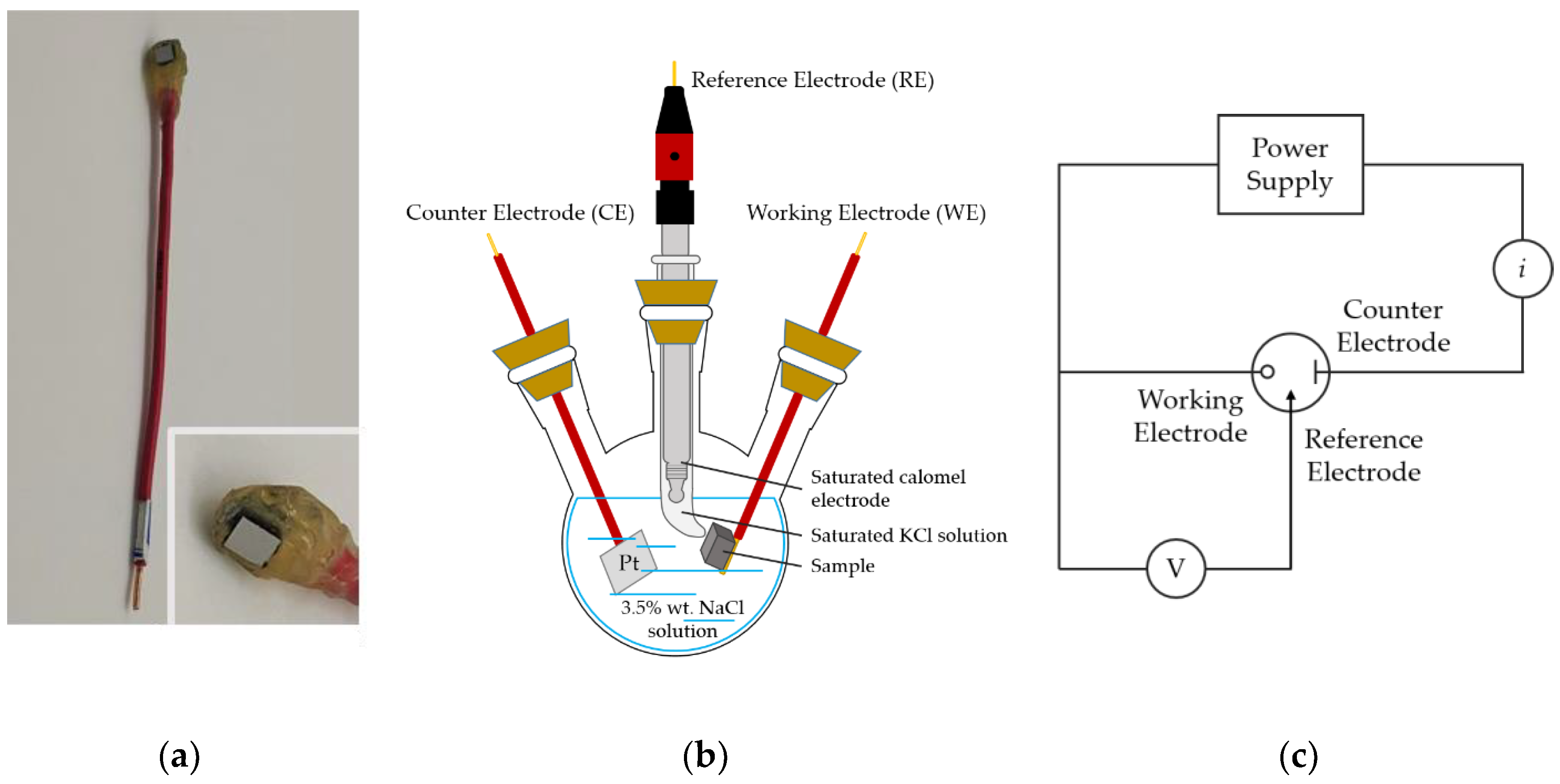
2. Materials and Methods
3. Results
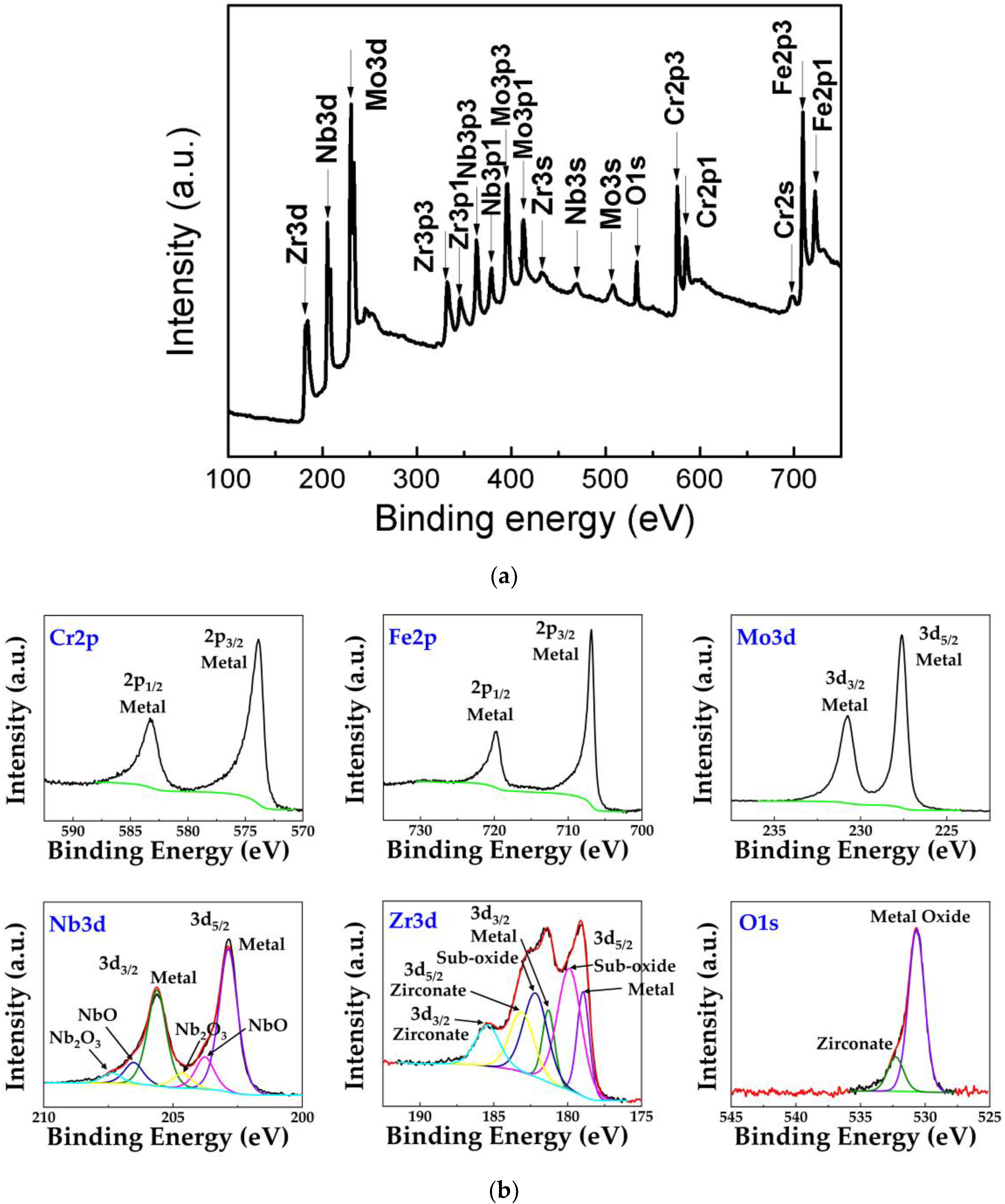
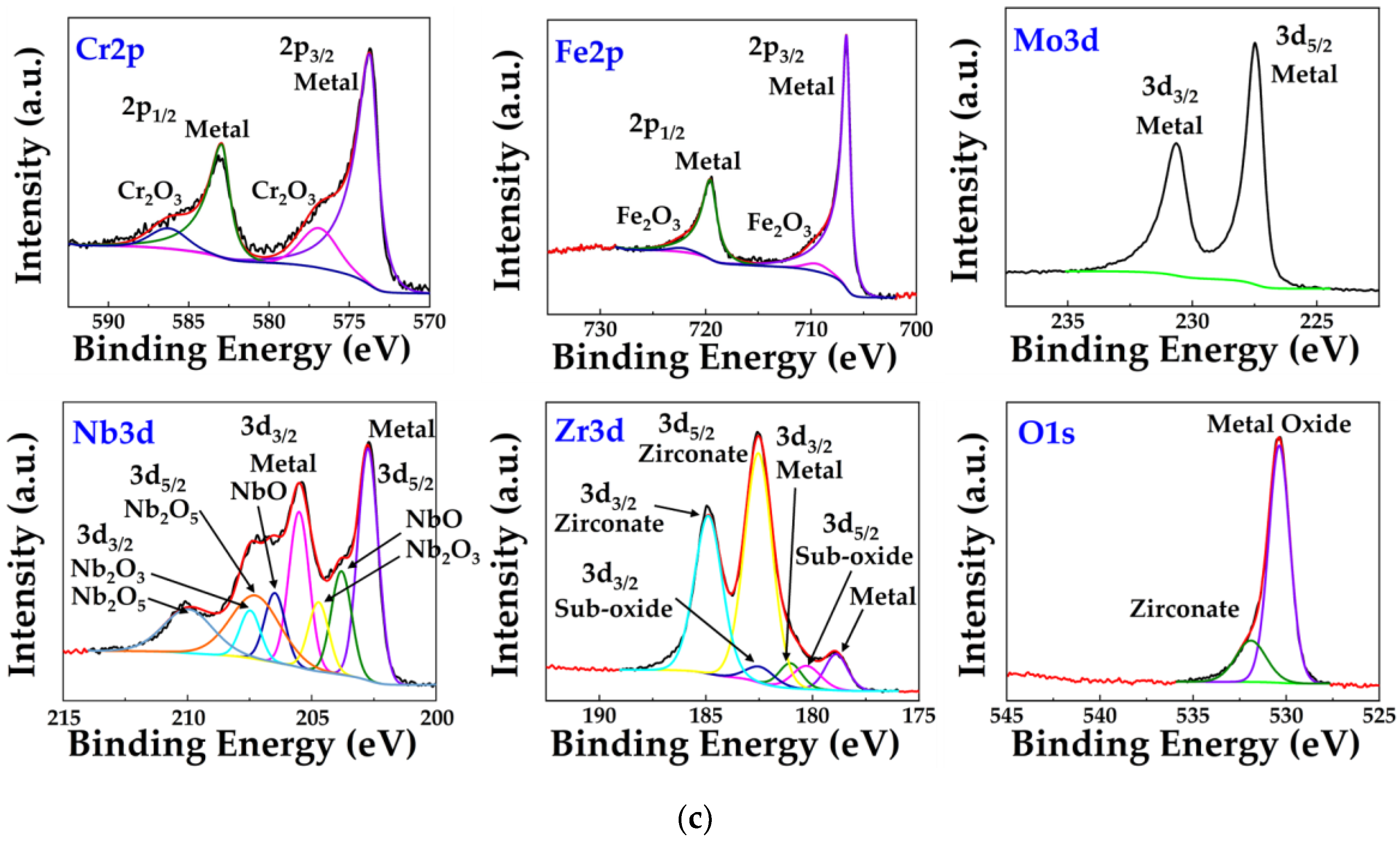
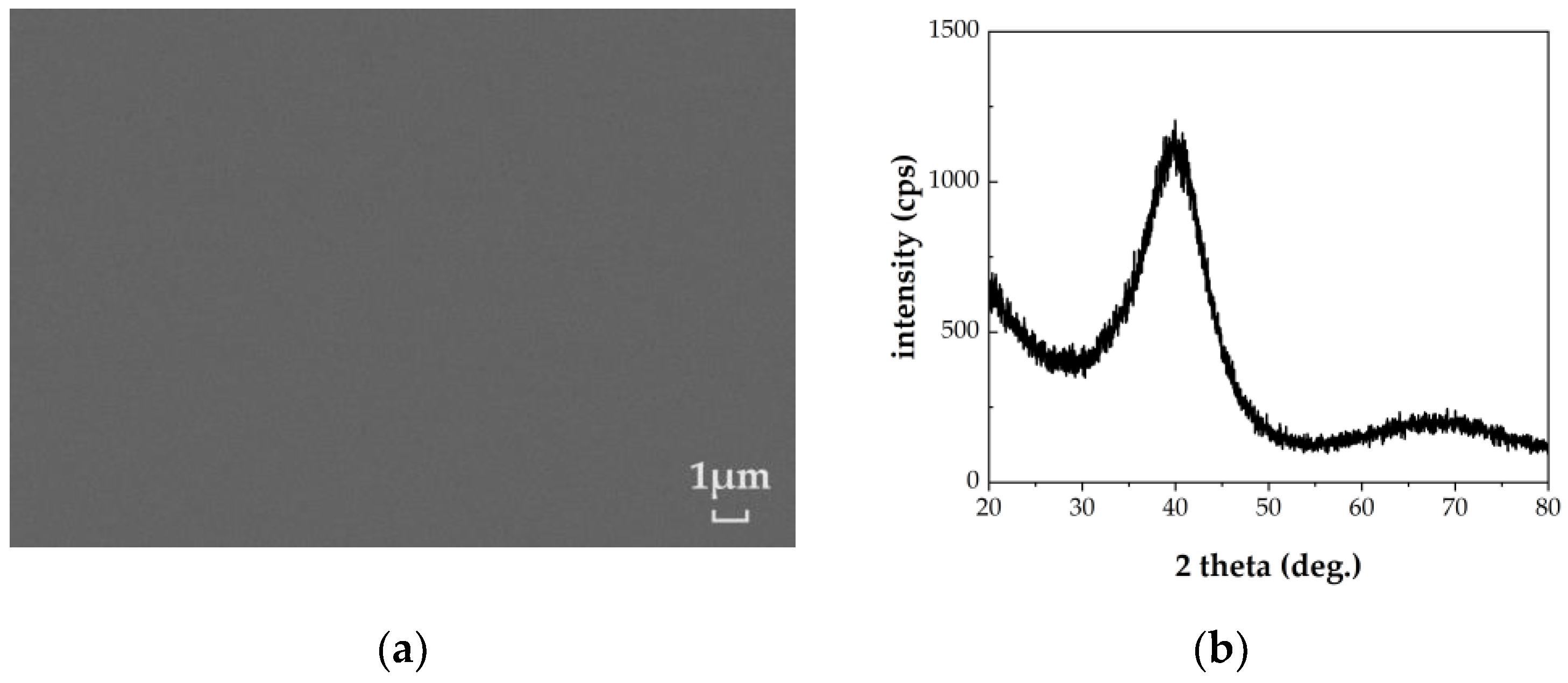
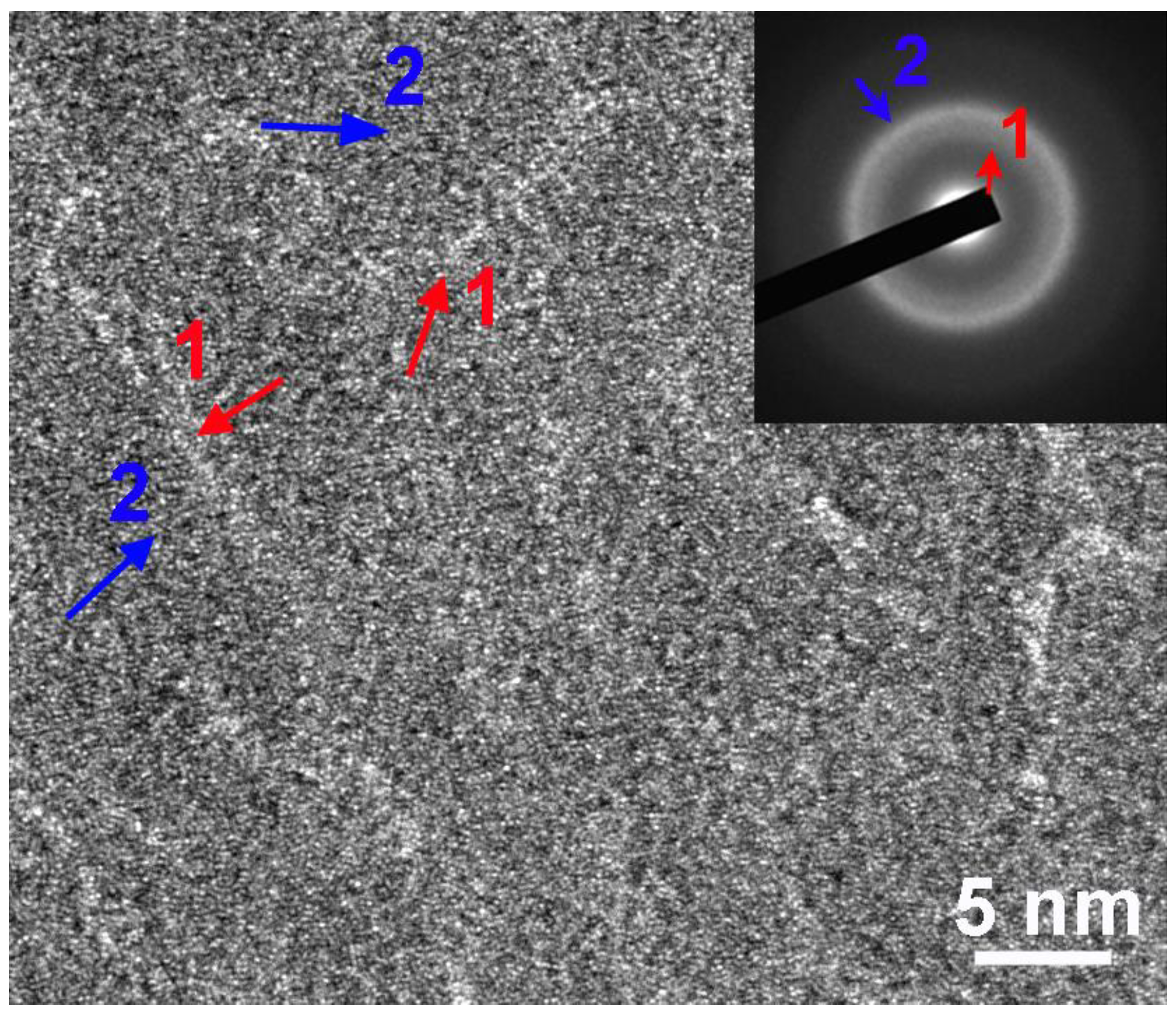
3.1. Formation and Structural Characterization of the (CrFeMoNbZr)Ox Thin Films
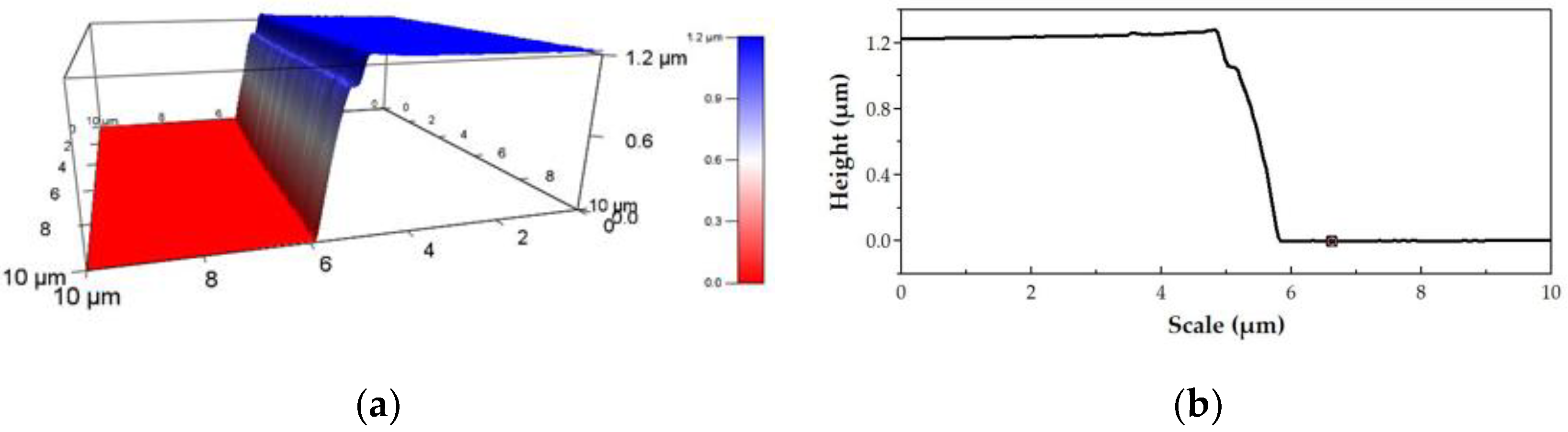
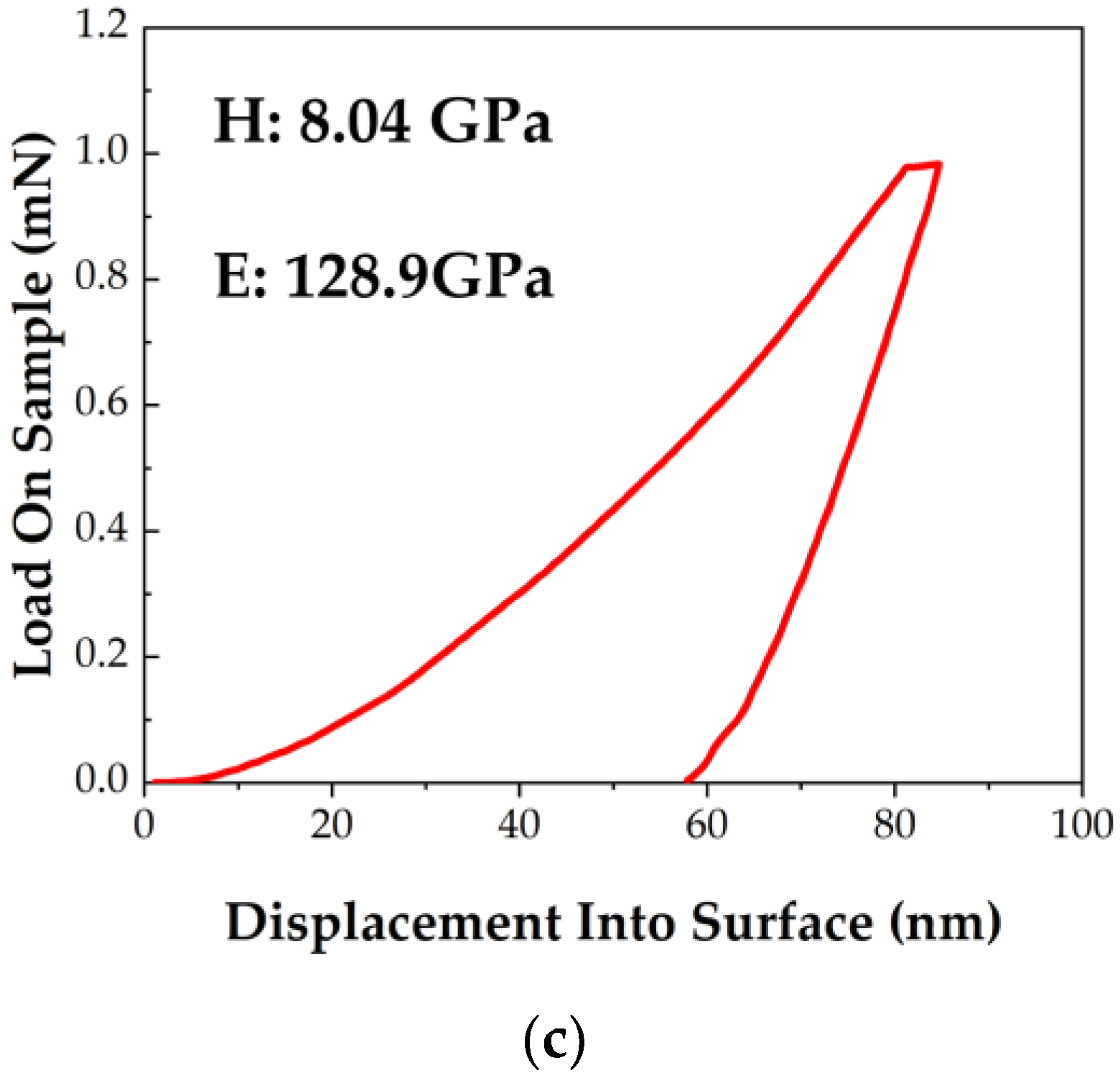
3.2. Mechanical Properties
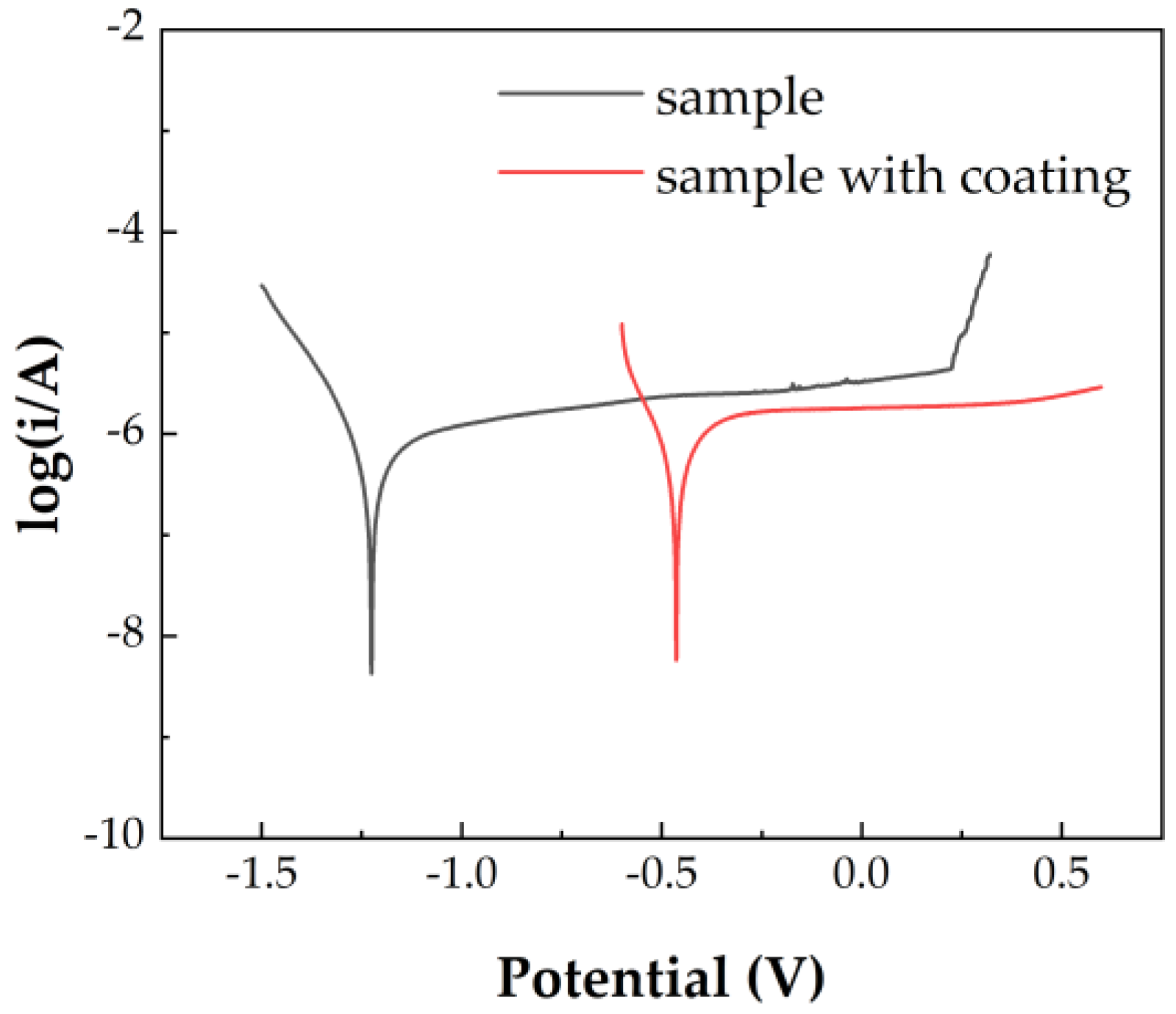
3.3. Corrosion Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Miracle, D.B. High-Entropy Alloys: A Current Evaluation of Founding Ideas and Core Effects and Exploring “Nonlinear Alloys”. JOM 2017, 69, 2130–2136. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys-A review. Int. