Setting of Natural Fracture Splitting Surface on Connecting Rod and Its Formation Mechanism
Abstract
1. Introduction
2. Process Conditions and Experiment
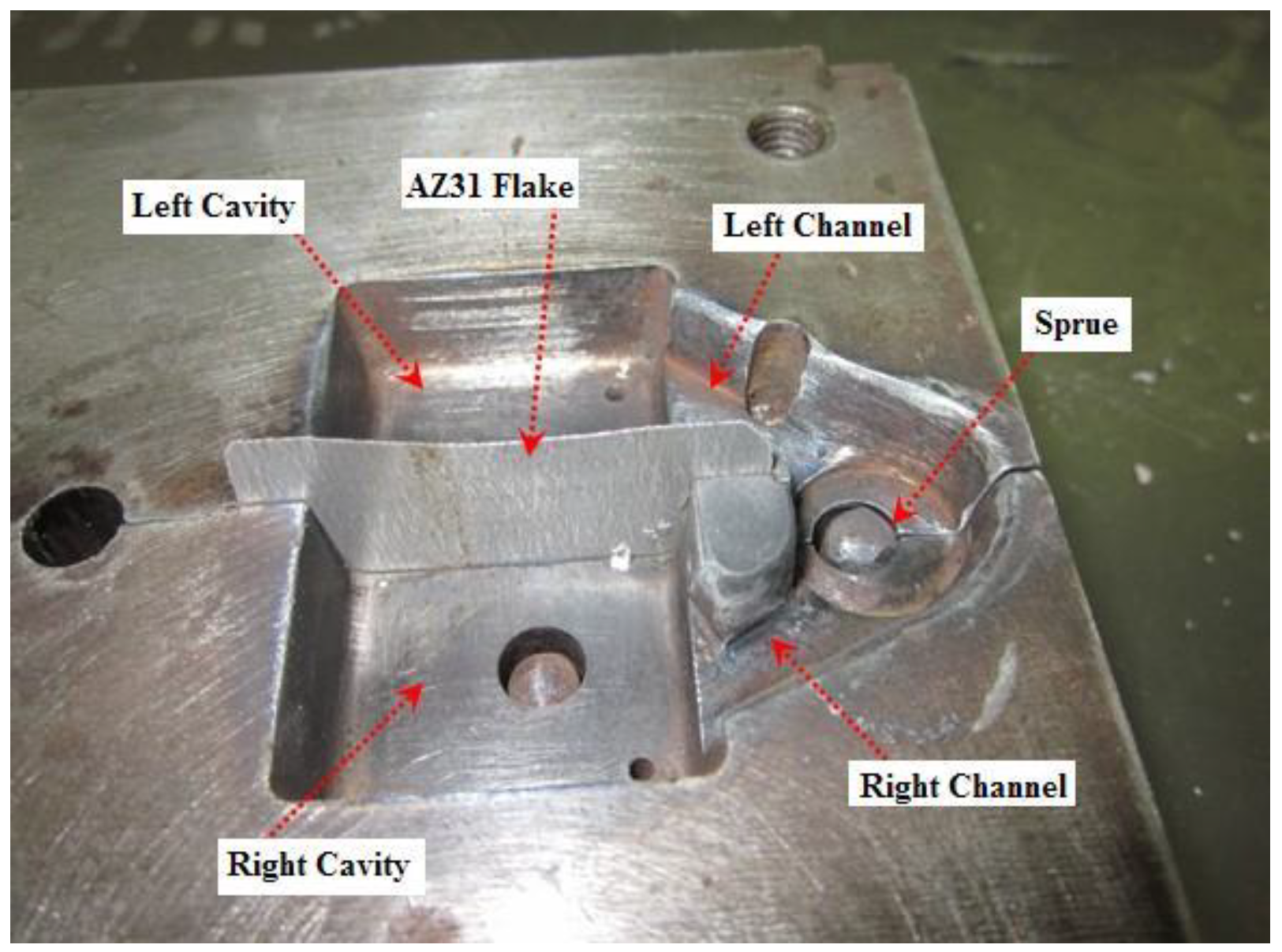
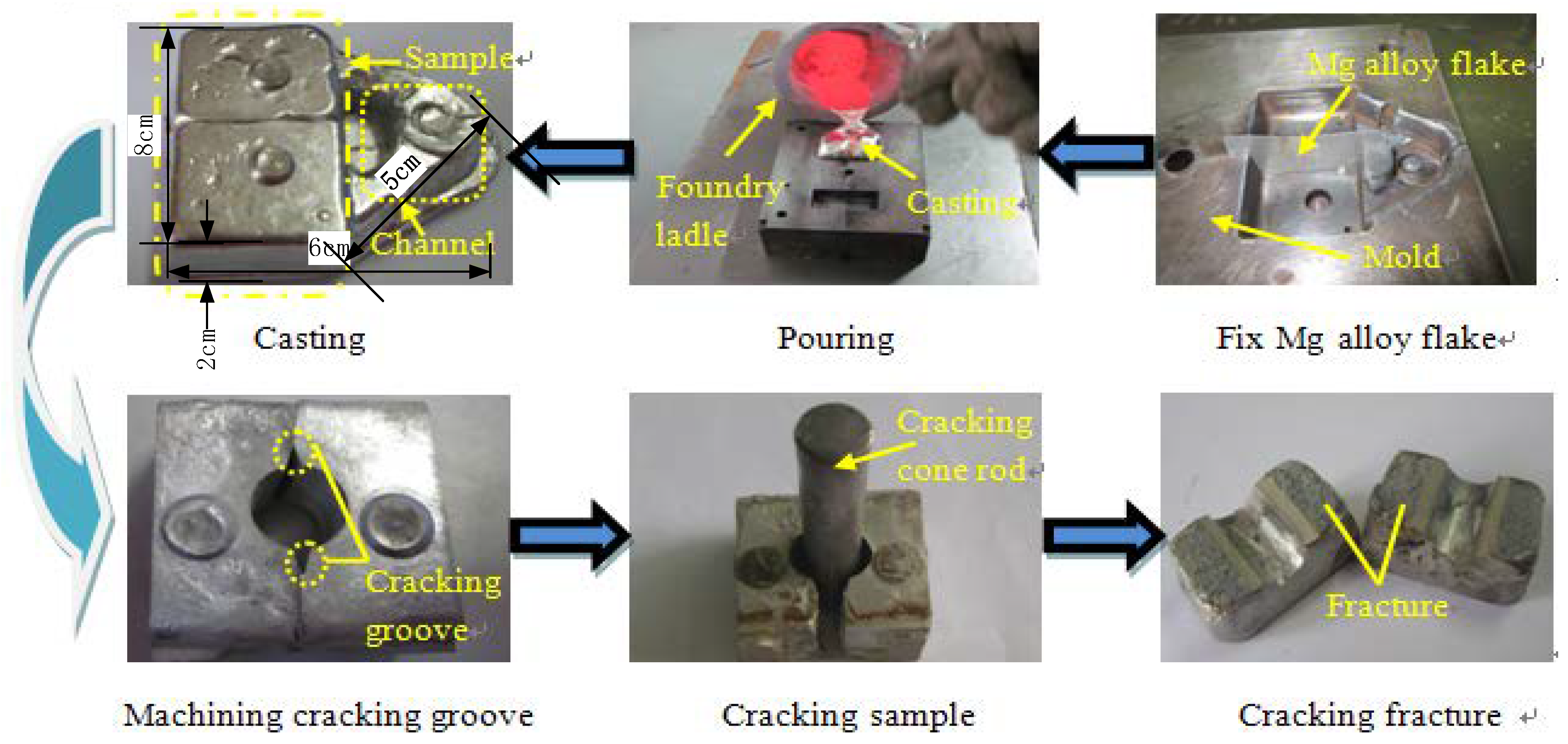
2.1. Experimental Equipment
2.2. Natural Fracture Splitting Process Experiment
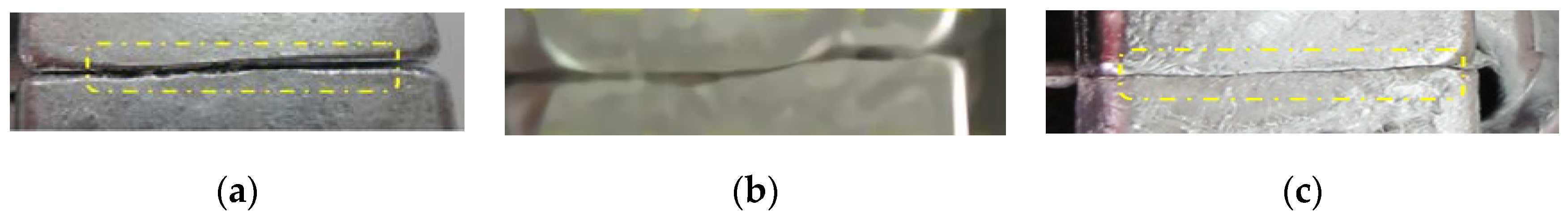
2.3. Influence of Magnesium Alloy Foil Thickness on the Quality of the Interface
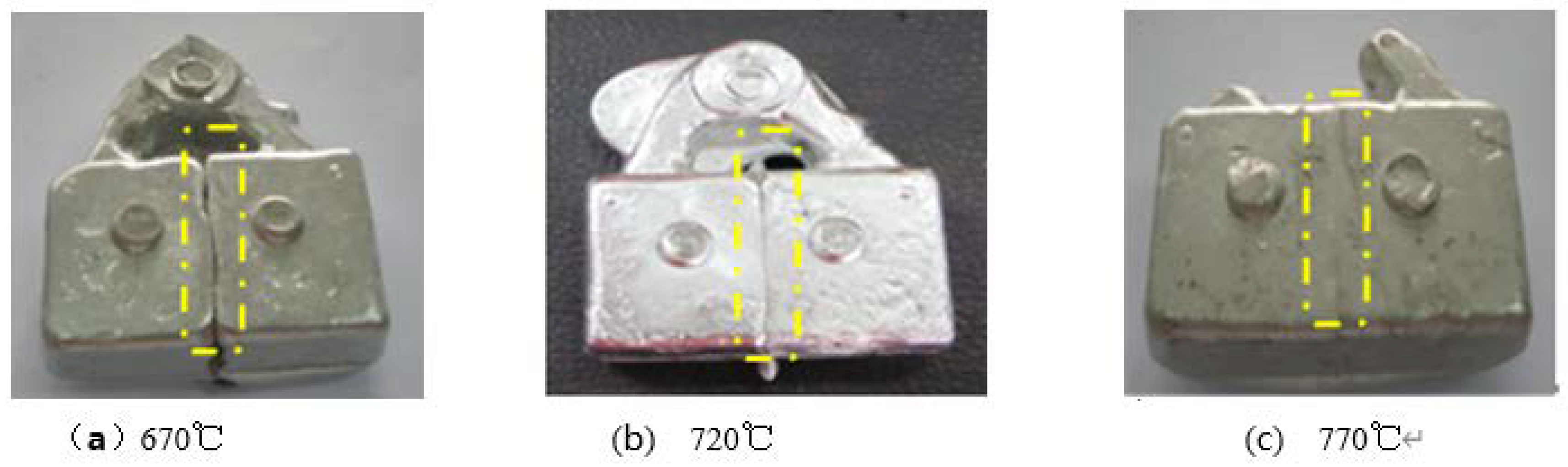
2.4. Influence of Casting Temperature on the Quality of the Interface
3. Fracture Surface Characteristics and Formation Mechanism of Fracture Splitting Surface
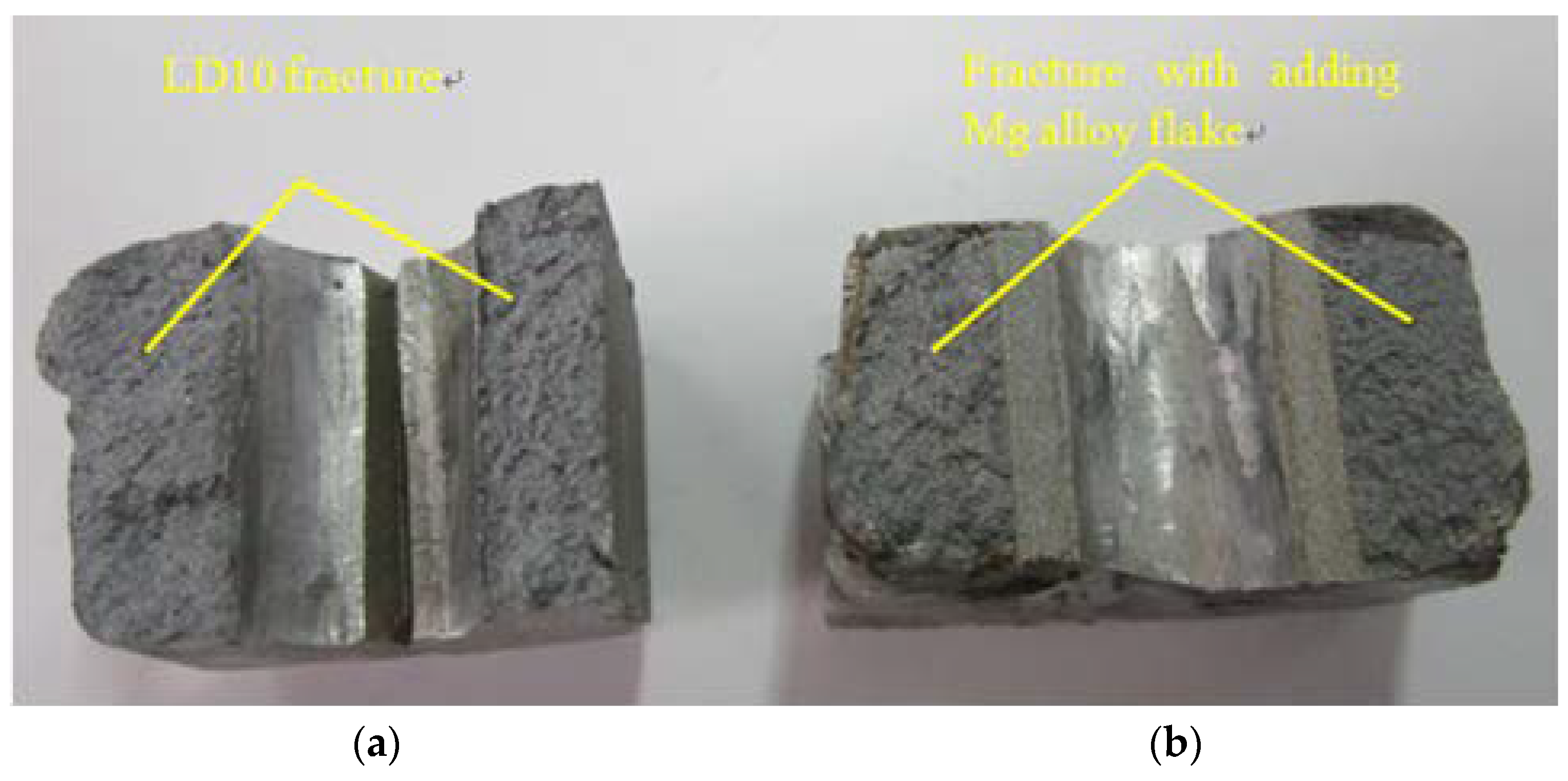
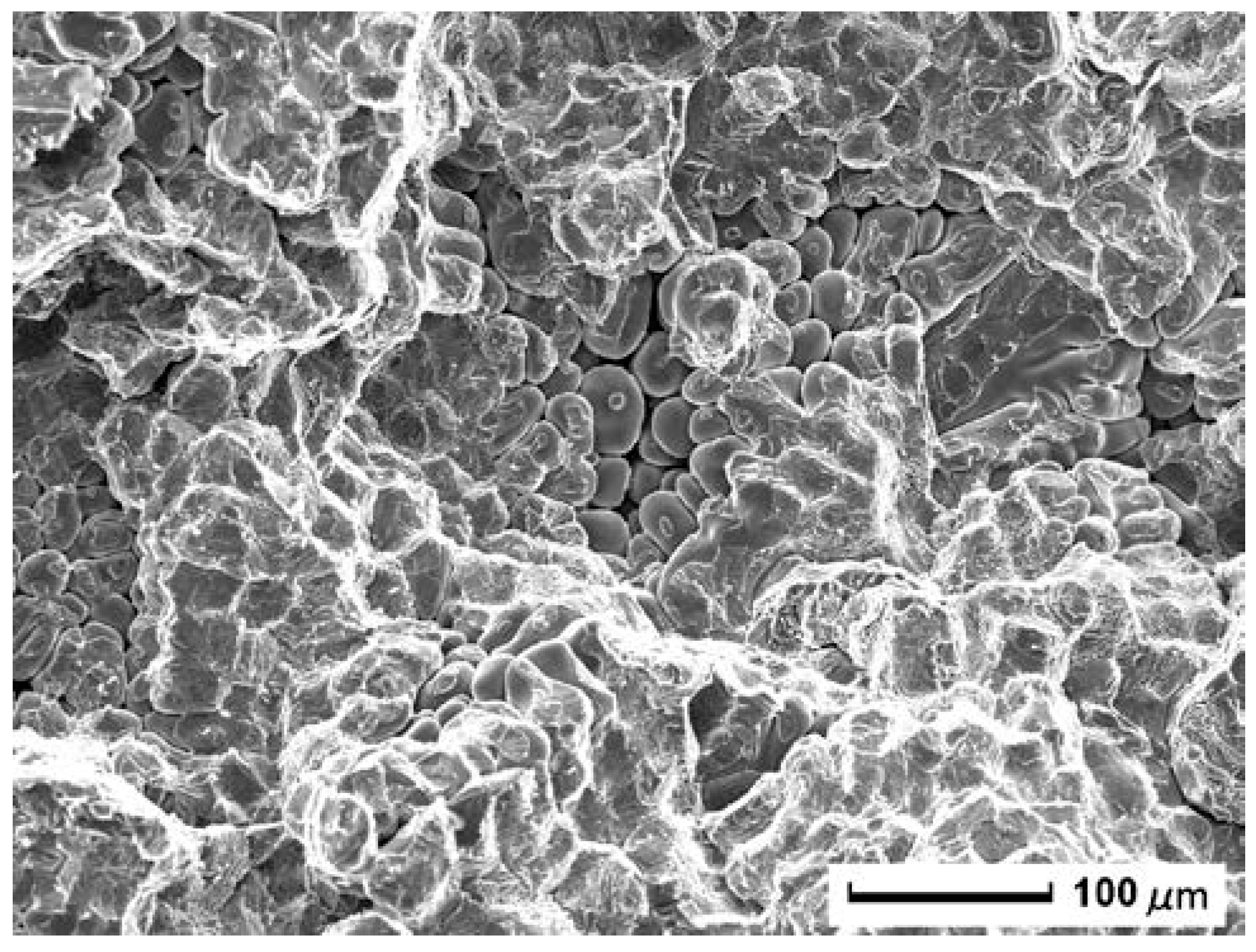
3.1. Fracture Surface Characteristics
3.2. Analysis of Formation Mechanism of Fracture Splitting Surface
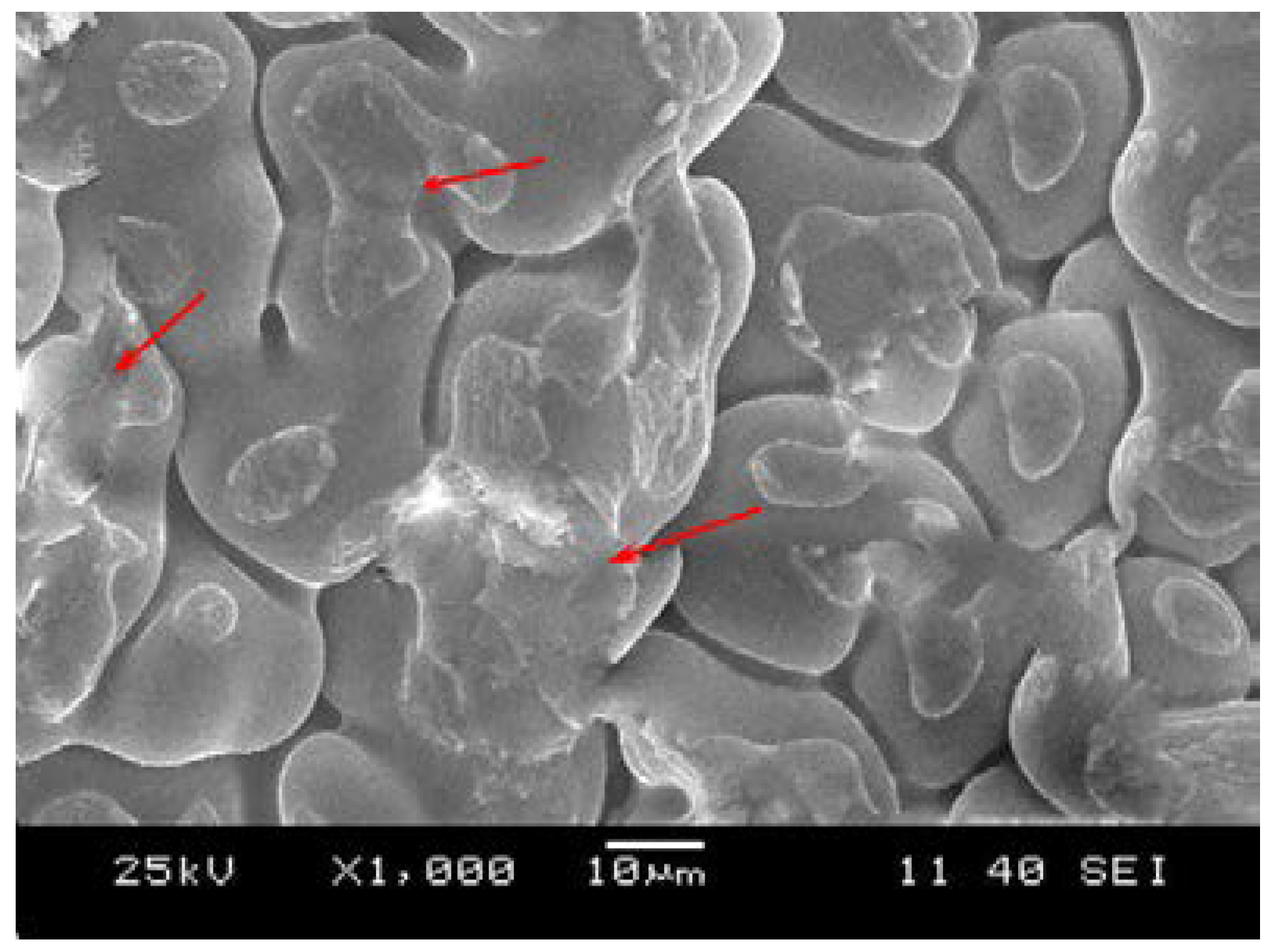
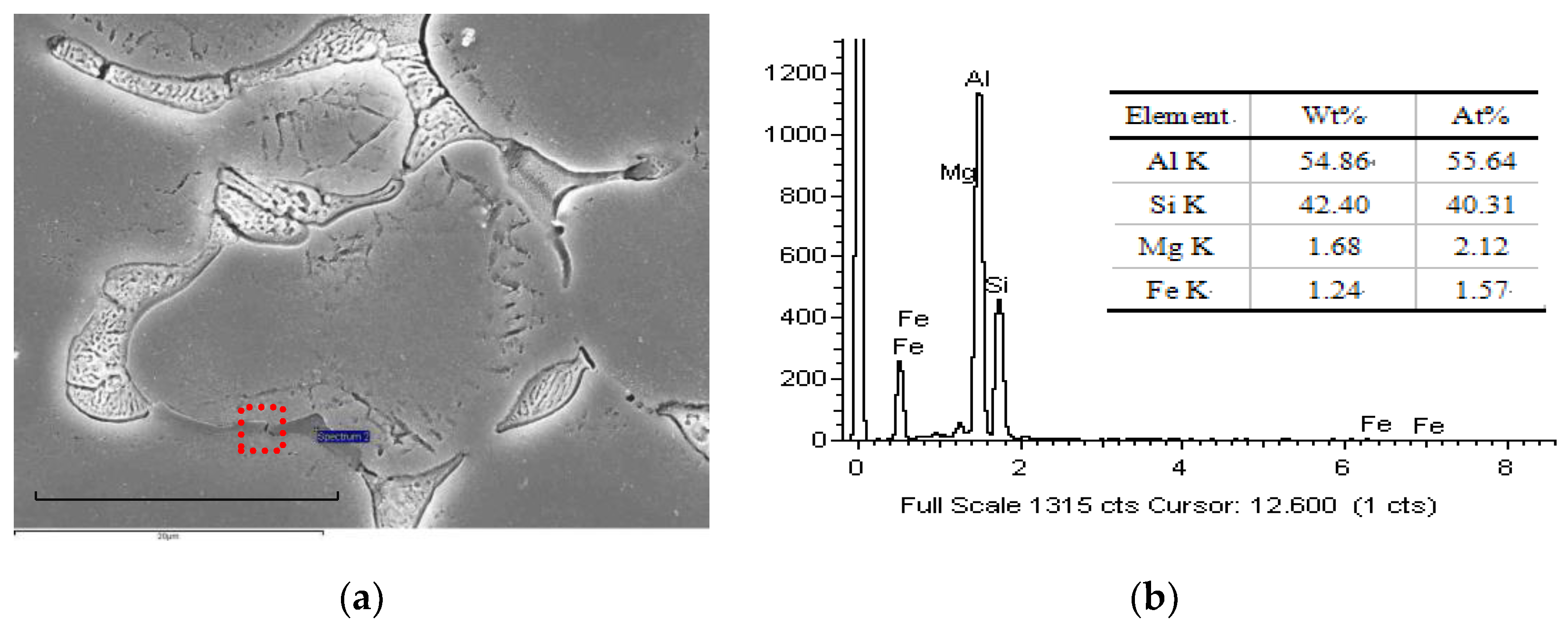
3.2.1. Formation of Oxidation Film Fracture Splitting Surface