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Feng, X.B.; Zhang, J.Y.; Xia, Z.R.; Fu, W.; Wu, K.; Liu, G.; Sun, J. Stable nanocrystalline NbMoTaW high entropy alloy thin films with excellent mechanical and electrical properties. Mater. Lett. 2018, 210, 84–87. [Google Scholar] [CrossRef]
- Gorban, V.F.; Andreev, A.A.; Shaginyan, L.R.; Firstov, S.A.; Karpets, M.V.; Danilenko, N.I. High-Entropy Coatings-Structure and Properties. J. Superhard Mater. 2018, 40, 88–101. [Google Scholar] [CrossRef]
- Chen, T.K.; Wong, M.S.; Shun, T.T.; Yeh, J.W. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Tech. 2005, 200, 1361–1365. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A Review on High Entropy Alloys Coatings: Fabrication Processes and Property Assessment. Adv. Eng. Mater. 2019, 21, 1900343. [Google Scholar] [CrossRef]
- Zhao, F.; Song, Z.X.; Zhang, G.F.; Hou, X.D.; Deng, D.W. Effects of substrate bias on structure and mechanical properties of AlCrTiWNbTa coatings. Surf. Eng. 2013, 29, 778–782. [Google Scholar] [CrossRef]
- Tunes, M.A.; Vishnyakov, V.M.; Camara, O.; Greaves, G.; Edmondson, P.D.; Zhang, Y.; Donnelly, S.E. A candidate accident tolerant fuel system based on a highly concentrated alloy thin film. Mater. Today Energy 2019, 12, 356–362. [Google Scholar] [CrossRef]
- Fritze, S.; Koller, C.M.; von Fieandt, L.; Malinovskis, P.; Johansson, K.; Lewin, E.; Mayrhofer, P.H.; Jansson, U. Influence of Deposition Temperature on the Phase Evolution of HfNbTiVZr High-Entropy Thin Films. Materials 2019, 12, 587. [Google Scholar] [CrossRef]
- Zhang, Y.; Tunes, M.A.; Crespillo, M.L.; Zhang, F.; Boldman, W.L.; Rack, P.D.; Jiang, L.; Xu, C.; Greaves, G.; Donnelly, S.E.; et al. Thermal stability and irradiation response of nanocrystalline CoCrCuFeNi high-entropy alloy. Nanotechnology 2019, 30, 294004. [Google Scholar] [CrossRef]