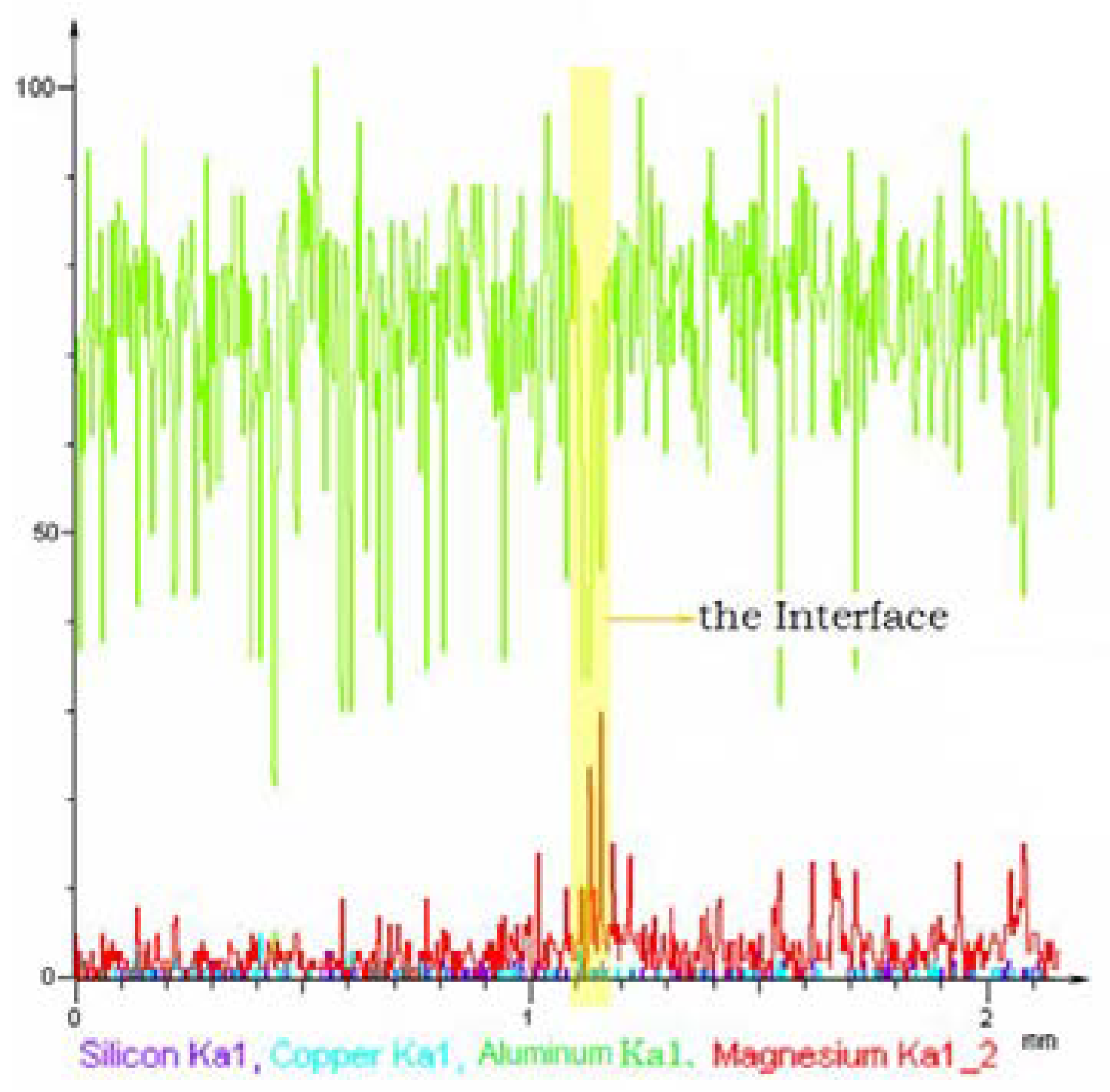
3.2.2. Formation of Liquid Film Fracture Splitting Surface
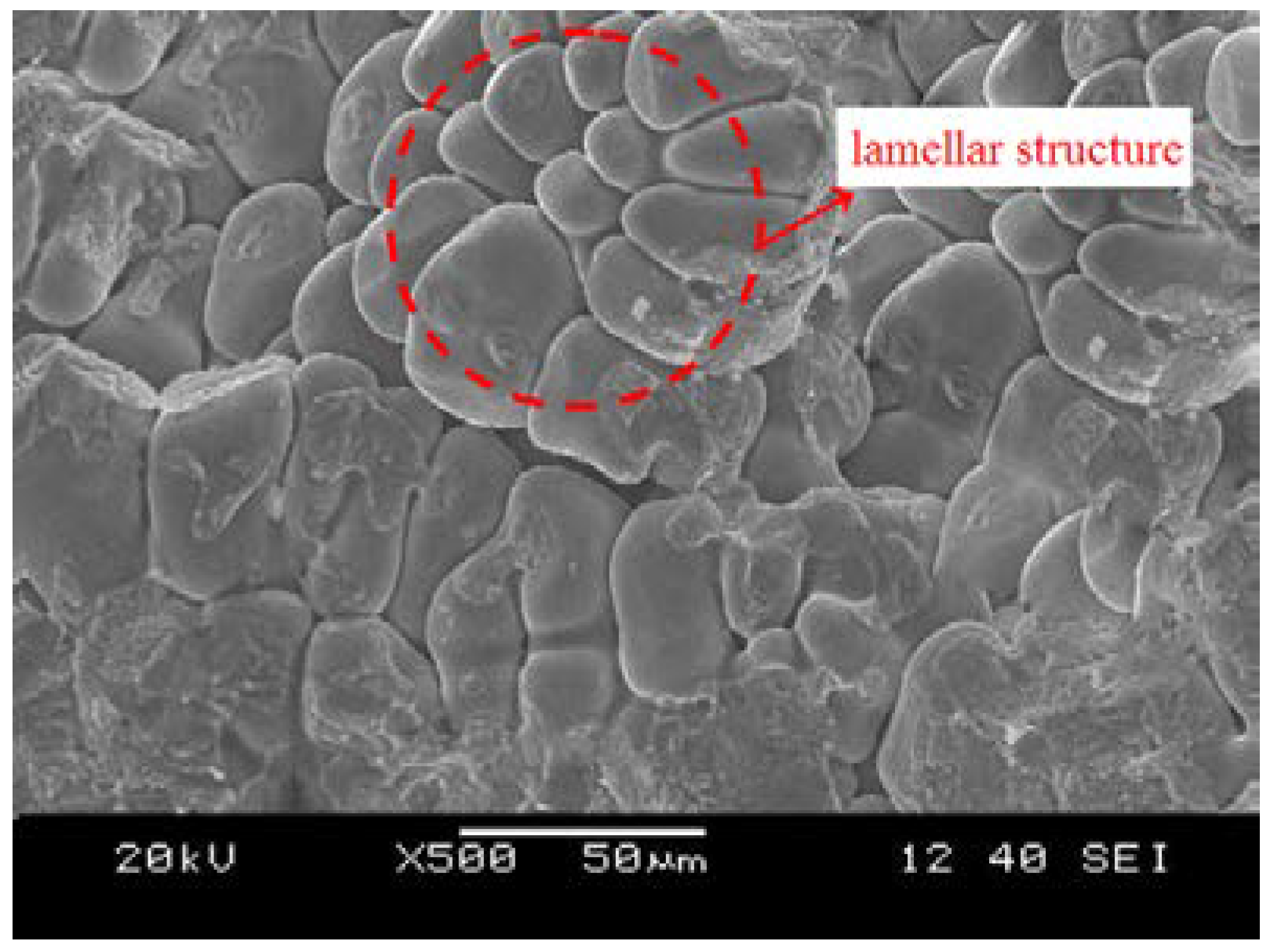
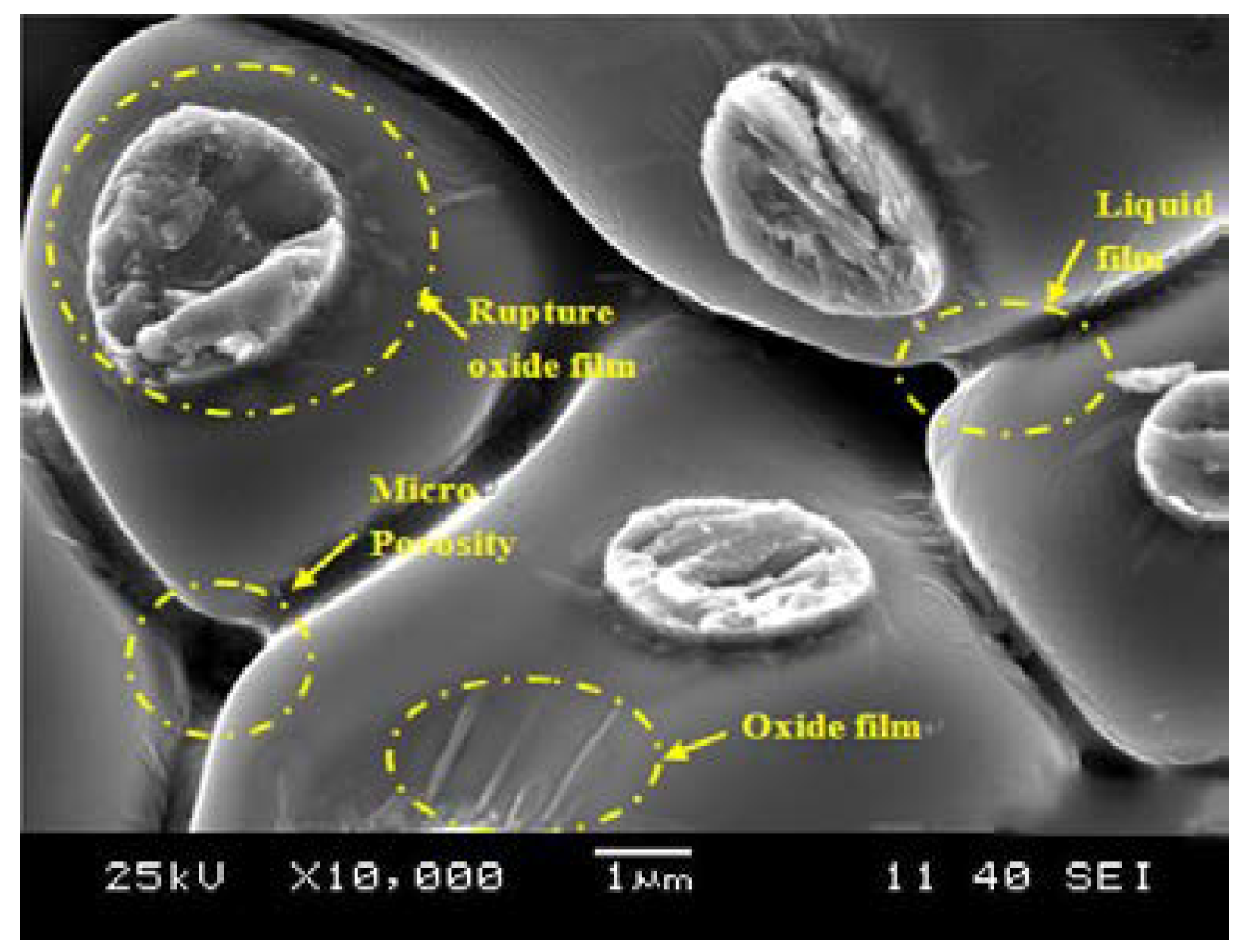
3.2.3. Formation of Micro-Porosity Fracture Splitting Surface
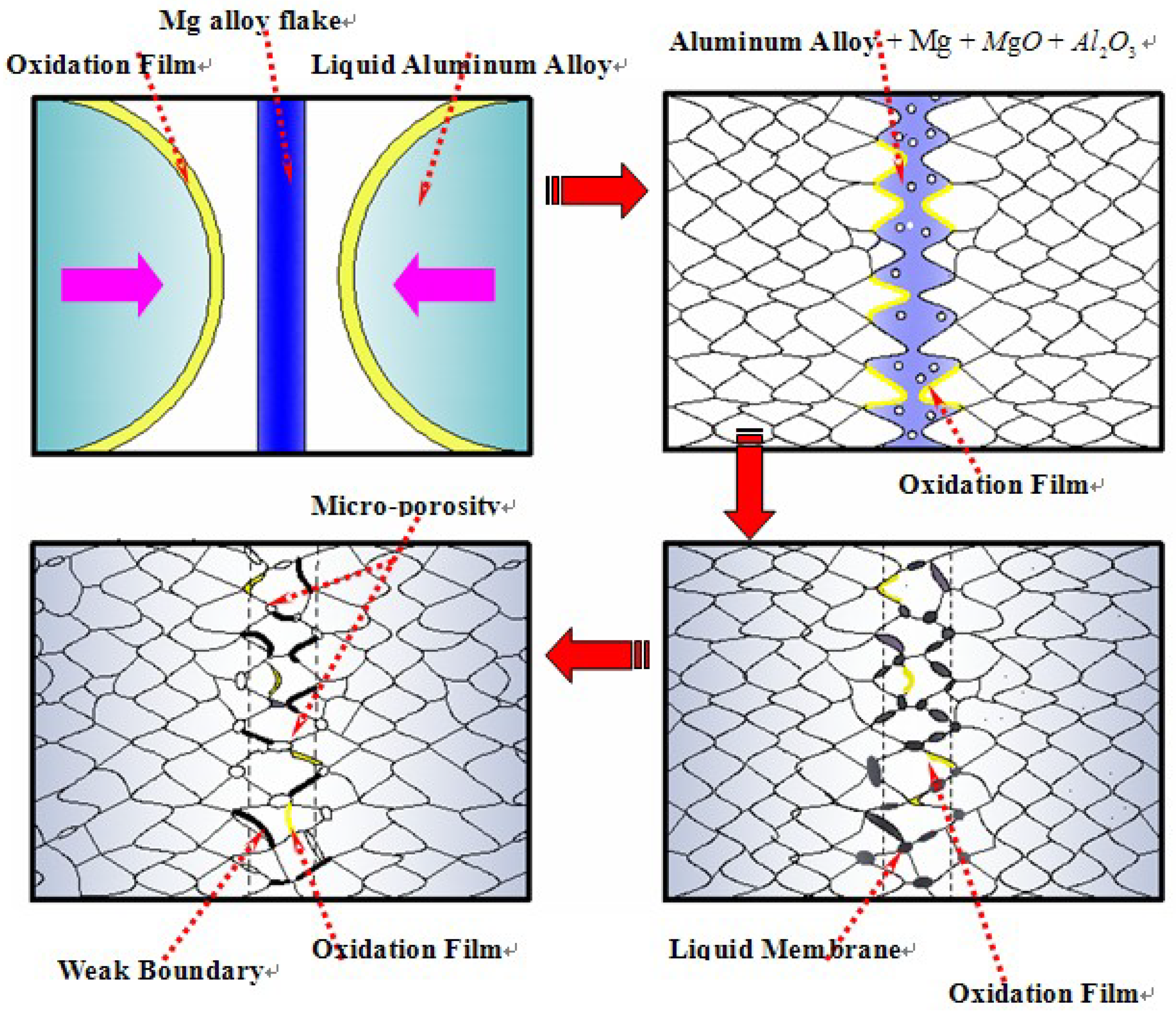
3.3. Comprehensive Analysis Of Natural Fracture Splitting Mechanism
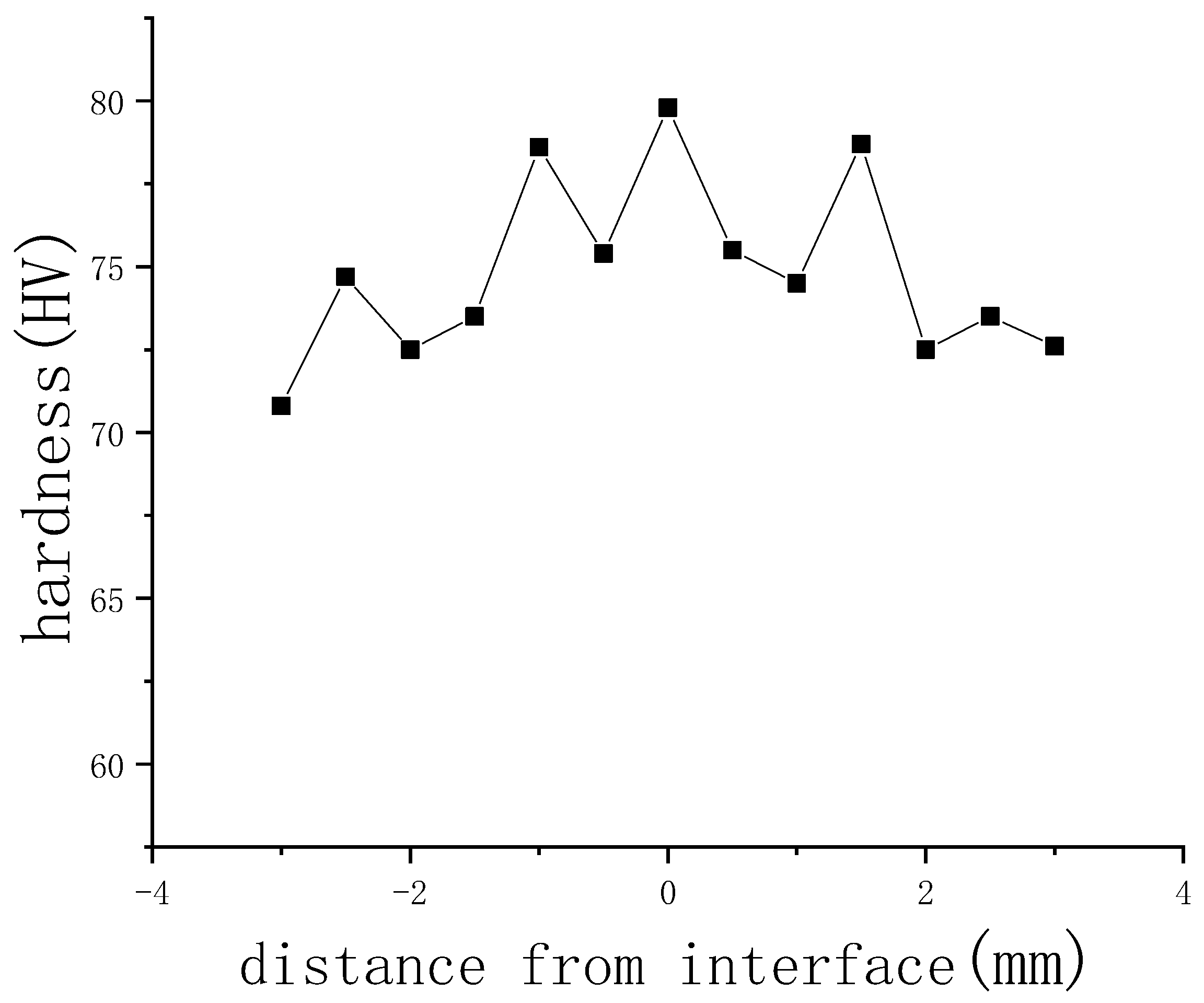
3.4. Mechanical Performance Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fukuda, S.; Eto, H. Development of fracture splitting connecting rod. JSAE Rev. 2002, 23, 101–104. [Google Scholar] [CrossRef]
- Shuqing, K.O.; Wenming, Y.S.C. Fracture splitting technology-the advanced method in manufacturing engine connecting rod. China Mech. Eng. 2001, 7, 32. [Google Scholar]
- Shuqing, K.; Wenming, J.; Zhengwei, G.; Shenhua, Y. The new technology and developing trend of IC engine connecting rod manufacturing. Chin. Intern. Combust. Engine Eng. 2001, 1, 6. [Google Scholar]
- Gu, Z.; Yang, S.; Ku, S.; Zhao, Y.; Dai, X. Fracture splitting technology of automobile engine connecting rod. Int. J. Adv. Manuf. Technol. 2005, 25, 883–887. [Google Scholar] [CrossRef]
- Yu, G.; Yang, S.; Kou, S.; Wang, M. Influence of starting notch geometry parameters for engine crankshaft bearing block on fracture splitting force. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering (RSETE), Nanjing, China, 24–26 June 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 3219–3223. [Google Scholar]
- Kubota, T.; Iwasaki, S.; Isobe, T. Fracture Split Method for Connecting Rod. U.S. Patent 6,961,997, 8 December 2005. [Google Scholar]
- Ji, Y.; Sun, X.; Zhao, F.; Zhang, C.; Jiang, B.; Liu, Y. Austenite grain growth behavior and its effects on fracture splitting performance of 36MnVS4 connecting rod for automobile engine. Kov. Mater. Met. Mater. 2018, 56, 113–120. [Google Scholar] [CrossRef]
- Weiwen, Z.; Cangshan, Z.; Xingzhao, W.; Jilong, M.; Shugui, Y.; Ge, Y. Double-stream-pouring technique of production of gradient materials by continuous casting. Chin. Sci. Bull. 1998, 43, 911–914. [Google Scholar] [CrossRef]
- Zheng, L.; Kou, S.; Yang, S.; Li, L.; Li, F. A study of process parameters during pulsed Nd: YAG laser notching of C70S6 fracture splitting connecting rods. Opt. Laser Technol. 2010, 42, 985–993. [Google Scholar] [CrossRef]
- Weber, M. Cost Effective Finishing of Power Forged Connecting Rods with the Fracture Splitting Method; SAE Technical Paper Series, No. 910157; Society of Automotive Engineers, Inc.: Detroit, MI, USA, 1991. [Google Scholar]
- Whittaker, D. The competitions for automotive connecting rod markets. Met. Powder Rep. 2001, 56, 32–37. [Google Scholar] [CrossRef]
- Jiang, D.M.; Wang, C.L.; Yu, J.; Gao, Z.Z.; Shao, Y.T.; Hu, Z.M. Cleavage and intergranular fracture in Al–Mg alloys. Scr. Mater. 2003, 49, 387–392. [Google Scholar] [CrossRef]