- Al-Olayyan, Y.; Fuchs, G.E.; Baney, R.; Tulenko, J. The effect of Zircaloy-4 substrate surface condition on the adhesion strength and corrosion of SiC coatings. J. Nucl. Mater. 2005, 346, 109–119. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Ceramic coating for corrosion (c3) resistance of nuclear fuel cladding. Surf. Coat. Technol. 2015, 281, 133–143. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Tunes, M.A.; da Silva, F.C.; Camara, O.; Schon, C.G.; Sagas, J.C.; Fontana, L.C.; Donnelly, S.E.; Greaves, G.; Edmondson, P.D. Energetic particle irradiation study of TiN coatings: Are these films appropriate for accident tolerant fuels? J. Nucl. Mater. 2018, 512, 239–245. [Google Scholar] [CrossRef]
- Imtyazuddin, M.; Mir, A.H.; Tunes, M.A.; Vishnyakov, V.M. Radiation resistance and mechanical properties of magnetron-sputtered Cr2AlC thin films. J. Nucl. Mater. 2019, 526, 151742. [Google Scholar] [CrossRef]
- Mráz, S.; Tyra, M.; to Baben, M.; Hans, M.; Chen, X.; Herrig, F.; Lambrinou, K.; Schneider, J.M. Thermal stability enhancement of Cr2AlC coatings on Zr by utilizing a double layer diffusion barrier. J. Eur. Ceram. Soc. 2020, 40, 1119–1124. [Google Scholar] [CrossRef]
- Khatkhatay, F.; Jiao, L.; Jian, J.; Zhang, W.R.; Jiao, Z.J.; Gan, J.; Zhang, H.B.; Zhang, X.H.; Wang, H.Y. Superior corrosion resistance properties of TiN-based coatings on Zircaloy tubes in supercritical water. J. Nucl. Mater. 2014, 451, 346–351. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Lin, M.-I.; Tsai, M.-H.; Shen, W.-J.; Yeh, J.