- Yang, D.; Ning, Y. Numerical analysis on crack tip of fracture splitting groove stress-strain field of main bearing block of engine. In Proceedings of the 2017 5th International Conference on Frontiers of Manufacturing Science and Measuring Technology (FMSMT 2017), Taiyuan, China, 24–25 June 2017; Atlantis Press: Paris, France, 2017. [Google Scholar]
- Jiang, F.; Zhang, Z.; Zhang, J.; Zhang, J.; Zhu, Y.; Sun, J. In-situ SEM observation of crack propagation along explosive clad interface. Chin. J. Nonferr. Met. 2005, 15, 1493. [Google Scholar]
- Kou, S.Q.; Wang, Y.J.; Yang, C.G.; Yang, S.H. Key techniques in numerical simulation and crack starting analysis for con-Rod fracture splitting under static loading. Chin. Intern. Combust. Engine Eng. 2008, 1, 14. [Google Scholar]
- Renguo, S.; Meikuang, T. A new method for predicting grain boundary segregation and intergranular embrittlement. Min. Metall. Eng. 1996, 16, 68–70. [Google Scholar]
- Liu, L.; Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Valtierra, S. Influence of oxides on porosity formation in Sr-treated Al-Si casting alloys. J. Mater. Sci. 2003, 38, 1255–1267. [Google Scholar] [CrossRef]
- Hui, W.; Chen, S.; Zhang, Y.; Shao, C.; Dong, H. Effect of vanadium on the high-cycle fatigue fracture properties of medium-carbon microalloyed steel for fracture splitting connecting rod. Mater. Des. 2015, 66, 227–234. [Google Scholar] [CrossRef]
- Kou, S.; Shi, Z.; Song, W. Fracture-splitting processing performance study and comparison of the C70S6 and 36MnVS4 connecting rods. SAE Int. J. Engines 2018, 11, 463–474. [Google Scholar] [CrossRef]
- Jiang, Y.; Sha, D.; Wang, K.; Qian, J. Properties and fracture splitting of cast steel matrix composites. Trans. Can. Soc. Mech. Eng. 2018, 43, 164–172. [Google Scholar] [CrossRef]


| Mg | Cu | Si | Mn | Fe | Ti | Zn | Al |
|---|---|---|---|---|---|---|---|
| 0.4–0.8 | 3.9–4.8 | 0.6–1.2 | 0.4–1 | ≤0.7 | ≤0.15 | ≤0.3 | Remain |
| Al | Zn | Mn | Cu | Fe | Be | Si | Mg |
|---|---|---|---|---|---|---|---|
| 3.0–3.1 | 0.9–1.0 | 0.2 | 0.05 | 0.05 | 0.02 | 0.15 | Remain |
| The AZ31 Foil Thickness (mm) | 0.12 | 0.24 | 0.30 |
|---|---|---|---|
| Casting temperature (°C) | 720 | 720 | 720 |
| Foil fusion state | Complete fusion | Failed fusion at the edge | Failed fusion |
| Fracture splitting surface bonding quality | High bonding strength | Failed bonding locally | Obvious overlap trace on surface |
| Fracture quality | Flaky brittle fracture | Poor fracture quality | Serious dropping dregs |
| Casting temperature (°C) | 670 | 720 | 770 |
|---|---|---|---|
| Casting situation | Without combustion of the AZ31 foil | Without combustion of the AZ31 foil | Combustion of the AZ31 foil |
| Fusion situation | Relatively large fusion trace | Relatively small fusion trace | Complete fusion |
| Temperature suitability | Relatively low | Suitable | Relatively high |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhang, Y. Setting of Natural Fracture Splitting Surface on Connecting Rod and Its Formation Mechanism. Metals 2020, 10, 590. https://doi.org/10.3390/met10050590
Zhang F, Zhang Y. Setting of Natural Fracture Splitting Surface on Connecting Rod and Its Formation Mechanism. Metals. 2020; 10(5):590. https://doi.org/10.3390/met10050590
Chicago/Turabian StyleZhang, Fengjun, and Yi Zhang. 2020. "Setting of Natural Fracture Splitting Surface on Connecting Rod and Its Formation Mechanism" Metals 10, no. 5: 590. https://doi.org/10.3390/met10050590
APA StyleZhang, F., & Zhang, Y. (2020). Setting of Natural Fracture Splitting Surface on Connecting Rod and Its Formation Mechanism. Metals, 10(5), 590. https://doi.org/10.3390/met10050590