-W. Evolution of structure and properties of multi-component (AlCrTaTiZr)O-x films. Thin Solid Films 2010, 518, 2732–2737. [Google Scholar] [CrossRef]
- Smithells, C.; Gale, W.; Totemeier, T. Smithells Metals Reference Book, 8th ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]
- Cheng, Y.Q.; Ma, E. Atomic-level structure and structure-property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Kittel, C.; McEuen, P.; McEuen, P. Introduction to Solid State Physics; Wiley New York: New York, NY, USA, 1996. [Google Scholar]
- Ohring, M. Materials Science of Thin Films: Deposition and Structure, 2nd ed.; Academic Press: San Diego, CA, USA, 2002; ISBN 0-12r-r524975-6. [Google Scholar]
- Zhao, S.; Wang, P.; Cheng, X.; Zhang, Y.; Wen, Z.; Zhang, Q.; Yao, K.-F.; Chen, N.; Wang, W.-H. Anomalous low-temperature transport property of oxygen containing high-entropy Ti-Zr-Hf-Cu-Ni metallic glass thin films. Sci. China Mater. 2019, 62, 907–912. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tang, X.; Jiang, G.; Fang, K.; Yao, K.; Zhang, Z.; Chen, N.; Shan, Z. A high-strength Co–Fe–Ta–B metallic-glass phase enabled tensile plasticity in Co–Fe–Ta–B–O oxide glass matrix nanocomposites. Appl. Phys. Lett. 2020, 116, 081903. [Google Scholar] [CrossRef]
- Chen, N.; Fang, K.; Zhang, H.; Zhang, Y.; Liu, W.; Yao, K.; Zhang, Z. Amorphous magnetic semiconductors with Curie temperatures above room temperature. J. Semicond. 2019, 40, 081510. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Luan, H.; Yang, X.; Wang, X.; Fang, K.; Li, J.; Jia, Y.; Yao, K.; Zhang, Z.; Chen, N. Formation and Properties of Amorphous Multi-Component (CrFeMoNbZr)Ox Thin Films. Metals 2020, 10, 599. https://doi.org/10.3390/met10050599
Gu X, Luan H, Yang X, Wang X, Fang K, Li J, Jia Y, Yao K, Zhang Z, Chen N. Formation and Properties of Amorphous Multi-Component (CrFeMoNbZr)Ox Thin Films. Metals. 2020; 10(5):599. https://doi.org/10.3390/met10050599
Chicago/Turabian StyleGu, Xiaoyu, Hengwei Luan, Xinglong Yang, Xinchao Wang, Kaixuan Fang, Jinfeng Li, Yuzhen Jia, Kefu Yao, Zhengjun Zhang, and Na Chen. 2020. "Formation and Properties of Amorphous Multi-Component (CrFeMoNbZr)Ox Thin Films" Metals 10, no. 5: 599. https://doi.org/10.3390/met10050599
APA StyleGu, X., Luan, H., Yang, X., Wang, X., Fang, K., Li, J., Jia, Y., Yao, K., Zhang, Z., & Chen, N. (2020). Formation and Properties of Amorphous Multi-Component (CrFeMoNbZr)Ox Thin Films. Metals, 10(5), 599. https://doi.org/10.3390/met10050599





